Abstract
Double-stranded RNA (dsRNA) is produced during the replication cycle of most viruses and triggers antiviral immune responses through Toll-like receptor 3 (TLR3). However, the molecular mechanisms and subcellular compartments associated with dsRNA-TLR3-mediated signaling are largely unknown. Here we show that c-Src tyrosine kinase is activated by dsRNA in human monocyte-derived dendritic cells, and is recruited to TLR3 in a dsRNA-dependent manner. DsRNA-induced activation of interferon-regulatory factor 3 and signal transducer and activator of transcription 1 was abolished in Src kinase-deficient cells, and restored by adding back c-Src, suggesting a central role of c-Src in antiviral immunity. We also provide evidence that TLR3 is localized in the endoplasmic reticulum of unstimulated cells, moves to dsRNA-containing endosomes in response to dsRNA, and colocalizes with c-Src on endosomes containing dsRNA in the lumen. These results provide novel insight into the molecular mechanisms of TLR3-mediated signaling, which may contribute to the understanding of innate immune responses during viral infections.
Keywords: dendritic cells, signal transduction, Src tyrosine kinase, Toll-like receptor 3
Introduction
Double-stranded RNA (dsRNA) is a molecular pattern produced during viral infections that stimulates production of type I interferons (IFNs), thus initiating antiviral immune responses (Jacobs and Langland, 1996). Also, endogenous RNAs forming secondary double-stranded structures that are released after necrosis and tissue damage can activate cellular IFN production, thus representing host-derived inducers of dsRNA-mediated inflammatory responses. In addition to IFN production, other cellular effects of dsRNA that contribute to antiviral defense mechanisms include termination of protein synthesis and induction of apoptosis, processes partly mediated by the IFN-inducible protein kinase PKR (Der et al, 1997). Certain viruses, such as Epstein–Barr virus, human immunodeficiency virus type 1, and human adenovirus, have evolved strategies to circumvent dsRNA-induced defense reactions in the responding cells, for example, by producing short, highly structured single-stranded RNAs and by inhibiting the host cell death machinery to impair the antiviral actions stimulated by dsRNA (Jacobs and Langland, 1996).
Several eukaryotic proteins interact with dsRNA to regulate gene expression during viral infections, for example, the dsRNA-activated protein kinase PKR, and the RNase III Dicer acting in RNA interference. Believed to be more important for mounting an antiviral innate immune response in response to dsRNA are the RNA helicases RIG-I and MDA5, and Toll-like receptor (TLR)3 (Tabeta et al, 2004; Kato et al, 2006). The TLR family consists of at least 11 family members, where the members sense the presence of microbial compounds ranging from lipids such as LPS (TLR4) and lipoproteins (TLR2 heterodimerizing with TLR1 or TLR6), proteins (flagellin, TLR5) to viral and bacterial nucleic acids (ss/ds RNA/DNA; interacting with TLR3, TLR7, TLR8, TLR9). TLRs are type I transmembrane receptors that contain an extracellular leucine-rich repeat domain and a highly conserved cytoplasmic Toll/IL-1 receptor (TIR) domain that is homologous to that of the interleukin-1 receptor family (Akira and Takeda, 2004). Signaling mediated by TLRs is initiated by recruitment and homophilic interaction with the different cytosolic TIR domain-containing TLR adapter proteins MyD88, TIRAP/Mal, TRIF/TICAM, or TRAM/TICAM2. Different TLRs can activate distinct cellular responses to pathogens, and the specific response of individual TLRs has been ascribed to differential usage of TLR adapter proteins. TLR3 responds to dsRNA and stimulates the IFN-β promoter, which is regulated by the transcription factors interferon regulatory factor (IRF)-3, NF-κB, and ATF-2/c-Jun (Oshiumi et al, 2003). Interestingly, TLR3-mediated signaling utilizes the TLR adapter protein TRIF and is independent of both the adapter protein MyD88 and the IL-1 receptor-associated protein kinases utilized by other TLRs, thus demonstrating TLR3-specific signaling mechanisms (Oshiumi et al, 2003; Yamamoto et al, 2003; Jiang et al, 2004). Both TLR3 and TRIF participate in the in vivo defense against viral infections (Hoebe et al, 2003; Tabeta et al, 2004). It has recently been found that TLR3-mediated IFN-β production and NF-κB activation diverge at TRIF (Jiang et al, 2004) and that TLR3 utilizes the adapter protein TRIF to engage downstream signaling proteins, such as TRAF3, the non-canonical IκB kinases, TBK-1 and IKKɛ to activate IRF-3, and TRAF6 or receptor interacting protein-1 (RIP-1) to stimulate NF-κB (Fitzgerald et al, 2003; Meylan et al, 2004; Hoebe and Beutler, 2006). Recently, tyrosine phosphorylation of TLR3 has been implicated in IRF-3 activation, but the identity of the tyrosine kinase involved in TLR3-mediated signaling has not been established.
C-Src is a member of the highly conserved Src family of protein tyrosine kinases, which consists of nonreceptor tyrosine kinases that display different expression patterns and have been implicated in numerous cellular processes, such as innate immune responses, and signaling induced by integrins, cytokines, antigens, and growth factors. C-Src contains an N-terminal myristoylation site targeting it to cellular membranes, and Src-homology (SH)2 and SH3 domains, which are implicated in its activation. The SH2 and SH3 domains bind to phosphotyrosine and proline-rich motifs, respectively, and mediate the interaction of c-Src with its upstream binding partners and its cellular substrates. C-Src is tyrosine phosphorylated at the two major sites Tyr416 and Tyr527, of which the autophosphorylation at Tyr416 is required for c-Src activity (Thomas and Brugge, 1997). Here we show that c-Src is required for TLR3-mediated activation of the transcription factors IRF-3 and signal transducer and activator of transcription 1 (STAT-1), indicating that c-Src is implicated in antiviral defense mechanisms. Furthermore, we show that TLR3 moves to endosomes upon dsRNA challenge, where it interacts with c-Src.
Results
The tyrosine kinase c-Src is activated by dsRNA, associates with TLR3, and is essential for dsRNA-elicited IRF-3 and STAT-1 activation
Phosphorylation of two specific tyrosine residues in the cytoplasmic domain of TLR3 has been shown to be essential for dsRNA-elicited induction of the human 561 gene, which depends on IRF-3 activation. However, the identity of the tyrosine kinase involved has not been determined. Therefore, we investigated if c-Src, a ubiquitously expressed member of the Src tyrosine kinase family (Thomas and Brugge, 1997), was involved in dsRNA-induced signaling. First, we found that dsRNA induced a time-dependent activation of c-Src in human monocyte-derived dendritic cells (mDCs) as assessed by phosphorylation of Tyr416 (Figure 1A), an event that is required for its kinase activity. Next, we examined if c-Src interacted with TLR3. Flag-tagged TLR3 was immunoprecipitated from dsRNA-treated HEK-TLR3Flag cells before immunoblotting for c-Src or activated Src (SrcpY416). Activated c-Src was recruited to TLR3 in a dsRNA-dependent manner (Figure 1B), suggesting that c-Src is involved in TLR3-mediated signaling.
Figure 1.
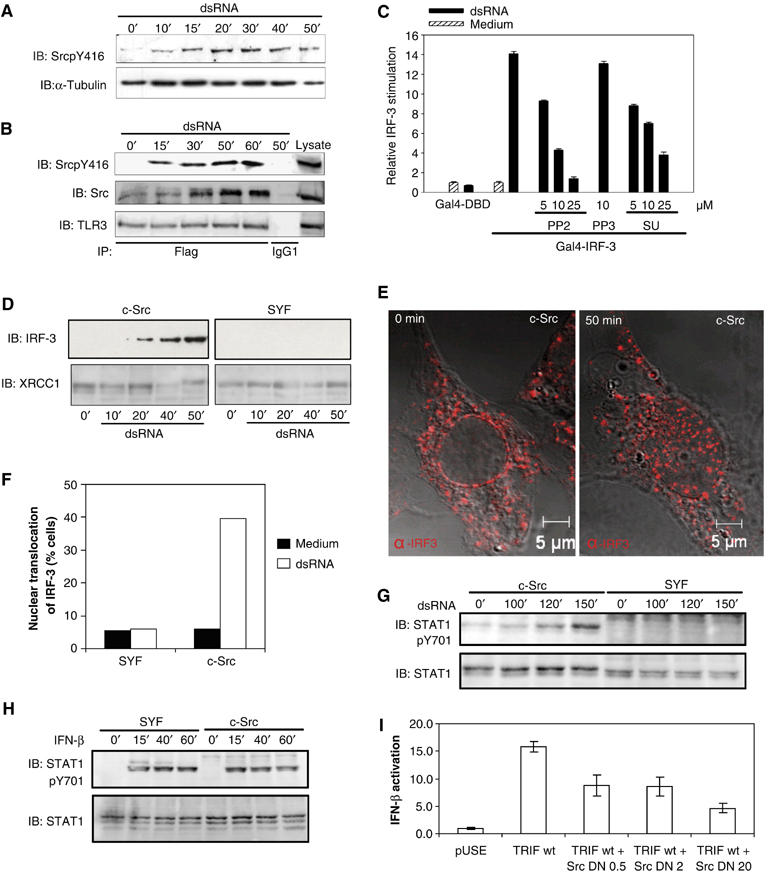
The tyrosine kinase c-Src is activated by dsRNA, associates with TLR3 and is required for dsRNA-induced IRF-3 and STAT-1 activation. (A) Human mDCs were incubated with dsRNA (100 μg/ml) and activation of c-Src was analyzed by immunoblotting (IB) with an antibody specific to activated phospho-Src. (B) HEK cells expressing Flag-tagged TLR3 were stimulated with dsRNA (100 μg/ml) and lysates were immunoprecipitated (IP) with anti-Flag or mouse IgG and immunoblotted (IB) for the indicated proteins. (C) HEK293 cells were transiently cotransfected with plasmids encoding TLR3 and a luciferase reporter gene containing the Gal4 upstream activation sequence, and expression vectors for Gal4-DBD or Gal4-IRF-3. After 24 h, cells were treated with PP2, PP3, or SU6656 before stimulation with dsRNA. (D) Nuclear extracts were prepared from dsRNA-treated SYF and c-Src-expressing control cells and analyzed by immunoblotting (IB) with an IRF-3 antibody. The blots were reprobed with the nuclear protein XRCC1 as a loading control. (E, F) Determination of IRF-3 localization in c-Src-, Yes-, and Fyn-deficient cells and c-Src-expressing control cells (c-Src) by confocal microscopy. (E) Cells were treated or not with dsRNA and stained intracellularly for IRF-3 (Alexa546). (F) Average percentages of IRF-3 nuclear translocation in SYF cells and c-Src-expressing control cells with nuclear IRF-3 as assessed by confocal microscopy. A total of 200 cells were counted under the different conditions. (G, H) DsRNA-elicited or IFN-β-induced activation of STAT-1 in SYF and c-Src-expressing cells was determined by immunoblotting with an antibody specific to activated STAT-1 (Y701) and STAT-1. (I) HEK293 cells were transiently cotransfected with vectors encoding TRIF, 0.5, 2, or 20 ng of kinase-inactive c-Src (K297R), and a luciferase reporter gene for IFN-β. Luciferase reporter gene activity was measured after 24 h.
The transcription factor IRF-3 plays an essential role in antiviral defense mechanisms through its regulation of IFN-β gene expression (Wathelet et al, 1998). To investigate the functional significance of the c-Src tyrosine kinase in TLR3-mediated signaling, we first examined the effect of pharmacological inhibitors of c-Src on dsRNA-induced activation of IRF-3. IRF-3 activation was assessed in an assay in which the yeast Gal4 DNA-binding domain (DBD) fused to IRF-3 lacking its own DBD (Wathelet et al, 1998) and the Gal4 upstream activation sequence coupled to luciferase were cotransfected into HEK293 cells. The Src family kinase inhibitors PP2 and SU6656 dose-dependently and significantly reduced dsRNA-elicited IRF-3 activation, whereas PP3, an inactive PP2 analogue, had no significant effect (Figure 1C), indicating that c-Src contributes to dsRNA-induced antiviral responses.
To further examine the importance of c-Src for cellular activation, we investigated dsRNA-elicited IRF-3 activation in immortalized mouse embryonic fibroblasts lacking expression of the Src family kinases Src, Yes, and Fyn (SYF cells). The dsRNA-elicited nuclear translocation of IRF-3 in SYF cells was compared to SYF cells in which c-Src has been introduced (Klinghoffer et al, 1999). Immunoblot analysis of nuclear extracts showed that dsRNA stimulated nuclear translocation of IRF-3 in Src-containing cells, but not in SYF cells lacking c-Src expression (Figure 1D). Also, visualization of IRF-3 localization in SYF and c-Src cells by confocal microscopy confirmed that dsRNA-induced nuclear translocation of IRF-3 was dependent on c-Src (Figure 1E and F).
The transcription factor STAT-1 regulates gene expression in response to type I IFNs and is essential for the ability to combat viral infections (Durbin et al, 1996). We found that dsRNA stimulated phosphorylation of Tyr701 on STAT-1 in c-Src-containing cells, whereas STAT-1 phosphorylation in cells devoid of c-Src could not be detected (Figure 1G). DsRNA-induced STAT-1 activation is dependent on IFN-β synthesis and subsequent signaling through the type I IFN receptor. Both dsRNA and LPS fail to activate STAT-1 in primary macrophages lacking the type I IFN receptor, showing the importance of IFN-α/β-mediated signaling for STAT-1 activation (Doyle et al, 2002). As a control, we checked that treatment with IFN-β resulted in Tyr701 phosphorylation both in SYF cells and in c-Src-expressing cells, thus showing that signaling from the type I IFN receptor to STAT-1 is operational in c-Src-deficient cells (Figure 1H). Taken together, these results suggest that c-Src is essential for the dsRNA-induced production of type I IFNs and activation of antiviral gene expression through IRF-3 and STAT-1.
TRIF is an essential adapter protein in TLR3-mediated signaling that is able to trigger IFN-β promoter activation (Oshiumi et al, 2003). Transfection of kinase-inactive c-Src (K297R) dose-dependently inhibited the induction of the IFN-β gene reporter by TRIF (Figure 1I), indicating that c-Src functions downstream of TRIF-elicited signaling.
C-Src regulates dsRNA-induced Akt kinase activation
To further evaluate the role of c-Src in TLR3-mediated dsRNA-induced signaling, we determined the effect of pharmacological inhibitors of Src family kinases on dsRNA-stimulated kinase activation. Akt kinase, a downstream target of phosphoinositide 3-kinase (PI3-K), is regulated in response to viral infections (Avota et al, 2001; Mannova and Beretta, 2005), and has recently been implicated in IRF-3 activation (Sarkar et al, 2004). First, we showed that Akt was activated by dsRNA in human mDCs (Figure 2A). The dsRNA-dependent Akt phosphorylation was not due to contaminating LPS, as the LPS antagonist Rhodobacter sphaeroides lipid A did not affect dsRNA-stimulated Akt activation or cytokine secretion (Supplementary Figure 1). The Src family kinase inhibitor PP2 markedly inhibited dsRNA-elicited Akt phosphorylation in human mDCs, whereas the inactive PP2 analogue, PP3, had no effect (Figure 2B). In contrast, PP2 failed to inhibit dsRNA-induced activation of p38 and p42/44 MAP kinase or JNK activation (Figure 2B). We also found that the p85 regulatory subunit of PI3-K, a critical upstream activator of Akt, was recruited to TLR3 in response to dsRNA (Figure 2C). This is in accordance with a previous report (Sarkar et al, 2004) and indicates that PI3-K transmits TLR3-mediated signaling. Taken together, our results show that (i) dsRNA elicits Akt activation, as previously shown to occur only through TLR2 and TLR4 (Arbibe et al, 2000; Bozinovski et al, 2002), (ii) c-Src regulates dsRNA-elicited Akt activation, which has not been reported earlier for any TLR-dependent Akt activation; and (iii) Akt and MAP kinase activation in response to dsRNA occurs through separate signaling pathways that diverge upstream of c-Src.
Figure 2.
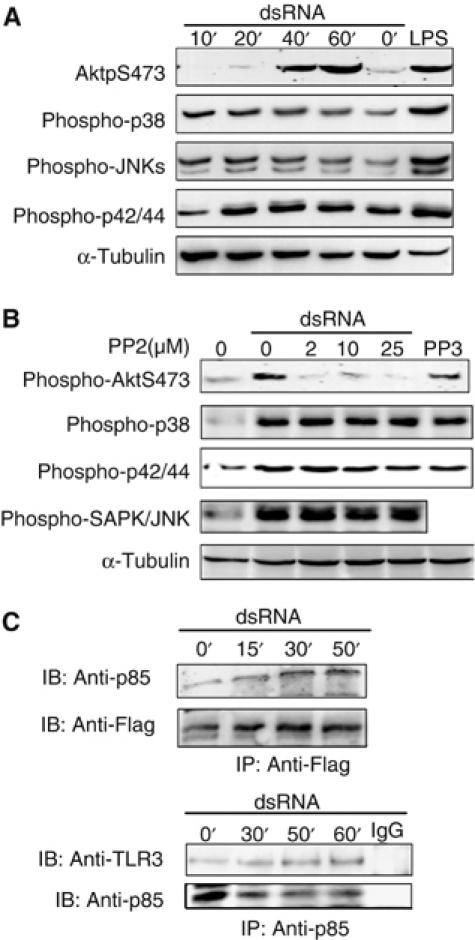
The tyrosine kinase c-Src conveys dsRNA-induced Akt activation. (A) Human mDCs were left untreated or treated with 100 μg/ml dsRNA for various times or 0.5 μg/ml LPS for 20 min. (B) Human mDCs were pretreated or not with PP2 or 25 μM PP3 before addition of 100 μg/ml dsRNA and treatment for 50 min. Cell lysates were immunoblotted (IB) for the indicated proteins. (C) HEK cells expressing Flag-tagged TLR3 were stimulated with dsRNA (100 μg/ml) for the indicated time periods and cell lysates were immunoprecipitated (IP) with anti-Flag, anti-p85, or control IgG and immunoblotted (IB) for the indicated proteins.
DsRNA is endocytosed through a clathrin-mediated pathway
The exact localization of TLR3 and dsRNA interactions and subsequent signaling events remains unclear. To examine potential cellular compartments for dsRNA/TLR3/c-Src associations, we first investigated the dynamics of viral dsRNA internalization and the subcellular localization of dsRNA in human mDCs. Cells were incubated with fluorescently labeled dsRNA corresponding to a sequence from human rhinovirus before imaging of the cells with confocal microscopy. The fluorescently labeled dsRNA stimulated an IFN-β reporter gene in TLR3-transfected cells, whereas no activation was observed in cells transfected with the TLR3 control vector. Also, the rhinovirus dsRNA induced IFN-β and TNF in human mDCs (Supplementary Figure 2), thus showing that the fluorescently labeled dsRNA is biologically active, acting through TLR3 to elicit immunomodulatory cytokines. DsRNA was internalized in vesicles that were transported from the cell periphery towards the center of the cell, coalesced in juxtanuclear areas (after 30 min; Figure 3A) and subsequently trafficked into tubular compartments that extended intracellularly (after 45 min; Figure 3A). To examine the subcellular identity of the tubular compartments, mDCs were incubated with Cy5-labeled dsRNA and fluorescent dextran. Fluorescently labeled dextran has previously been used to identify and position lysosomes (Astarie-Dequeker et al, 2002; Latz et al, 2004). As judged from the significant overlap between Cy5-labeled dsRNA and fluorescently labeled dextran (Figure 3B), the tubular compartments were identified as lysosomes. The tubular lysosomes were highly dynamic structures that altered morphology and individual tubules extended towards the cell periphery and subsequently retracted back to the center of the cell every 10–15s (data not shown). Indeed, lysosomes and MHC class II compartments have been shown to undergo large structural changes and form tubular structures in macrophages, B cells, and DCs in response to certain stimuli (Kleijmeer et al, 2001; Chow et al, 2002). This event may facilitate, for example, phagosome–lysosome fusion and MHC class II transport to the cell surface.
Figure 3.
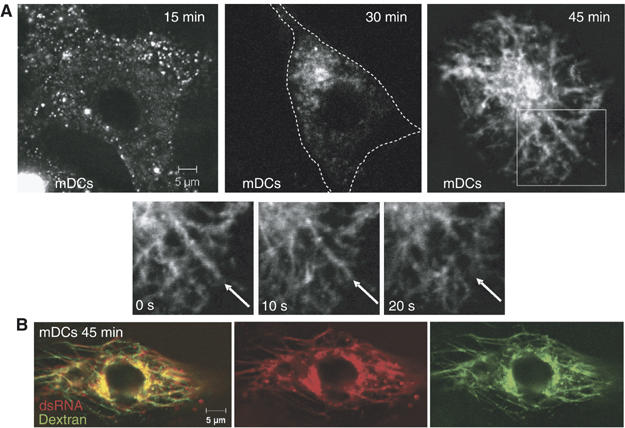
DsRNA is internalized in vesicular compartments and traffics to tubular lysosomes. Confocal images of human mDCs incubated with (A) Cy5-labeled dsRNA and (B) Cy5-labeled dsRNA and Alexa543-labeled dextran for 45 min.
To examine the uptake pathways of dsRNA, human mDCs were incubated with fluorescently labeled dsRNA and stained intracellularly with protein markers of early (EEA1) and late (lysosome-associated membrane protein 1 (LAMP1)) endosomes. DsRNA consecutively colocalized with EEA1 and LAMP1 (Figure 4A and B). We found that the internalization of dsRNA followed that of transferrin (Figure 5A), known to be internalized through a clathrin-mediated mechanism (Conner and Schmid, 2003). In contrast, endocytosed dsRNA failed to show significant overlap with albumin (Figure 5B), which is taken up by a caveolin-dependent pathway (Conner and Schmid, 2003). In support of these data, dsRNA was not found in caveolin-1-positive vesicles (Figure 5C).
Figure 4.
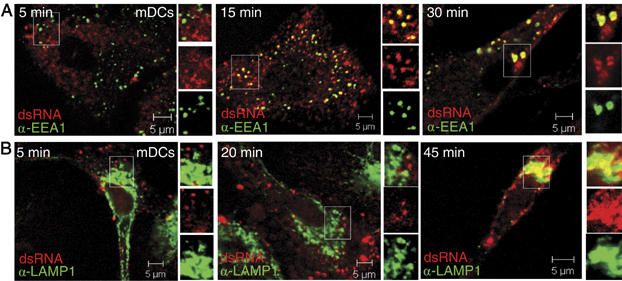
DsRNA sequentially colocalizes with early and late endosome marker proteins. (A, B) Confocal images of human mDCs incubated with Cy5-labeled dsRNA as indicated and stained intracellularly for EEA1 (FITC; A) or LAMP1 (Alexa647; B).
Figure 5.
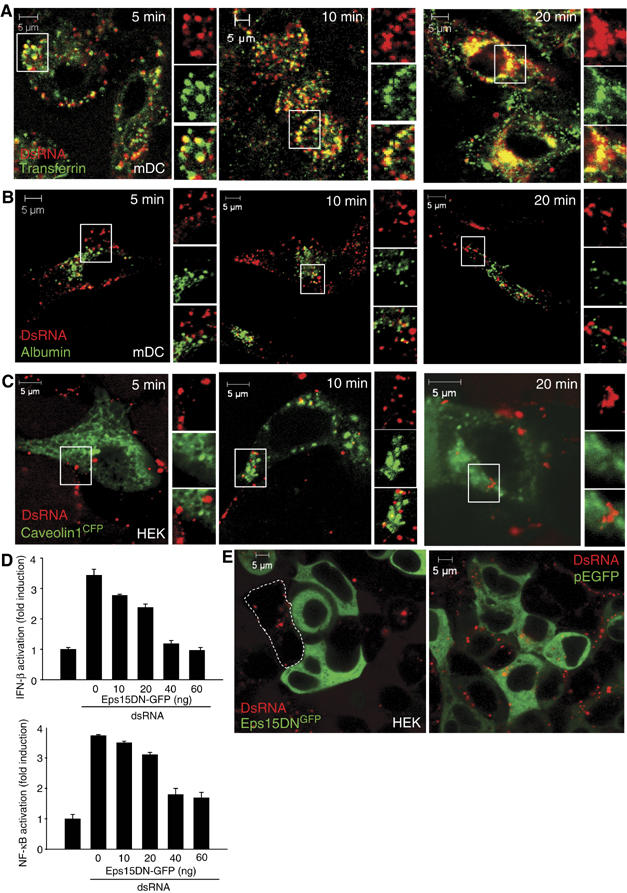
DsRNA follows a clathrin-dependent uptake pathway. (A, B) Human mDCs were coincubated with Cy5-labeled dsRNA and transferrin (A) or albumin (B) and analyzed by confocal microscopy. (C) HEK cells expressing CFP-tagged caveolin-1 were incubated with Cy5-labeled dsRNA and analyzed by confocal microscopy. (D) HEK cells were transfected with plasmids encoding E-GFP (60, 50, 40, 20, 0 ng), Eps15DNGFP (0, 10, 20, 40, 60 ng), Flag-tagged TLR3 (40 ng) and a human IFN-β (40 ng) or an NF-κB (40 ng) luciferase reporter gene. After 36 h, cells were stimulated or not with dsRNA (100 μg/ml) for 6 h and luciferase gene activity was measured. (E) Confocal images of HEK cells expressing Eps15DNGFP or pEGFP incubated with fluorescently labeled dsRNA.
Transfection of a dominant negative version of Eps15, a scaffolding molecule that is necessary for clathrin-mediated coat assembly and endocytosis (Benmerah et al, 1999), impaired the dsRNA-induced IFN-β and NF-κB activation (Figure 5D). Moreover, cells expressing a GFP-tagged version of Eps15DN failed to internalize dsRNA, whereas cells lacking Eps15DNGFP or cells transfected with pEGFP readily endocytosed dsRNA (Figure 5E). Taken together, these results show that dsRNA-elicited immune responses depend on internalization of dsRNA by clathrin-mediated, caveolin-independent endocytosis. CpG DNA was recently found to be internalized by similar mechanisms (Latz et al, 2004), and indeed we observed extensive overlap between fluorescently labeled dsRNA and CpG DNA when human mDCs were incubated with these nucleic acid species for various intervals (Supplementary Figure 3).
C-Src is recruited to dsRNA-containing endosomes
To determine the cellular localization of c-Src in relation to dsRNA endocytosis, we utilized a c-Src-GFP fusion protein. The subcellular localization of the c-Src-GFP fusion protein follows that of endogenously expressed c-Src and SrcGFP has been found to retain normal activity (Sandilands et al, 2004). HEK293 cells were transfected with GFP-tagged c-Src and EEA1Tomato as a marker for early endosomes, and treatment with dsRNA clearly induced colocalization of c-SrcGFP with EEA1Tomato in live cells (Figure 6A). Also, a high degree of colocalization of c-SrcGFP with LAMP1-positive endosomes was observed after intracellular staining of dsRNA-treated HEK293 cells with anti-LAMP1 (Figure 6B). Hence, c-Src localizes to the membranes of early and late endosomes in dsRNA-treated cells. Similarly, upon treatment of mDCs with fluorescently labeled dsRNA, c-Src associated with LAMP1-positive, late endosomes containing dsRNA (Figure 6C), thus confirming our results in primary cells. Moreover, c-Src localized to endosomes was activated, as assessed by staining with antibodies to phosphorylated c-Src (data not shown). C-Src was also present on the plasma and nuclear membranes. Taken together, our results demonstrate that c-Src and TLR3 associate in a dsRNA-dependent manner (Figure 1B), and that c-Src is recruited to dsRNA-containing endosomes.
Figure 6.
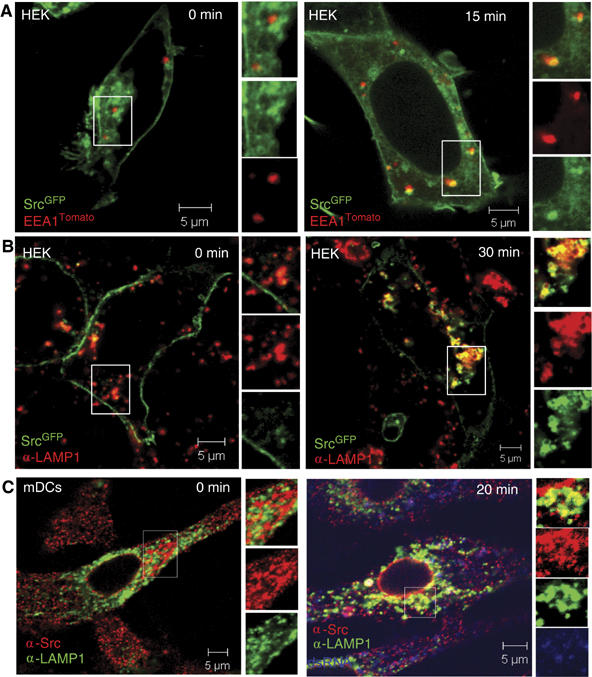
The tyrosine kinase c-Src colocalizes with dsRNA on endosomes. HEK cells were transfected with c-SrcGFP (A, B) and EEA1Tomato (A), treated with dsRNA and directly visualized by confocal microscopy (A) or stained intracellularly with LAMP1 (Alexa546; B). (C) Human mDCs were stimulated with Cy5-labeled dsRNA and stained intracellularly with anti-Src (Alexa546) and anti-LAMP1 (FITC) before confocal microscopy.
TLR3 associates with c-Src on dsRNA-containing endosomes
The localization of TLR3 and its ligand, viral dsRNA, has not been unambiguously shown. To examine the localization of TLR3 and to determine the cellular site of interaction between dsRNA, TLR3, and c-Src, we first generated HeLa cells stably expressing a chimera of the yellow fluorescent protein (YFP) fused to the carboxy terminus of human full-length TLR3. HeLa-TLR3YFP cells were incubated with the cholera toxin B subunit, which binds to the glycosphingolipid GM-1 on the plasma membrane, or a nuclear stain before analysis by confocal microscopy. TLR3 was not expressed at the plasma membrane or in the nucleus, but was abundantly expressed on interconnecting membranes in the cytoplasm that extended from the nuclear membrane to the plasma membrane (Figure 7A and B). Transient expression of the CFP-tagged sequence of calreticulin, an endoplasmic reticulum (ER)-resident protein, or a CFP-tagged Golgi-resident protein (β-galactosyl transferase) in HeLa-TLR3YFP cells revealed near-complete coexpression of TLR3 with the ER marker protein (Figure 7D), whereas TLR3 was not expressed in the Golgi apparatus (Figure 7C). Likewise, complete colocalization of TLR3YFP with the ER-associated protein calnexin was observed after staining of HeLa-TLR3YFP cells with anti-calnexin (Figure 7E). Complete colocalization was also observed when TLR9CFP, recently shown to be expressed in the ER (Latz et al, 2004), was transfected in HeLa-TLR3YFP cells (Supplementary Figure 4). It is possible that the ER localization observed for TLR3YFP was due to overexpression of the protein chimera, as overexpressed membrane proteins may accumulate in the ER owing to misfolding. However, YFP-tagged TLR4 did not localize to the ER even though overexpressed to similar extents as TLR3YFP (Supplementary Figure 5). Also, TLR3YFP was fully functional in mediating dsRNA-induced NF-κB activation (data not shown) and IFN-β promoter activation (Supplementary Figure 6), suggesting that TLR3YFP is expressed at a subcellular site where it binds its ligand and transmits signaling. Nevertheless, to confirm the expression pattern of TLR3 in primary cells, human mDCs were stained with antibodies recognizing TLR3 and the ER protein calnexin. The fluorescent signals for TLR3 and calnexin broadly colocalized (Figure 7F), confirming the results obtained in HeLa-TLR3YFP cells and suggesting that endogenously expressed TLR3 localizes to the ER of unstimulated human mDCs. An isotype-matched control antibody did not stain mDCs (data not shown).
Figure 7.
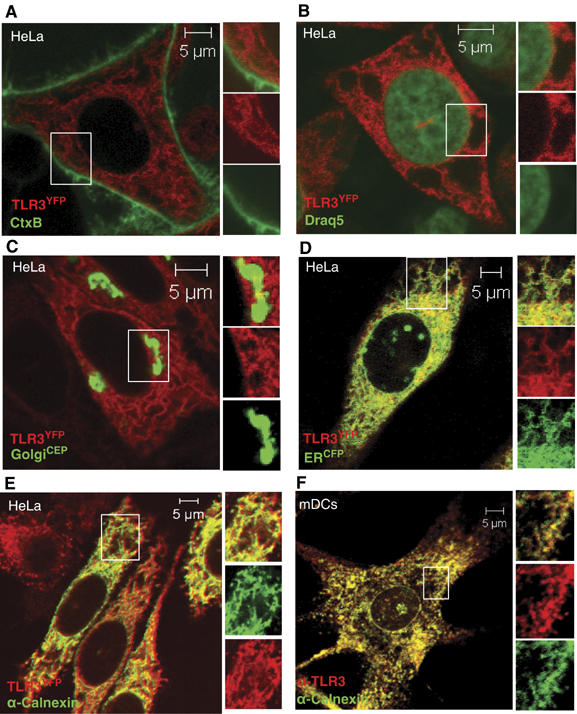
TLR3 is localized in the ER in resting cells. (A, B) HeLa cells expressing YFP-tagged TLR3 were stained with cholera toxin subunit B (A) or draq 5 (B) and visualized by confocal microscopy. (C–E) HeLa cells expressing TLR3YFP protein chimera (red) transiently transfected with a CFP-tagged Golgi protein marker (C), a CFP-tagged ER protein marker (D), or stained intracellularly for the ER-resident protein calnexin (E). (F) Untreated human mDCs were permeabilized and stained with anti-TLR3 (TLR3.7; Alexa647) and anti-calnexin (FITC) and examined by confocal microscopy.
To establish the cellular site of interaction between dsRNA and TLR3, fluorescently labeled dsRNA was added to HeLa cells stably expressing TLR3YFP. Interestingly, upon addition of dsRNA, TLR3YFP encircled the surface of vesicles that carried dsRNA in their lumen (Figure 8A). These TLR3-containing vesicles were identified as early endosomes, as they expressed the PI-3 phosphate-binding module FYVE (Figure 8B), which is expressed on early endosomes. It should be noted that ectopic expression of the FYVE finger domain has been shown to modulate the size of endosomes, resulting in somewhat larger endosomes, possibly owing to FYVE-mediated displacement of endogenous PI-3 phosphate-binding proteins involved in defining the early endosome (Gillooly et al, 2000). Moreover, addition of dsRNA to human mDCs resulted in LAMP1-positive endosomes that contained TLR3 (Figure 8C). Importantly, we also found that TLR3 colocalized with c-Src on these vesicular structures in mDCs (Figure 8D). These results show that TLR3 moves from the ER to endosomes, thus associating with dsRNA and c-Src on endosomes.
Figure 8.
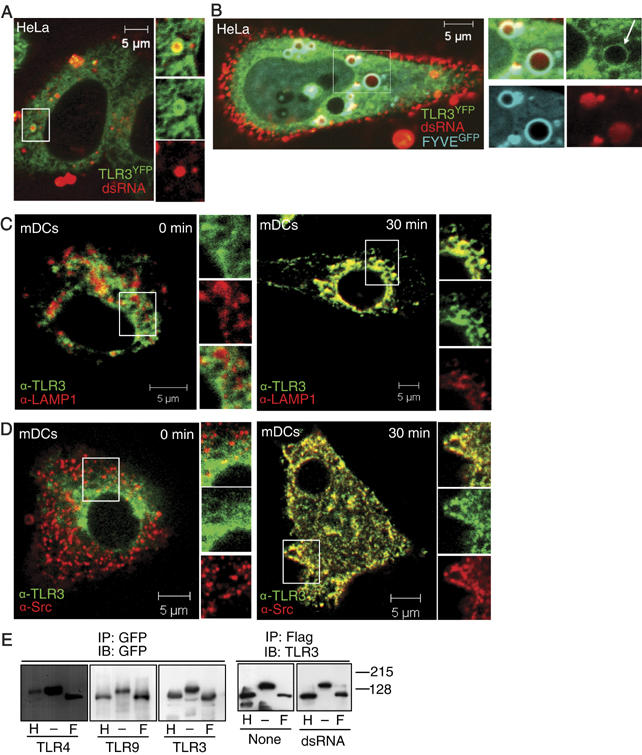
TLR3 colocalizes with dsRNA and c-Src on endosomes. (A, B) Confocal images of HeLa cells expressing TLR3YFP incubated with (A) Cy5-conjugated dsRNA for 10 min or (B) transfected with CFP-tagged FYVE and treated with Cy5-labeled dsRNA for 10 min. (C, D) Human mDCs were treated with dsRNA or not and stained intracellularly for TLR3 (Alexa546) and LAMP1 (Alexa647; C) or TLR3 (Alexa546) and c-Src (Alexa647; D). (E) Whole-cell lysates of HEK cells expressing TLR3Flag , TLR9GFP, and TLR4GFP, and HeLa cells expressing TLR3YFP were immunoprecipitated with anti-GFP or anti-Flag. Immunoprecipitates were left untreated (−) or were incubated with Endo H (H) or PNGase F (F) and immunoblotted for GFP or TLR3 as indicated.
The presence of TLR3 on endosomes could result from transport from the ER through the trans-Golgi secretory pathway or from direct recruitment from the ER, as recently shown to occur for ER-resident proteins during phagocytosis (Gagnon et al, 2002; Guermonprez et al, 2003). To examine this aspect, we utilized peptide-N-glycosidase F (PNGase F), which cleaves complex as well as neutral glycoproteins, that is, proteins that have been modified both in the ER and in the Golgi, and endoglycosidase H (Endo H), which is unable to deglycosylate proteins that have been glycosylated in the Golgi apparatus. Immunoprecipitated TLR3, both from untreated and dsRNA-stimulated cells, as well as TLR9, was sensitive to both PNGase F and Endo H. In contrast, TLR4 was resistant to Endo H (Figure 8E) as expected from its presence in the Golgi and at the plasma membrane (Latz et al, 2002). Similar changes in migration patterns after deglycosylation were observed for YFP- and Flag-tagged TLR3. Thus, the glycosylation observed is intrinsic to TLR3 and independent of the molecular tags associated with TLR3. Hence, our results suggest that TLR3 residing on endosomes is recruited from the ER, and not through the Golgi apparatus.
Discussion
Establishing innate immune responses to pathogens requires a series of coordinated events, including recognition and binding of pathogen-associated molecular patterns, uptake and elimination of pathogens in the endosomal/lysosomal compartments, as well as signal transduction leading to the induction of immune response genes. In this study, we show that c-Src is necessary for antiviral gene expression by IRF-3 and STAT-1, that activated c-Src associates with TLR3 in response to dsRNA, and that the cellular site of interaction between c-Src and TLR3 is dsRNA-containing endosomes. These results reveal novel features about the TLR3 signaling pathway. Regarding the mechanism by which c-Src signals to IRF-3, we do not presently know at which exact step in the TLR3-mediated IRF-3 activation pathway c-Src exerts its function or the direct downstream target that is phosphorylated by c-Src. However, c-Src appears to function downstream of or at the level of the adapter protein TRIF, as kinase-inactive c-Src abrogated TRIF-induced IFN-β promoter activation. TRIF has been suggested to function as a docking platform that interacts with several signaling proteins in addition to TLR3, for example, TBK-1, TRAF3, RIP-1, and RIP-3, to initiate divergent signaling pathways leading to IRF-3 or NF-κB activation (Fitzgerald et al, 2003; Jiang et al, 2004; Meylan et al, 2004). C-Src could associate directly with TLR3 or through TRIF by hitherto unidentified mechanisms such as tyrosine phosphorylation of TRIF and association with the c-Src SH2 domain. Also, TRIF harbors proline-rich motifs that might associate with the SH3 domain of c-Src. However, as only the N-terminal part of TRIF appears to be able to activate the IFN-β promoter, c-Src would be expected to associate with the N-terminal part of TRIF, thus mediating IFN-β synthesis. Hence, it is possible that c-Src forms a complex with TLR3 and TRIF with its associated partners that modulate TBK-1/IKKɛ-mediated phosphorylation of IRF-3. Recently, TLR3 was shown to be phosphorylated in response to dsRNA treatment (Sarkar et al, 2004), but whether c-Src phosphorylates TLR3 is not known. Upon transient transfection of Src wild type together with hnRNP K, a known substrate of c-Src (Ostareck-Lederer et al, 2002), we were indeed able to detect Src-elicited phosphorylation of hnRNP K. Immunoblot analysis confirmed that both c-Src and TLR3 were overexpressed, yet we were not able to detect c-Src-dependent TLR3 phosphorylation (data not shown). We found that Akt is activated in response to dsRNA and that c-Src mediates Akt activation, but not dsRNA-elicited activation of p38, p42/44 MAP kinases, or JNKs. This illustrates that c-Src differentially regulates TLR3-mediated signaling. Hence, c-Src phosphorylates a protein(s) that mediates Akt activation, but is not required for activation of p38, p42/44 MAP kinases, or JNKs. Interestingly, c-Src has recently been shown to directly phosphorylate Akt to control its activation (Chen et al, 2001), and this mechanism might also be functional in response to dsRNA.
The involvement of c-Src in TLR-induced Akt activation has not previously been reported. In this context, a recent study by Sarkar et al (2004) showed that the PI3-K–Akt pathway is necessary for maximal phosphorylation and activation of IRF-3 in response to dsRNA. Hence, our results extend these findings and show that triggering of IRF-3 activation through the PI3-K–Akt pathway is dependent on c-Src.
Although their role has been debated, Src family kinases have previously been implicated in the regulation of immune responses induced by LPS. Mice deficient for the Src family kinases Hck and Fgr are resistant to endotoxic shock, whereas mice expressing constitutively active Hck display enhanced immune responses to LPS (Lowell and Berton, 1998; Ernst et al, 2002). However, the molecular mechanisms underlying these immunoprotective effects of Src-family kinases are not understood.
It is generally believed that the nucleic acid recognizing TLRs, TLR3, TLR7, TLR8, and TLR9, reside in the endosomal membrane and that binding to their ligands occurs in the endosomal lumen. However, it is only TLR9 that has unambiguously been shown to localize to endosomes (Ahmad-Nejad et al, 2002; Latz et al, 2004). Our study is the first to show that TLR3 associates with viral dsRNA in endosomes. The intracellular localization of TLR9 was recently found to depend on its transmembrane region (Barton et al, 2006). Regarding possible molecular determinants that could direct TLR3 to endosomal/lysosomal compartments, it has previously been found that the linker region in TLR3 is associated with intracellular localization of TLR3 (Funami et al, 2004). TLR3 also contains several tyrosine-based sorting signals (YXXØ) within its cytosolic domain, which generally direct transmembrane proteins to endosomal–lysosomal organelles. Also, ubiquitination of the cytosolic domain of transmembrane proteins may serve as an endosome–lysosome sorting signal, and TLR3 has indeed been found to be ubiquitinated (Chuang and Ulevitch, 2004).
We found that c-Src is essential for TLR3-mediated antiviral gene expression and is recruited to TLR3 on endosomes, thus leading to the assembly of an active signaling complex on the cytoplasmic tail of TLR3. Hence, TLR3 and c-Src transmit antiviral signaling from endosomes containing dsRNA as their cargo. Our results are based on colocalization of c-Src with the endosomal markers EEA1 and LAMP1. As LAMP1 stains both late endosomes and lysosomes, we cannot directly exclude signaling from lysosomes. However, a recent study by Honda et al (2005) shows that retention of CpG in endosomal vesicles is necessary for stimulation of IRF-7 and IFN production through TLR9 and the TLR adapter protein MyD88. In contrast, CpG that was rapidly transferred from late endosomes to lysosomes failed to activate the MyD88–IRF-7 pathway and IFN production. Based on these and our results, and considering the homology between TLR9 and TLR3, we might speculate that TLR3 signaling to IRF-3 occurs from the membranes of early and late endosomes, but perhaps not lysosomes. It should be noted that there is much evidence showing that signaling occurs on endosomal membranes. In particular, the endosomal signaling of receptor tyrosine kinases, for example, the epidermal growth factor (EGF) receptor, has been extensively studied. Activated, tyrosine-phosphorylated EGF receptors, bound to EGF, have been found to preserve their dimerization and kinase activity within endosomes (Sorkin et al, 1988; Burke et al, 2001). Also, the kinases Raf, MEK, and p42/44 MAP kinase are present on endosomes (Andresen et al, 2002).
Regarding the mechanism by which TLR3 translocates to endosomes from the ER, we show that it does not involve the secretory pathway through the Golgi apparatus (Figure 8E). Translocation of TLR3 to endosomes could occur by direct delivery of ER membranes to the endosomes in a way similar to what has been suggested for ER-mediated phagocytosis (Burke et al, 2001; Conner and Schmid, 2003). However, Touret et al (2005) failed to observe contact between the ER and the plasma membrane. Furthermore, their results indicate that the plasma membrane is the main constituent of phagosomes, and that the contribution of the ER membrane to phagosomes/endosomes is quantitatively small. Hence, ER proteins like TLR3 infrequently occur on phagosomes/endosomes. It will also be interesting to explore the receptors and signaling that regulate the transport of TLR3 from the ER to endosomes.
In conclusion, our study provides novel results on the molecular mechanisms for endosomal sensing of dsRNA by TLR3, and identify c-Src as a novel TLR3-interacting protein that localizes to endosomes and contributes to dsRNA-induced signaling.
Materials and methods
Reagents
DsRNA (a 34 bp sequence from human rhinovirus 16) was labeled at the 3′ end with Cy5 (Dharmacon). Synthetic dsRNA (Poly IC) was from Amersham Biosciences. Alexa488-labeled transferrin and albumin, Alexa543-labeled dextran, and rhodamine-labeled cholera toxin B subunit were from Molecular Probes. Fluorescein isothiocyanate (FITC)-conjugated anti-EEA1, Alexa647-conjugated anti-LAMP1, and antibodies to EEA1, LAMP1, and GFP were from BD Transduction Laboratories. Antibodies to calnexin and XRCC1 were from Abcam and the monoclonal antibody to Flag (M2) was from Sigma. The monoclonal antibodies to TLR3 were from Imgenex and Hycult (clone TLR3.7). Antibodies against phosphorylated forms of kinases and against STAT-1 and IRF-3 were purchased from Cell Signaling Technology. Antibodies to c-Src and α-tubulin were obtained from Santa Cruz Biotechnology. PP2, PP3, and SU6656 were purchased from Calbiochem. Endo H and PNGase F were from Roche Molecular Biochemicals.
Plasmids
YFP-labeled cDNA of human TLR3 was constructed using the primers 5′-ATCCAAGAAGCTTATGAGACAGACTTTGCCTT GTATC-3′ and 5′-TTCGGTACTCGAGATGTACAGAGTTTTTGGAT CCAAGTGC-3′. The PCR fragment was cloned into the HindIII/XhoI sites of pcDNA3-YFP. The FYVE, caveolin-1, and Eps15DN fluorescent constructs have been described previously (Benmerah et al, 1999). The plasmids for luciferase reporter constructs containing the full IFN-β p125 promoter, and the Gal4/IRF-3 system, were gifts from Kate Fitzgerald (Umass Med School, Worcester, MA) and Tom Maniatis (Harvard University, Cambridge, MA), respectively. The c-Src-GFP construct was a generous gift from Emma Sandilands (Sandilands et al, 2004). The EEA1Tomato construct, made by recombining pDest-Tomato and pEntr-EEA1-CT, was kindly provided by Harald Stenmark (The Norwegian Radium Hospital). TLR4-YFP and TLR9-YFP constructs have been reported earlier (Latz et al, 2002, 2004).
Cell culture and generation of mDCs
HEK293 and HeLa cells were maintained in DMEM supplemented with 10% FCS, 20 μg/ml garamycin, and 2 mM L-glutamine and cultured at 37°C in 8% CO2. SYF mouse embryonic fibroblasts from the triple Src knockout mouse lacking Src, Yes, and Fyn and the added-back version of the SYF cells, with the wild-type c-Src gene introduced were obtained from ATCC and maintained under the same conditions as HEK293 and HeLa cells. Peripheral blood monocytes were isolated from human A+ buffy coats by Lymfoprep (Nycomed) density gradient centrifugation. Immature mDCs were prepared from peripheral blood monocytes by culturing in RPMI supplemented with 10% A+ serum, IL-4 (500 U/ml), and GM-CSF (800 U/ml) for 6 days as previously reported (Sallusto and Lanzavecchia, 1994). The purity of the cells was assessed by FACS analysis as described (Sallusto and Lanzavecchia, 1994). DsRNA upregulated CD40, CD83, and CD86 (data not shown), confirming that dsRNA induced mDC maturation.
Isolation of nuclear extracts
SYF and c-Src cells were seeded in 100-mm plates 24 h before treatment. After stimulation, the cells were lysed in 250 μl of lysis buffer containing 50 mM Tris pH 7.5, 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, 2 mM EDTA, 40 mM β-glycerophosphate, 100 mM NaF, 200 μM Na3VO4, 10 μg/ml leupeptin, 1 μM pepstatin, and 1 mM PMSF. Lysates were centrifuged at 10 000 r.p.m. for 15 min at 4°C. Pellets were lysed in 70 μl of lysis buffer containing 10% SDS and 10 mM Tris–HCl, pH 6.8, using a Hamilton injector. The nuclear extracts were clarified by centrifugation at 13 000 r.p.m. for 20 min at room temperature.
Transfection assays
HEK293 cells were seeded in 96-well plates 24 h before transfection with Genejuice (Novagen) following the manufacturer's instructions. Forty nanograms of each plasmid was used unless otherwise indicated. The Renilla luciferase pRL-TK vector (Promega) was cotransfected for normalization. Reporter gene activity was measured using the Dual Luciferase Assay System (Promega).
Immunoprecipitation
Cells were seeded into 100-mm plates 24 h before treatments. After stimulation, the cells were lysed in 1 ml of lysis buffer containing 50 mM Tris pH 7.5, 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, 2 mM EDTA, 40 mM β-glycerophosphate, 100 mM NaF, 200 μM Na3VO4, 10 μg/ml leupeptin, 1 μM pepstatin, and 1 mM PMSF. Lysates were clarified by centrifugation and incubated with 1–4 μg antibody overnight at 4°C. The immunocomplexes were recovered by brief centrifugation after 1 h incubation with protein G Sepharose (Nycomed Amersham) at 4°C, washed four times with lysis buffer, resuspended in denaturing sample buffer, and subjected to SDS–PAGE and immunoblotting.
Immunoblotting
Following treatment, cells were washed once in PBS and lysed in lysis buffer as described above. Cell lysates were clarified by centrifugation and separated on 6 or 10% SDS–PAGE. The proteins were electrophoretically transferred to Hybond-C nitrocellulose membranes (Nycomed Amersham) in 25 mM Tris, 192 mM glycine, and 20% ethanol. The membranes were blocked in Tris-buffered saline containing 5% nonfat dry milk and 0.25% Tween 20 for 1 h at 22°C before incubation with the indicated antibodies overnight at 4°C. After washing three times with Tris-buffered saline containing 0.25% Tween 20, immunoreactive proteins were detected using HRP-conjugated secondary antibody and ECL detection reagent (Pierce).
Confocal microscopy
Confocal microscopy studies were performed with a Zeiss Axiovert 100-M inverted microscope equipped with an LSM 510 laser-scanning unit and a 1.4 NA × 63 Plan-Apochromat oil-immersion objective. Cells were seeded on glass-bottomed 35-mm tissue culture dishes (Matek). Before imaging of living cells, fresh RPMI medium supplemented with 10 mM HEPES (Gibco) was added. The cell chamber was heated to 37°C using a Tempcontrol Digital 37-2 device (Warner Instruments). To minimize photobleaching, laser power was typically 20% under maximum and the pinhole was set to 0.8–1.2. Multi-tracking was used for dual or triple color imaging.
DsRNA uptake studies
Adherent mDCs were incubated with Cy5-conjugated dsRNA (3–5 μM) in growth medium for various time periods, washed twice with PBS, and immediately imaged by confocal microscopy at 37°C. The cells were coincubated with dsRNA and Alexa488-labeled transferrin or albumin (10 μg/ml) for 5 min. To identify lysosomal compartments, mDCs were incubated with fluorescently labeled dextran (250 μg/ml).
Immunofluorescence
Cells were fixed in PBS containing 1% paraformaldehyde for 15 min at room temperature, or in 100% methanol for 1 min on ice. Nonspecific antibody sites were blocked with PBS containing 2.5% BSA, 20% A+ serum, and 0.2% saponin for 30 min at room temperature. For staining, cells were incubated with antibodies diluted in PBS containing 2.5% BSA, 20% A+ serum, and 0.2% saponin for 30 min at room temperature.
Deglycosylation experiments
TLR3, TLR4, and TLR9 were immunoprecipitated from cell lysates of HEK293 cells expressing TLR3Flag, TLR9GFP, and TLR4GFP, and HeLa cells expressing TLR3YFP using anti-Flag or anti-GFP antibodies. The glycosylation pattern of TLRs was determined by incubating the immunoprecipitates with Endo H or PNGase F for 50 min at 37°C. The proteins were separated by SDS–PAGE and subjected to immunoblotting.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr S Akira (Osaka University, Osaka, Japan) for the HEK293 cells stably expressing Flag-tagged human TLR3. This work was supported by the National Programme for Research in Functional Genomics in Norway (FUGE, to MWA), the Research Council of Norway (to MWA and TE), the Cancer Fund at St Olav's Hospital (to MWA and TE), the Faculty of Medicine, NTNU (to IBJ and MWA), the Norwegian Cancer Society (to TE), and the National Institutes of Health (to EL). We are grateful to Dr Kate Fitzgerald (Umass Med School, Worcester, MA) for providing the TRIF construct and for critically reading the manuscript.
References
- Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H (2002) Bacterial CPG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol 32: 1958–1968 [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4: 499–511 [DOI] [PubMed] [Google Scholar]
- Andresen BT, Rizzo MA, Shome K, Romero G (2002) The role of phosphatidic acid in the regulation of the Ras/MEK/Erk signaling cascade. FEBS Lett 531: 65–68 [DOI] [PubMed] [Google Scholar]
- Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG (2000) Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol 1: 533–540 [DOI] [PubMed] [Google Scholar]
- Astarie-Dequeker C, Carreno S, Cougoule C, Maridonneau-Parini I (2002) The protein tyrosine kinase Hck is located on lysosomal vesicles that are physically and functionally distinct from CD63-positive lysosomes in human macrophages. J Cell Sci 115: 81–89 [DOI] [PubMed] [Google Scholar]
- Avota E, Avots A, Niewiesk S, Kane LP, Bommhardt U, ter MV, Schneider-Schaulies S (2001) Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat Med 7: 725–731 [DOI] [PubMed] [Google Scholar]
- Barton GM, Kagan JC, Medzhitov R (2006) Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol 7: 49–56 [DOI] [PubMed] [Google Scholar]
- Benmerah A, Bayrou M, Cerf-Bensussan N, utry-Varsat A (1999) Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci 112 (Part 9): 1303–1311 [DOI] [PubMed] [Google Scholar]
- Bozinovski S, Jones JE, Vlahos R, Hamilton JA, Anderson GP (2002) Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem 277: 42808–42814 [DOI] [PubMed] [Google Scholar]
- Burke P, Schooler K, Wiley HS (2001) Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol Biol Cell 12: 1897–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y (2001) Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem 276: 31858–31862 [DOI] [PubMed] [Google Scholar]
- Chow A, Toomre D, Garrett W, Mellman I (2002) Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418: 988–994 [DOI] [PubMed] [Google Scholar]
- Chuang TH, Ulevitch RJ (2004) Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol 5: 495–502 [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL (2003) Regulated portals of entry into the cell. Nature 422: 37–44 [DOI] [PubMed] [Google Scholar]
- Der SD, Yang YL, Weissmann C, Williams BR (1997) A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA 94: 3279–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S, Vaidya S, O'Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G (2002) IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17: 251–263 [DOI] [PubMed] [Google Scholar]
- Durbin JE, Hackenmiller R, Simon MC, Levy DE (1996) Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84: 443–450 [DOI] [PubMed] [Google Scholar]
- Ernst M, Inglese M, Scholz GM, Harder KW, Clay FJ, Bozinovski S, Waring P, Darwiche R, Kay T, Sly P, Collins R, Turner D, Hibbs ML, Anderson GP, Dunn AR (2002) Constitutive activation of the SRC family kinase Hck results in spontaneous pulmonary inflammation and an enhanced innate immune response. J Exp Med 196: 589–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T (2003) IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4: 491–496 [DOI] [PubMed] [Google Scholar]
- Funami K, Matsumoto M, Oshiumi H, Akazawa T, Yamamoto A, Seya T (2004) The cytoplasmic ‘linker region' in Toll-like receptor 3 controls receptor localization and signaling. Int Immunol 16: 1143–1154 [DOI] [PubMed] [Google Scholar]
- Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, Bergeron JJ, Desjardins M (2002) Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110: 119–131 [DOI] [PubMed] [Google Scholar]
- Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H (2000) Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J 19: 4577–4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van EP, Amigorena S (2003) ER–phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425: 397–402 [DOI] [PubMed] [Google Scholar]
- Hoebe K, Beutler B (2006) TRAF3: a new component of the TLR-signaling apparatus. Trends Mol Med 12: 187–189 [DOI] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B (2003) Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424: 743–748 [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T (2005) IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434: 772–777 [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Langland JO (1996) When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219: 339–349 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Mak TW, Sen G, Li X (2004) Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci USA 101: 3533–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441: 101–105 [DOI] [PubMed] [Google Scholar]
- Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky AY, Ossendorp F, Melief CJ, Stoorvogel W, Geuze HJ (2001) Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol 155: 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P (1999) Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J 18: 2459–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT (2004) TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5: 190–198 [DOI] [PubMed] [Google Scholar]
- Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T (2002) Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4–MD-2–CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem 277: 47834–47843 [DOI] [PubMed] [Google Scholar]
- Lowell CA, Berton G (1998) Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src-family kinases Hck and Fgr. Proc Natl Acad Sci USA 95: 7580–7584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannova P, Beretta L (2005) Activation of the N-Ras–PI3K–Akt–mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J Virol 79: 8742–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J (2004) RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol 5: 503–507 [DOI] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T (2003) TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol 4: 161–167 [DOI] [PubMed] [Google Scholar]
- Ostareck-Lederer A, Ostareck DH, Cans C, Neubauer G, Bomsztyk K, Superti-Furga G, Hentze MW (2002) c-Src-mediated phosphorylation of hnRNP K drives translational activation of specifically silenced mRNAs. Mol Cell Biol 22: 4535–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179: 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC (2004) RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell 7: 855–869 [DOI] [PubMed] [Google Scholar]
- Sarkar SN, Peters KL, Elco CP, Sakamoto S, Pal S, Sen GC (2004) Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat Struct Mol Biol 11: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Sorkin AD, Teslenko LV, Nikolsky NN (1988) The endocytosis of epidermal growth factor in A431 cells: a pH of microenvironment and the dynamics of receptor complex dissociation. Exp Cell Res 175: 192–205 [DOI] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B (2004) Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA 101: 3516–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513–609 [DOI] [PubMed] [Google Scholar]
- Touret N, Paroutis P, Terebiznik M, Harrison RE, Trombetta S, Pypaert M, Chow A, Jiang A, Shaw J, Yip C, Moore HP, van der WN, Houben D, Peters PJ, de CC, Mellman I, Grinstein S (2005) Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell 123: 157–170 [DOI] [PubMed] [Google Scholar]
- Wathelet MG, Lin CH, Parekh BS, Ronco LV, Howley PM, Maniatis T (1998) Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol Cell 1: 507–518 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


