Abstract
The Set1-containing complex, COMPASS, methylates histone H3 on lysine 4 (K4) in Saccharomyces cerevisiae. Despite the preferential association of K4-trimethylated H3 with regions of the genome that are transcribed by RNA polymerase II, transcriptional silencing is one of the few cases in S. cerevisiae where histone-methylation defects have a clear effect on gene expression. To better understand the role of COMPASS in transcriptional silencing, we have determined which members of COMPASS are required for silencing at the ribosomal DNA locus (rDNA), a telomere, and the silent mating loci (HM) using Northern analyses. Our findings indicate that most members of COMPASS are required for silencing at the rDNA and telomere, while none are required for silencing of endogenous genes at the HM loci. To complement gene-expression analysis, quantitative Western blot experiments were performed to determine the members of COMPASS that are required for methylation of histone H3. While most are required for trimethylation, cells lacking certain COMPASS proteins maintain reduced levels of K4 mono- and dimethylated H3, suggesting that some COMPASS members have redundant function. Finally, we show Paf1 is required for silencing and K4-methylated H3 at the rDNA, suggesting a possible direct role for K4-methylated H3 in gene silencing.
TRANSCRIPTIONAL silencing is a form of repression of RNA polymerase II (Pol II)-transcribed genes that occurs in a regional, rather than a promoter-specific, manner. In Saccharomyces cerevisiae, three genomic regions where transcriptional silencing occurs have been identified: the homothallic mating (HM) loci, telomeres, and the ribosomal DNA locus (rDNA) (reviewed in Rusche et al. 2003). Silencing at each of these genomic regions requires Sir2, an NAD-dependent histone deacetylase. At the HM loci and telomeres, Sir2 functions as a member of the silent information regulator (Sir) complex, consisting of Sir2, Sir3, and Sir4. In many cases, the requirements for transcriptional silencing at the rDNA are different from those at the HM loci and telomeres. While Sir2 is required for rDNA silencing, Sir3 and Sir4 are not (Bryk et al. 1997; Fritze et al. 1997; Smith and Boeke 1997). At the rDNA, Sir2 functions as a member of the RENT complex that also contains Net1, a protein required for nucleolar stability, and Cdc14, a protein phosphatase. RENT associates with the rDNA, but does not associate with the HM loci or telomeres (Straight et al. 1999).
Post-translationally modified histones are required for transcriptional silencing in S. cerevisiae (reviewed in Strahl and Allis 2000; Jenuwein and Allis 2001). Deacetylated histones play a direct role in transcriptional silencing. Sir2 deacetylates lysine residues in the tails of histones H3 and H4, creating a chromatin domain that is favorable for the binding of Sir3 and Sir4 (reviewed in Rusche et al. 2003) and refractory to the function of Pol II (Chen and Widom 2005). K4-methylated histone H3 is also important for gene silencing in S. cerevisiae. Set1 is a histone methyltransferase that is required for the mono-, di-, and trimethylation of lysine 4 (K4) of histone H3 in S. cerevisiae (Briggs et al. 2001; Roguev et al. 2001; Krogan et al. 2002; Nagy et al. 2002; Santos-Rosa et al. 2002). Set1 belongs to a multi-subunit complex called COMPASS, whose members include Set1, Bre2, Sdc1, Shg1, Spp1, Swd1, Swd2, and Swd3 (Roguev et al. 2001; Krogan et al. 2002; Nagy et al. 2002). Of the eight proteins in COMPASS, only Swd2, a WD-40 motif-containing protein, is essential for viability as it is required for correct termination of Pol II transcription (Cheng et al. 2004; Dichtl et al. 2004). The remaining seven members of COMPASS are not essential, meaning that cells lacking one or more of the seven nonessential members of COMPASS are viable, as are strains that lack K4-methylated histone H3.
Cells lacking Set1 do not form a functional COMPASS complex (Roguev et al. 2001) and yet have few phenotypes. One of the most prominent defects of set1Δ cells is the inability to silence Pol II-transcribed genes at telomeres and the rDNA (Nislow et al. 1997; Bryk et al. 2002; Krogan et al. 2002; Nagy et al. 2002). Several studies have examined the requirement for some of the COMPASS members in silencing at telomeres (Nislow et al. 1997; Miller et al. 2001; Krogan et al. 2002; Nagy et al. 2002; Schneider et al. 2005). However, conflicting results from these studies have left the question of which COMPASS proteins are required for silencing at telomeres unresolved. The roles of the COMPASS members other than Set1 in transcriptional silencing at the rDNA or of endogenous genes at the HM loci have not yet been determined.
High levels of K4-trimethylated histone H3 are associated with genes that have been recently transcribed by Pol II (Bernstein et al. 2002; Santos-Rosa et al. 2002; Boa et al. 2003). Recruitment of COMPASS to actively transcribed genes occurs through interactions with the Pol II-elongation complex Paf1C (Krogan et al. 2003; Ng et al. 2003b). In addition, Paf1C is required for ubiquitylation of histone H2B by Ubc2 (Rad6), a modification that is a prerequisite for methylation of histone H3 present at the 5′-end of actively transcribed genes by COMPASS (Briggs et al. 2002; Ng et al. 2003a; Wood et al. 2003). Cells lacking Paf1 or Ubc2 fail to accumulate K4-trimethylated H3 at Pol II-transcribed genes (Dover et al. 2002; Sun and Allis 2002; Krogan et al. 2003; Ng et al. 2003a,b; Wood et al. 2003). Despite a wealth of knowledge about the regulation of K4 methylation of histone H3, the function of K4-methylated histone H3 at actively transcribed genes remains unclear.
A recent article examining the role of COMPASS in methylation of histone H3 after induction of the MET16 gene stated that Set1, Swd1, and Swd3 were required for K4-dimethylated H3, while Set1, Swd1, Swd3, Sdc1, and Spp1 were required for K4-trimethylated H3 (Morillon et al. 2005). Other studies reported that Bre2 is required for K4 trimethylation of histone H3 and that loss of K4-trimethylated H3 in cells lacking Bre2 can be restored partially in the presence of a specific dominant allele of SET1 (Schlichter and Cairns 2005; Schneider et al. 2005). While these and other studies have looked at a subset of COMPASS members, quantitative analyses to determine the role of each of the seven nonessential COMPASS members in K4 mono-, di-, or trimethylation of histone H3 have not been reported.
Here, we examine the requirement for individual COMPASS members in transcriptional silencing and K4 mono-, di-, and trimethylation of histone H3. Our data show that with the exception of Shg1, all members of COMPASS are required for transcriptional silencing at the rDNA. In contrast to the rDNA, we found that all members of COMPASS are required for silencing of a Pol II gene at the telomere on the left arm of chromosome VII, while none are required for silencing of endogenous genes located at the HM loci. Using strains lacking one or more of the genes encoding subunits of COMPASS, we show that COMPASS complexes lacking one or more subunits maintain the ability to methylate histone H3. These findings indicate a redundancy in the roles of several COMPASS members in the mono- and dimethylation of histone H3 on K4. Similar to the requirement for Paf1C in targeting COMPASS to genes transcribed by RNA Pol II, Paf1 is required for gene silencing and wild-type levels of K4-methylated histone H3 at the rDNA, suggesting a possible direct role for K4-methylated H3 in gene silencing at the rDNA.
MATERIALS AND METHODS
Media:
Standard yeast media were prepared as described (Rose et al. 1990). YPADT is YPD media supplemented with l-tryptophan (20 mg/liter) and adenine sulfate (20 mg/liter). Synthetic complete (SC) media and SC containing 1 mg/liter 5-fluoroorotic acid (SC + 5-FOA) (Boeke et al. 1987) were used to measure the level of expression of a URA3 gene at the telomere on the left arm of chromosome VII.
Yeast strains:
S. cerevisiae strains are shown in Table 1. MBY1198, MBY1217, and MBY1238 have been described previously (Bryk et al. 2002). All other strains were made for this study. A set of six strains, each with one of the genes encoding a COMPASS protein deleted and replaced with the KANMX4 gene from pRS400 (Brachmann et al. 1998), was made by PCR-mediated gene disruption (Schneider et al. 1995) in MBY1198. A shg1Δ∷KANMX4 spp1Δ∷LEU2 double mutant was made by PCR-mediated gene disruption (Schneider et al. 1995) in MBY1666 (see Table 1). Other multiple COMPASS gene deletion mutants were made by genetic crosses. The URA3 gene was integrated into the ADH4 locus at the left end of chromosome VII (URA3–TEL–VIIL) in MBY1198 and the COMPASS gene deletion strains using plasmid ADH4UCA-IV, kindly provided by Dan Gottschling (Gottschling et al. 1990). PAF1 was deleted and replaced with KANMX4 from pRS400 or URA3 from pRS406 (Brachmann et al. 1998), and SIR2 was deleted and replaced with KANMX4 from pRS400 by PCR-mediated gene disruption (Schneider et al. 1995). Strains containing gene deletions or insertions were checked by restriction digest of PCR-amplified genomic DNA and genetic crosses to verify Mendelian segregation of KANMX4, LEU2, or URA3 marking the gene deletions and URA3 marking the telomere on the left end of chromosome VII.
TABLE 1.
Yeast strains
| Strain | Genotype |
|---|---|
| MBY1198 | MATα ade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 Ty1his3AI-236 Ty1ade2AI-515 |
| MBY1206 | MATaade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 Ty1his3AI-236 Ty1ade2AI-515 |
| MBY1217 | MBY1198 set1Δl∷TRP1 |
| MBY1235 | MBY1206 set1Δl∷TRP1 |
| MBY1238 | MBY1198 sir2Δ∷KANMX4 |
| MBY1635 | MBY1198 bre2Δ∷KANMX4 |
| MBY1636 | MBY1198 sdc1Δ∷KANMX4 |
| MBY1666 | MBY1198 shg1Δ∷KANMX4 |
| MBY1678 | MBY1198 spp1Δ∷KANMX4 |
| MBY1681 | MBY1198 swd1Δ∷KANMX4 |
| MBY1683 | MBY1198 swd3Δ∷KANMX4 |
| MBY1752 | MBY1198 paf1Δ∷URA3 |
| MBY1792 | MATamURA3-LEU2∷RDN1 his3Δ200 leu2Δ met15Δ0 trp1Δ63 ura3Δ0 |
| MBY1804 | MATamURA3-LEU2∷leu2Δ1 his3Δ200 met15Δ0 trp1Δ63 ura3Δ0 |
| MBY1831 | MATaade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 sir2Δ∷KANMX4 SET1-N-myc3 Ty1his3AI-236 Ty1ade2AI-515 |
| MBY1847 | MBY1198 bre2Δ∷KANMX4 sdc1Δ∷KANMX4 |
| MBY1912 | MBY1198 adh4∷URA3–TEL–VIIL |
| MBY1914 | MBY1217 adh4∷URA3–TEL–VIIL |
| MBY1916 | MBY1635 adh4∷URA3–TEL–VIIL |
| MBY1917 | MBY1636 adh4∷URA3–TEL–VIIL |
| MBY1919 | MBY1666 adh4∷URA3–TEL–VIIL |
| MBY1922 | MBY1678 adh4∷URA3–TEL–VIIL |
| MBY1923 | MBY1681 adh4∷URA3–TEL–VIIL |
| MBY1925 | MBY1683 adh4∷URA3–TEL–VIIL |
| MBY1927 | MATα ade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 Ty1ade2AI-515 bre2Δ∷KANMX4 shg1Δ∷KANMX4 |
| MBY1929 | MATaade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 Ty1ade2AI-515 sdc1Δ∷KANMX4 shg1Δ∷KANMX4 |
| MBY1930 | MATaade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 Ty1ade2AI-515 bre2Δ∷KANMX4 sdc1Δ∷KANMX4 shg1Δ∷KANMX4 |
| MBY1931 | MATaade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 Ty1his3AI-236 Ty1ade2AI-515 sdc1Δ∷KANMX4 spp1Δ∷KANMX4 |
| MBY1933 | MATaade2Δ∷hisG his3Δ200 leu2Δ0 met15Δ0 trp1Δ63 ura3Δ0 Ty1his3AI-236 Ty1ade2AI-515 bre2Δ∷KANMX4 spp1Δ∷KANMX4 |
| MBY1935 | MBY1198 shg1Δ∷KANMX4 spp1Δ∷LEU2 |
| MBY1964 | MBY1804 paf1Δ∷KANMX4 |
| MBY1979 | MBY1792 paf1Δ∷KANMX4 |
Plate assay for expression of the telomeric URA3 gene:
Cultures containing 5 ml of YPADT medium were seeded with wild-type or COMPASS gene deletion cells containing URA3–TEL–VIIL and grown to saturation. Ten-fold serial dilutions of each culture were made in sterile milliQ water and 5 μl of each dilution was spotted onto SC and SC + 5-FOA agar. Plates were photographed after 2–3 days of incubation at 30°.
Western blot analysis:
Cells grown to early-log phase (1–2 × 107 cells/ml) in 50 ml YPADT medium were washed with milliQ water and resuspended in 0.25 ml RIPA buffer (150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 50 mm Tris–HCl, pH 8.0) (Harlow and Lane 1999) containing 1% SDS and protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 1.67 μg/ml aprotonin, 1.67 μg/ml pepstatin, 0.33 μg/ml leupeptin, 0.4 μg/ml bestatin). Cells were disrupted at 4° with acid-washed glass beads in a mini-bead-beater (Biospec Products, Bartlesville, OK). Lysates were incubated on ice for 30 min and clarified by centrifugation at 12,000 rpm at 4° for 30 min. Proteins from clarified whole-cell extracts (4–100 μg) were separated on 15% SDS–polyacrylamide gels, transferred to PVDF membrane, and probed with α-histone H3 (ab1791, Abcam; 1:2500), α-K4-monomethyl H3 (ab8895, Abcam; 1:125 and 1:2000), α-K4-dimethyl H3 (07-030, Upstate Cell Signaling; 1:5000), or α-K4-trimethyl H3 (ab8580, Abcam; 1:5000). Antibody binding was detected using HRP-conjugated α-rabbit secondary antibodies [Promega (Madison, WI); 1:2000] and an Immun-Star chemiluminescence kit (Bio-Rad, Hercules, CA). Western blots were quantified on a Fujifilm LAS-3000 image analyzer using ImageGauge and IJAR software.
Northern blot analysis:
Total RNA was isolated from yeast cells as described previously (Bryk et al. 1997). Northern analysis was performed as described (Swanson et al. 1991). Strand-specific 32P-labeled RNA probes were used to detect Ty1his3AI, total Ty1, and PYK1 mRNAs (Curcio and Garfinkel 1992). ACT1 (+564–+1200), URA3 (+134–+679), a1 (+173–+405), and α2 (+166–+523) probes were made by PCR amplification of yeast genomic DNA and then purified from agarose gels and labeled with [α-32P]dATP by random priming (Ausubel et al. 1988). Northern blots were quantified on a Molecular Dynamics (Sunnyvale, CA) Storm 860 phosphorimager using ImageQuant software.
Chromatin immunoprecipitation/slot-blot analysis:
Chromatin immunoprecipitations (ChIP) were performed according to Bryk et al. (2002) with some modifications. Cultures were grown to midlog phase, and crosslinked chromatin was prepared and sheared by sonication at 4° to an average size of 500 bp with 12 20-sec pulses using a Branson (Danbury, CT) Sonifier 250 at a 1.5 power setting, 100% duty cycle. Sonicated chromatin was incubated with 6 μl of α-K4-monomethyl H3 (ab8895, Abcam), 15 μl of α-K4-dimethyl H3 (07-030, Upstate Cell Signaling), or 5 μl of α-K4-trimethyl H3 (ab8580, Abcam) for 16 hr at 4°. Immune complexes were isolated after incubation at 4° for 2 hr with Protein A or Protein G sepharose beads (Amersham Biosciences). Formaldehyde crosslinks were reversed by incubation of the immunoprecipitated or input chromatin samples for 12–16 hr at 65°. Next, the chromatin was treated with proteinase K, and the DNA was purified by phenol and chloroform extraction, precipitated with ethanol, and analyzed by slot blot. The 32P-labeled probes specific for the nontranscribed spacer of the rDNA locus (rDNA NTS) and the telomere on the right arm of chromosome VI (TelVIR) were described previously (Bryk et al. 2002). Percentage immunoprecipitation (%IP) is the ratio of the signal from immunoprecipitated DNA to the signal from input DNA. The %IP was determined by quantification of the blots on a Storm 860 phosphorimager (Molecular Dynamics) using ImageQuant software. The %IP values for the wild-type and spp1Δ extracts were corrected for background by subtracting the %IP value obtained for set1Δ cells, where no H3 K4 methylation occurs.
Oligonucleotides:
The sequences of oligonucleotides are available upon request.
RESULTS
Several COMPASS members are required for silencing in the rDNA:
Previous work has shown that SET1 is required for gene silencing at the rDNA in S. cerevisiae (Briggs et al. 2001; Bryk et al. 2002; Fingerman et al. 2005). To determine the requirement for other members of COMPASS in rDNA silencing, a set of strains with each lacking one of the seven nonessential genes encoding a COMPASS protein was constructed. Each of these strains contains a single copy of a Pol II-transcribed Ty1his3AI element in the rDNA (rDNA–Ty1his3AI element) (Bryk et al. 1997). The level of mRNA from the rDNA–Ty1his3AI element in total RNA isolated from the seven COMPASS deletion strains and the wild-type strain MBY1198 (Table 1) was measured to determine if the deleted COMPASS gene was required to silence Pol II transcription in the rDNA (Figure 1). The low level of rDNA–Ty1his3AI transcript in total RNA from wild-type cells reflects transcriptional silencing (Figure 1, top). We measured the level of ACT1 mRNA as a normalization control. In the COMPASS deletion strains, with the exception of the shg1Δ mutant, the level of the rDNA–Ty1his3AI transcript relative to ACT1 mRNA was increased 2.5- to 5.3-fold above that of the wild-type strain. In contrast, the value of the ratio of the rDNA–Ty1his3AI:ACT1 mRNA from shg1Δ cells was similar to the value from wild-type cells (Figure 1). Haploid S. cerevisiae strains contain ∼30 endogenous Ty1 elements (Wilke et al. 1992). As a control to show that the transcriptional defects in the COMPASS deletion mutants were specific to the rDNA, we monitored steady-state mRNA from the endogenous Ty1 elements using a Ty1-specific probe (Figure 1, middle). Importantly, the level of total Ty1 mRNA was nearly equivalent in the COMPASS deletion mutants and the wild-type strain, indicating that the COMPASS proteins are required for silencing of the Ty1his3AI element in the rDNA and do not regulate expression of Ty1 elements located outside of the rDNA. In summary, these results indicate that SET1, BRE2, SDC1, SPP1, SWD1, and SWD3 are required for transcriptional silencing of the rDNA–Ty1his3AI element and that SHG1 is not required for rDNA silencing. These findings are consistent with the results of quantitative transposition assays that measure the level of expression of the rDNA–Ty1his3AI element (data not shown).
Figure 1.
Several COMPASS members are required for rDNA silencing. Total RNA from wild-type cells and COMPASS deletion cells that each contain a single Ty1his3AI element in the rDNA was probed with a 32P-labeled HIS3 RNA probe to detect rDNA–Ty1his3AI message (top), a Ty1 RNA probe to detect total Ty1 message (middle), or an ACT1 probe as a loading control (bottom). Transcript levels for Ty1his3AI and total Ty1 were normalized to the ACT1 loading control. The average values of rDNA–Ty1his3AI:ACT1 and total Ty1:ACT1 mRNA for each mutant relative to the ratio in wild-type cells are shown. The average values of rDNA–Ty1his3AI:ACT1 (±SE; n = 3 or 4) were set1Δ:wild type (WT) 2.5 (±0.1); bre2Δ:WT 4.0 (±0.6); sdc1Δ:WT 3.3 (±0.5); shg1Δ:WT 1.4 (±0.1); spp1Δ:WT 5.3 (±1.1); swd1Δ:WT 2.7 (±0.4); and swd3Δ:WT 4.4 (±1.0). The values of total Ty1:ACT1 mRNA were set1Δ:WT 1.3 (±0.2); bre2Δ:WT 1.7 (±0.4); sdc1Δ:WT 1.4 (±0.3); shg1Δ:WT 1.1 (±0.3); spp1Δ:WT 1.2 (±0.3); swd1Δ:WT 0.9 (±0.2); and swd3Δ:WT 1.4 (±0.2).
All COMPASS members are required for silencing of a URA3 gene integrated at a telomere:
Several studies have analyzed the requirement for a subset of COMPASS members in telomeric silencing (Nislow et al. 1997; Miller et al. 2001; Krogan et al. 2002; Nagy et al. 2002; Schneider et al. 2005). Despite the use of similar strains and plate-growth assays, some of the results are contradictory. To resolve these discrepancies, in addition to plate assays, we used a direct method to determine the genetic requirements for telomeric silencing by measuring the level of RNA transcript from a Pol II-transcribed gene inserted at a telomere. We integrated the URA3 gene at a telomere in a wild-type strain and the COMPASS deletion strains (materials and methods; Table 1) and performed Northern analysis to evaluate the role of COMPASS members in telomeric silencing by comparing the levels of URA3 mRNA in the wild-type and COMPASS deletion strains (Figure 2A). The low level of URA3 transcript in total RNA from the wild-type strain indicates that the URA3 gene was silenced at the telomere. We measured the level of ACT1 mRNA as a normalization control. In the COMPASS deletion mutants, the ratio of URA3:ACT1 mRNA was increased 3.7- to 9.7-fold above that of the wild-type strain (Figure 2A).
Figure 2.
A role for COMPASS in silencing at the telomere on the left arm of chromosome VII. (A) Total RNA from wild-type and COMPASS deletion strains that carry URA3 at the telomere on the left arm of chromosome VII (materials and methods, Table 1) were subject to Northern analysis using radiolabeled DNA probes to measure URA3 or ACT1 mRNA. Blots were quantified and the ratios of URA3 transcript (normalized to ACT1 mRNA) in mutant relative to wild-type cells were determined and are shown below the URA3 panel. The values (±SE; n = 3 or 4) are set1Δ:WT 6.0 (±1.1); bre2Δ:WT 6.7 (±0.7); sdc1Δ:WT 6.6 (±0.6); shg1Δ:WT 3.7 (±0.1); spp1Δ:WT 4.2 (±1.3); swd1Δ:WT 7.4 (±0.04); and swd3Δ:WT 9.7 (±2.2). (B) A representative plate assay (n = 3) indicates a requirement for COMPASS in silencing at a telomere. Growth on SC media verifies that equivalent numbers of wild-type and COMPASS deletion cells were plated. Growth on SC + 5-FOA indicates silencing of the URA3 gene, since 5-FOA is toxic to cells that express the URA3 gene.
To complement the Northern analysis, plate-growth assays were performed (Figure 2B). The chemical 5-FOA is converted by the URA3 gene product to the toxic analog 5-fluorouracil. Growth of cells on media containing 5-FOA (SC + 5-FOA) indicates that the URA3 gene is silenced at the telomere, whereas lack of growth on 5-FOA media means that URA3 is expressed and that telomeric silencing is defective. In plate assays using wild-type cells, the level of expression of the telomeric URA3 gene was extremely low, as evidenced by robust growth on SC + 5-FOA (Figure 2B). In contrast, in COMPASS deletion mutant cells, the URA3 gene was expressed at higher levels, leading to a 10,000-fold reduction in growth on SC + 5-FOA in the set1Δ, sdc1Δ, swd1Δ, and swd3Δ mutants, a 100- to 1000-fold reduction in the bre2Δ mutant, and a 10- to 100-fold reduction in the spp1Δ mutant. In the shg1Δ cells, a 5- to 10-fold reduction in growth was observed on SC + 5-FOA (Figure 2B), a result that is consistent with the Northern analysis showing that deletion of SHG1 has the smallest effect of all COMPASS deletion mutants on the level of mRNA from the telomeric URA3 gene (Figure 2A). Our findings using Northern blotting and plate assays demonstrate that all members of COMPASS are required for transcriptional silencing of the URA3 gene when located at the telomere. However, there is assay-dependent variability in the degree of the loss-of-silencing phenotype observed in the COMPASS deletion mutants.
COMPASS is not required for silencing of endogenous genes at the HM loci:
Mating-type-specific genes present at the HM loci are not expressed in wild-type cells. Thus, the a1 gene is silenced when present at HMR (referred to as the HMRa1 gene), but is expressed when present at the MAT locus (i.e., in a MATa strain). Likewise, the α2 gene is silenced when present at HML, but is expressed when located at the MAT locus in MATα cells. To examine the role of COMPASS in transcriptional silencing at HMR, we measured the level of HMRa1 transcript in wild-type MATα cells (WT MATα) and COMPASS deletion MATα cells (set1Δ MATα, bre2Δ MATα, sdc1Δ MATα, etc.) by Northern analysis (Figure 3A). The lack of detectable HMRa1 transcript in total RNA from wild-type MATα cells indicates that the HMRa1 gene was silenced. Similarly, HMRa1 transcript was not detected in total RNA from the COMPASS deletion mutants, indicating that transcriptional silencing of HMRa1 does not require COMPASS. As controls, total RNA from a MATa wild-type strain where the a1 gene is present at the MAT locus and a MATα sir2Δ strain in which deletion of the gene encoding the silencing factor SIR2 causes loss of silencing at the HMRa1 locus were included on the blot. In both wild-type MATa cells (WT MATa) and MATα sir2Δ cells (sir2Δ MATα), a1 transcripts were detected (Figure 3A), indicating that the a1 mRNA from the MATa or HMR locus can be detected by Northern analysis if present. Similar results were obtained when examining HMLα2 RNA from wild-type MATa and set1Δ MATa cells. No HMLα2 transcript was present in total RNA from wild-type or set1Δ cells; however, α2 transcripts were detected in total RNA from wild-type MATα cells (WT MATα) and sir2Δ MATa cells (Figure 3B). Together, these data indicate that COMPASS is not required for transcriptional silencing of endogenous genes present at the HM loci.
Figure 3.
COMPASS is not required for silencing at the HM loci. (A) Silencing of the HMRa1 gene does not require COMPASS. Total RNAs from wild type (WT), sir2Δ, COMPASS deletion MATα cells (with the a1 gene at HMR), and MATa wild-type cells (with the a1 gene at MAT; WT MATa) were analyzed to monitor levels of a1 transcript. ACT1 mRNA was measured as a loading control. (B) Silencing of the α2 gene does not require Set1. Northern analyses were performed on total RNAs from WT MATa, set1Δ MATa, WT MATα, and sir2Δ MATa cells to examine the requirement for Set1 in silencing of the HMLα2 gene.
Overlapping requirements for COMPASS proteins in methylation of histone H3 on K4:
To determine the requirement for COMPASS members in the methylation of histone H3 on K4, we analyzed whole-cell extracts isolated from wild-type cells and COMPASS deletion mutants by quantitative Western blotting. Total histone H3 was measured using antisera that recognizes the C terminus of histone H3, and K4-monomethylated histone H3, K4-dimethylated histone H3, and K4-trimethylated histone H3 were measured using antisera that recognize one of the three forms of K4-methylated histone H3 (Figure 4A; materials and methods). The data indicate that Set1, Swd1, and Swd3 are required for mono-, di-, and trimethylation of histone H3 on K4 and that Bre2 and Sdc1 are required for trimethylation of histone H3 on K4. While the levels of K4 mono- and K4-dimethylated H3 in extracts from bre2Δ or sdc1Δ cells were lower than the levels found in extracts from wild-type cells, the analysis revealed that K4 mono- and dimethylation of histone H3 can occur in the absence of BRE2 or SDC1. All three forms of K4-methylated histone H3 were present in spp1Δ cells and in shg1Δ cells, albeit at levels lower than in wild-type cells. Consistent with the small effects of SHG1 on gene silencing at the rDNA and telomere (Figures 1 and 2), extracts from shg1Δ cells contained the highest levels of K4 mono-, di-, and trimethylated H3 of all the COMPASS deletion mutants.
Figure 4.
K4-methylated H3 is dependent on several members of the COMPASS complex. Representative data from quantitative Western analysis measuring the levels of K4-methylated H3 in whole-cell extracts from (A) wild-type cells and single COMPASS deletion mutants and (B) wild-type cells and multiple-deletion mutants using antisera specific for K4-monomethylated H3 (α-mono), K4-dimethylated H3 (α-di), or K4-trimethylated H3 (α-tri). Antisera specific for the C terminus of histone H3 (α-H3) was used to ensure equivalent loading of cell extracts in each lane. The values of the ratio of the levels of K4-methylated H3 from mutant cells relative to wild-type cells for the blot are shown. The lack of a value indicates that K4-methylated H3 was not detected in mutants. The data for all the replicates are as follows. The average (±SE) when n = 3 or the average (range) when n = 2 is K4-monomethylated H3, bre2Δ:WT 0.15 (±0.04), sdc1Δ:WT 0.16 (±0.04), shg1Δ:WT 0.80 (±0.08), spp1Δ:WT 0.44 (±0.06), bre2Δ sdc1Δ:WT 0.31 (±0.09), bre2Δ shg1Δ:WT 0.03 (±0.01), sdc1Δ shg1Δ:WT 0.07 (±0.03), bre2Δ sdc1Δ shg1Δ:WT 0.05 (±0.03), shg1Δ spp1Δ:WT 0.27 (0.10–0.44, n = 2); K4-dimethylated H3, bre2Δ:WT 0.08 (±0.06), sdc1Δ:WT 0.08 (±0.04), shg1Δ:WT 0.80 (±0.11), spp1Δ:WT 0.36 (±0.05), bre2Δ sdc1Δ:WT 0.05 (±0.01), bre2Δ shg1Δ:WT 0.0035 (0.0026–0.0044, n = 2), sdc1Δ shg1Δ:WT 0.0018 (0.0011–0.0026, n = 2), bre2Δ sdc1Δ shg1Δ:WT 0.0019 (0.0008–0.0029, n = 2), shg1Δ spp1Δ:WT 0.12 (±0.03); K4-trimethylated H3, shg1Δ:WT 0.47 (±0.05), spp1Δ:WT 0.05 (±0.01).
The analysis shown in Figure 4A suggests that a core set of COMPASS members, including Set1, Swd1, and Swd3, is essential for the mono- and dimethylation of histone H3 on K4 and that these core proteins, along with Bre2 and Sdc1, are required for trimethylation of histone H3 on K4. Further, while K4 mono-, di-, and trimethylated forms of histone H3 were detected in extracts from cells lacking Spp1, the level of K4-trimethylated H3 was <10% of the level detected in wild-type extracts, suggesting an important role for Spp1 in trimethylation of H3 on K4. To further understand the requirements for Bre2, Sdc1, Spp1, and Shg1 in the methylation of histone H3, we constructed and characterized double and triple gene-deletion mutants (Figure 4B). Western analysis on extracts from cells lacking both BRE2 and SDC1 showed that these cells have no detectable K4-trimethylated histone H3 and reduced levels of K4 mono- and dimethylated histone H3. The levels of K4 mono- and dimethylated histone H3 in the bre2Δ sdc1Δ double mutant were similar to those in the corresponding single-deletion mutants (Figure 4A), suggesting that BRE2 and SDC1 are redundant with each other with respect to their role in methylation of histone H3. These data indicate that, in cells with an otherwise complete COMPASS complex, Bre2 and Sdc1 are not absolutely required for K4 mono- or dimethylation of H3.
To understand the contribution of Shg1 to the histone H3 methylation, we analyzed extracts from multiple-deletion mutant cells lacking BRE2 and SHG1 (bre2Δ shg1Δ), SDC1 and SHG1 (sdc1Δ shg1Δ), or BRE2, SDC1 and SHG1 (bre2Δ sdc1Δ shg1Δ) (Figure 4B). The levels of K4-methylated H3 were reduced to a similar degree in extracts from these multiple-deletion mutants with the levels of K4-monomethylated H3 at <10% of wild type and the levels of K4-dimethylated histone H3 at <1% of wild type. Interestingly, cells lacking SHG1 (shg1Δ) maintained levels of K4 mono- and dimethylated histone H3 that were not significantly different from the levels in wild-type cells (Figure 4A) and cells lacking Bre2 and/or Sdc1 maintained higher levels of K4 mono- and dimethylated H3 when Shg1 was present (Figure 4, A and B). Thus, these data indicate that, in the absence of Bre2 and/or Sdc1, Shg1 is important for the function and/or stability of the COMPASS complex.
Next, we analyzed the role of Spp1 in COMPASS by measuring the levels of K4-methylated H3 in cells lacking BRE2 and SPP1 (bre2Δ spp1Δ), SDC1 and SPP1 (sdc1Δ spp1Δ), or SHG1 and SPP1 (shg1Δ spp1Δ) (Figure 4B). Our data show that extracts from cells lacking Spp1 and Bre2 or Spp1 and Sdc1 were devoid of K4-methylated histone H3, indicating that H3 K4 mono- and K4 dimethylation requires either Spp1 and Bre2 or Spp1 and Sdc1. Cells lacking both Shg1 and Spp1 contained no K4-trimethylated histone H3 and reduced levels of K4 mono- and dimethylated H3 compared to the single-deletion mutants (see Figure 4A). These data reveal that neither Spp1 nor Shg1 is absolutely required for trimethylation of K4 of histone H3. However, the lack of K4-trimethylated H3 and reduced K4 mono- and dimethylated H3 in the spp1Δ shg1Δ double mutant indicates that Spp1 and Shg1 make independent contributions to the function or stability of COMPASS. Consistent with the silencing defects of the single spp1Δ mutant and the H3-methylation defects of the double mutant, using Northern analysis we determined that the double shg1Δ spp1Δ mutant was defective for rDNA silencing (data not shown).
Bulk K4-methylated histone H3 in spp1Δ cells mirrors the levels associated with the rDNA and a telomere:
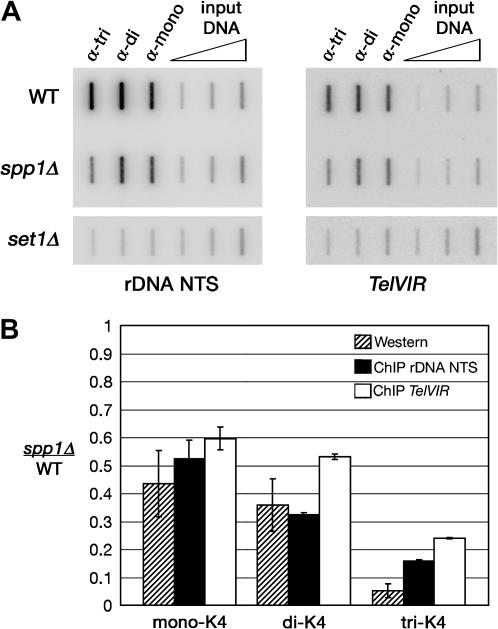
To determine if the levels of K4-methylated histone H3 measured in whole-cell extracts reflected the levels present at silent loci, we performed ChIP analyses to measure K4-methylated H3 at the rDNA NTS and the telomere at the right arm of chromosome VI (TelVIR) in wild-type, spp1Δ, and set1Δ cells. Input and immunoprecipitated DNA was analyzed using a slot blot to determine the fraction of rDNA NTS or TelVIR DNA associated with K4-methylated histone H3 (materials and methods; Figure 5A). Consistent with the reduced levels of bulk K4-methylated H3 in spp1Δ cells, the association of the three forms of K4-methylated H3 with the rDNA NTS and TelVIR was lower in spp1Δ cells than in wild-type cells. To facilitate a comparison of ChIP and Western data, in Figure 5B the values of the ratio (spp1Δ/wild type) of the level of rDNA NTS or TelVIR associated with K4-methylated H3 as measured by ChIP (Figure 5A) are represented graphically alongside the values of K4-methylated H3 in spp1Δ cells relative to wild-type cells measured by Western analysis (Figure 4A). This representation shows that in spp1Δ cells the levels of bulk K4-methylated H3 were reduced and, correspondingly, the levels of K4-methylated H3 were lower at the rDNA NTS and TelVIR.
Figure 5.
Reduced levels of K4-methylated H3 were present at silent loci in spp1Δ cells. (A) ChIP analysis shows that the association of K4-methylated H3 with silent loci was reduced in spp1Δ cells. Immunoprecipitated and input DNA were analyzed by slot blot using a 32P-labeled DNA probe specific for the rDNA NTS or telomere on the right end of chromosome VI (TelVIR). Open triangles represent increasing amounts of input DNA used to show linearity of the hybridization. The ratios (spp1Δ:WT) of %IP of the rDNA NTS from two independent experiments are K4-monomethylated H3: 0.57, 0.48; K4-dimethylated H3: 0.32, 0.33; and K4-trimethylated H3: 0.16, 0.15. The ratios (spp1Δ:WT) of %IP of TelVIR from two independent experiments are K4-monomethylated H3: 0.63, 0.57; K4-dimethylated H3: 0.52, 0.54; and K4-trimethylated H3: 0.24, 0.24. (B) Graph of the average ratio (spp1Δ:WT) of the levels of bulk K4-methylated histone H3 measured by Western analyses (Figure 4A) and the average ratio (spp1Δ:WT) of %IP of rDNA NTS and TelVIR associated with K4-methylated histone H3 as measured by ChIP (Figure 5A). Error bars, standard deviation for three independent Western blots, and range for two independent ChIP experiments are shown.
PAF1 is required for rDNA silencing:
Studies have implicated the Paf1 complex in methylation of histone H3 on K4 and silencing at telomeres (Krogan et al. 2003; Ng et al. 2003b). Because PAF1 was reported to be required for methylation of histone H3 on K4, we reasoned that cells lacking PAF1 would exhibit defects in rDNA silencing. Total RNA from wild-type and paf1Δ cells carrying a URA3 marker (mURA3) inside the rDNA or outside the rDNA (Smith and Boeke 1997) was analyzed by Northern analysis to determine if PAF1 is required for rDNA silencing (Figure 6A). The data indicate that the ratio of the mURA3 (inside rDNA):ACT1 mRNA increased 2.9-fold in the paf1Δ cells compared to the wild-type cells, consistent with a defect in rDNA silencing. The ratio of mURA3 (outside rDNA):ACT1 mRNA was 1.1 in paf1Δ cells, similar to wild-type cells. Thus, these data show that PAF1 is required for transcriptional silencing at the rDNA.
Figure 6.
Paf1 is required for rDNA silencing and association of K4-methylated H3 with the rDNA. (A) RNA from the mURA3 gene inside the rDNA was increased in paf1Δ strains. Northern analyses were performed on total RNA from wild-type and paf1Δ cells that have mURA3 inside the rDNA (left) or outside the rDNA at the leu2Δ1 locus (right). For three independent experiments, the average ratio (paf1Δ normalized to wild type) (±SE) of mURA3 mRNA (inside rDNA) relative to ACT1 mRNA was 2.9 (±0.2) and of mURA3 mRNA (outside rDNA) to ACT1 mRNA was 1.1 (±0.2). (B) Levels of K4-methylated histone H3 associated with the rDNA NTS were reduced in paf1Δ cells. ChIP experiments were performed with formaldehyde-crosslinked extracts from wild-type, paf1Δ, and set1Δ cells using antisera specific for K4-dimethylated H3 (α-di) or K4-trimethylated H3 (α-tri). The average value of %IP for the paf1Δ cells relative to the %IP for the wild-type cells (±SE) from three independent experiments was 0.37 (±0.03) for K4-dimethylated histone H3 and 0.18 (±0.04) for K4-trimethylated histone H3.
The levels of K4-methylated histone H3 at the rDNA were measured by ChIP in wild-type, paf1Δ, and set1Δ cells. Analysis of immunoprecipitated DNA showed that the levels of K4 di- and trimethylated histone H3 at the rDNA NTS in paf1Δ cells were reduced relative to those in wild-type cells (Figure 6B). The level of K4-dimethylated H3 at the rDNA in the paf1Δ strain was 37% of that in wild type, while the level of K4-trimethylated H3 was 18% of that in wild type. The presence of K4-methylated histone H3 in the paf1Δ strain is consistent with the results of Schlichter and Cairns (2005), who also detected K4-methylated forms of histone H3 in whole-cell extracts prepared from paf1Δ strains.
DISCUSSION
We have examined the requirements for individual members of the COMPASS complex in methylation of histone H3 and in transcriptional silencing at the rDNA, telomeres, and the silent mating loci in S. cerevisiae. We found that most members of the COMPASS complex, with the exception of Shg1, are required for transcriptional silencing at the rDNA (Figure 1). In contrast to the rDNA, we found that all members of the COMPASS complex are required for silencing of a gene at a telomere (Figure 2) and that none were required for silencing of the endogenous a1 and α2 genes at HMR and HML, respectively (Figure 3). To complement the silencing data, we performed a comprehensive analysis examining the contribution of each nonessential COMPASS member to mono-, di-, and trimethylation of lysine 4 on histone H3, using quantitative Western blotting experiments. Given that the levels of all three forms of K4-methylated histone H3 were altered to different extents in the COMPASS deletion mutants (Figure 4), we cannot assign which forms of K4-methylated H3 are required for gene silencing. However, it is clear that loss of and reduction of K4 mono-, di-, and trimethylated H3 are associated with loss of silencing at the rDNA and telomeres. Our results from the analysis of multiple-deletion mutants suggest that some members of COMPASS have redundant function (Figure 4B), hinting at the possibility of functional COMPASS subcomplexes in vivo. In addition, there is a requirement for Paf1 in rDNA silencing (Figure 6A). Considering that Paf1 is required to target COMPASS to actively transcribed genes (Krogan et al. 2003; Ng et al. 2003b) and that reduced levels of K4 di- and trimethylated H3 were present at the rDNA in paf1Δ cells (Figure 6B), one possibility is that Paf1 recruits COMPASS to the rDNA and other silent loci.
In previous work, we examined the role of Set1 in silencing at the rDNA (Bryk et al. 2002). Here we have determined the role of the other six members of COMPASS in silencing at the rDNA. Our data indicate that, with the exception of Shg1, all members of COMPASS are required for rDNA silencing. rDNA silencing occurs in shg1Δ cells where the level of K4-trimethylated H3 is ∼50% of wild type, a result that suggests that silent chromatin at the rDNA is tolerant of approximately twofold changes in the level of K4-trimethylated H3. Given that the levels of all three forms of K4-methylated histone H3 are reduced in the COMPASS deletion mutants found to be required for rDNA silencing (Figure 4), our data cannot confirm or refute a previous report that only K4-trimethylated H3 is required for rDNA silencing (Fingerman et al. 2005).
We examined the requirement for COMPASS in silencing at the telomere on the left arm of chromosome VII using Northern analysis and plate-growth assays. Our results show that all COMPASS members are required for telomeric silencing (Figure 2). These data are in agreement with growth assays reported by Krogan et al. (2002) and Schneider et al. (2005), who examined the role of COMPASS in telomeric silencing, although it is important to note that RNA analysis was not performed and Shg1 was not tested in the earlier studies. Similar to these previous studies, our plate-growth assays showed partial loss of silencing in bre2Δ cells and spp1Δ cells. Our Northern analysis indicated a clear requirement for Bre2 and Spp1 in telomeric silencing, as URA3 transcript levels in total RNA from bre2Δ and spp1Δ cells were 6.7- and 4.2-fold greater than wild type, respectively (Figure 2). Further, our data revealed that shg1Δ mutants and spp1Δ mutants, which are the least affected in the telomeric silencing assays (Figure 2), had levels of K4 mono-, di-, and trimethylated H3 that were closest to wild type (Figure 4A). ChIP experiments with a spp1Δ mutant (Figure 5) and a shg1Δ mutant (data not shown) showed that the levels of K4-methylated H3 at TelVIR reflected the levels measured in whole-cell extracts. Together, these data suggest that in spp1Δ cells and shg1Δ cells, the level of K4-methylated H3 is proportional to the degree of loss of telomeric silencing.
The role for Shg1 in transcriptional silencing at telomeres is intriguing, given that Shg1 is not required at the rDNA. We conclude that while the subtle reduction in levels of K4-methylated H3 in the shg1Δ mutant does not disrupt rDNA silencing, it is sufficient to derepress Pol II gene transcription at telomeres. This is consistent with the suggestion that silencing at telomeres is highly sensitive to changes in the levels of silencing factors in S. cerevisiae (Aparicio et al. 1991). Silencing at telomeres and at the HM loci requires several factors, including Sir3 and histone H4. Mutations in SIR3 suppress mutations in the gene encoding histone H4 (HHF2) and thereby restore silencing at HML but not at telomeres (Aparicio et al. 1991). Likewise, telomeric silencing may be more sensitive to changes in the levels of K4-methylated H3, resulting in the requirement for Shg1 in silencing at telomeres, and not at the rDNA.
To address the role of COMPASS in silencing at the HM loci, we measured the levels of the HMRa1 and HMLα2 gene transcripts in COMPASS mutants using Northern analysis. Previously it was reported that K4 methylation was required for silencing of Pol II-transcribed marker genes inserted into the HM loci (Nislow et al. 1997; Santos-Rosa et al. 2004; Fingerman et al. 2005). While one study stated that Set1 was required to repress expression of an inserted marker gene at a modified HML locus (Santos-Rosa et al. 2004), a second study concluded that Set1 was required for silencing of marker genes inserted into HML and HMR (Fingerman et al. 2005). Our analysis here shows that COMPASS and K4-methylated H3 are not required to silence endogenous genes present at the HM loci (Figure 3). These findings are consistent with the ability of our set1Δ strains of opposite mating type to mate in genetic crosses (data not shown). We conclude that while K4-methylated H3 may be required to regulate the expression of exogenous marker genes inserted into genetically altered HM loci, it does not regulate silencing of endogenous genes present at the HM loci.
A number of subunits of the COMPASS complex are dispensable for methylation of histone H3, with requirements for mono- and dimethylation being more relaxed than those for trimethylation. Our data showed that a core set of COMPASS subunits—Set1, Swd1, and Swd3—was essential for all forms of K4-methylated H3. The results of the quantitative Western analyses showed that K4 mono- and dimethylation of histone H3 can occur in the absence of any single member of COMPASS other than the core members. However, with the exception of the shg1Δ mutant, the levels of K4 mono- and dimethylated H3 were reduced significantly in the COMPASS deletion mutants compared to wild-type cells. Although the levels of K4-methylated H3 were least affected in the shg1Δ mutant, our studies with multiple-deletion mutants revealed that, in the absence of Bre2 and/or Sdc1, Shg1 is important for the function and/or stability of the COMPASS complex.
Studies using multiple-deletion mutants have given us the means to examine genetic interactions among members of COMPASS. From these studies (Figure 4), we conclude that Shg1 and Spp1 make independent contributions to the histone methylation reaction and/or stability of COMPASS. The results of studies with multiple-deletion mutants suggest that some members of COMPASS share overlapping functions. We found that when SPP1 was deleted there was a requirement for Bre2 and Sdc1 in mono- and dimethylation, indicating that, together, Bre2 and Sdc1 can complement the loss of Spp1 in the H3 K4 mono- and dimethylation reactions. Recent ChIP studies showed that Pol II accumulates at the 5′-end of the MET16 and RPS11B genes in spp1Δ and sdc1Δ cells, suggesting that Spp1 and Sdc1 function similarly in regulating the distribution of Pol II along active genes (Morillon et al. 2005). These data are consistent with our results showing that some COMPASS members have redundant functions.
Data from our genetic analysis suggest the possibility of several partially functional COMPASS subcomplexes, consisting of core members Set1, Swd1, and Swd3, together with various combinations of other COMPASS subunits. Our results using single and multiple COMPASS deletion mutants revealed that H3 K4 methylation can occur in the absence of one or more COMPASS proteins. Further, biochemical studies have shown that functional COMPASS complexes lacking one member of COMPASS can be isolated from single COMPASS gene deletion mutants (Roguev et al. 2001; Schneider et al. 2005). While we do not have proof of COMPASS subcomplexes in wild-type cells, the possibility of multiple COMPASS subcomplexes with different functions goes hand in hand with genomic-survey studies that show that K4-dimethylated H3 is associated with active and inactive genes and is the predominant form of K4-methylated histone H3 in S. cerevisiae (Bernstein et al. 2002; Santos-Rosa et al. 2002). Indeed, relaxed requirements to form numerous K4 dimethylation-competent COMPASS subcomplexes may contribute to the maintenance of high levels of K4-dimethylated H3 over the genome and lend support to speculation that the methylation activity of COMPASS is regulated by the availability of COMPASS proteins. Biochemical experiments to isolate COMPASS subcomplexes from wild-type cells and ChIP analysis to measure the levels of K4-methylated H3 at transcriptionally active and inactive genomic regions in cells lacking one or more COMPASS members will help us determine if COMPASS subcomplexes exist and function in vivo.
Acknowledgments
The authors appreciate the input and contributions of Fred Winston, in whose lab this work was initiated. We acknowledge Cristine Heaps for access to a Fujifilm LAS-3000 image detection system, Dan Gottschling for reagents, Andrea Fuller for assisting with the construction of a paf1Δ strain, J. Ruth German and Christina Zelasko from the National Science Foundation (NSF)-funded Research Experience for Undergraduates program in the Department of Biochemistry at Texas A&M University (NSF DBI-0139246), and Jessica Kilgore for work on telomeric silencing and Western analysis. This research was supported by the American Heart Association (beginning grant-in-aid 0365004Y), the American Cancer Society (RSG-04-049-01-GMC), and the National Institutes of Health (GM-070930).
References
- Aparicio, O. M., B. L. Billington and D. E. Gottschling, 1991. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66: 1279–1287. [DOI] [PubMed] [Google Scholar]
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman et al., 1988. Current Protocols in Molecular Biology. Greene Publishing Associates/John Wiley & Sons, New York.
- Bernstein, B. E., E. L. Humphrey, R. L. Erlich, R. Schneider, P. Bouman et al., 2002. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc. Natl. Acad. Sci. USA 99: 8695–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boa, S., C. Coert and H. G. Patterton, 2003. Saccharomyces cerevisiae Set1p is a methyltransferase specific for lysine 4 of histone H3 and is required for efficient gene expression. Yeast 20: 827–835. [DOI] [PubMed] [Google Scholar]
- Boeke, J. D., J. Trueheart, G. Natsoulis and G. R. Fink, 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175. [DOI] [PubMed] [Google Scholar]
- Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li et al., 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
- Briggs, S. D., M. Bryk, B. D. Strahl, W. L. Cheung, J. K. Davie et al., 2001. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 15: 3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, S. D., T. Xiao, Z. W. Sun, J. A. Caldwell, J. Shabanowitz et al., 2002. Gene silencing: trans-histone regulatory pathway in chromatin. Nature 418: 498. [DOI] [PubMed] [Google Scholar]
- Bryk, M., M. Banerjee, M. Murphy, K. E. Knudsen, D. J. Garfinkel et al., 1997. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 11: 255–269. [DOI] [PubMed] [Google Scholar]
- Bryk, M., S. D. Briggs, B. D. Strahl, M. J. Curcio, C. D. Allis et al., 2002. Evidence that Set1, a factor required for methylation of histone H3, regulates rDNA silencing in S. cerevisiae by a Sir2-independent mechanism. Curr. Biol. 12: 165–170. [DOI] [PubMed] [Google Scholar]
- Chen, L., and J. Widom, 2005. Mechanism of transcriptional silencing in yeast. Cell 120: 37–48. [DOI] [PubMed] [Google Scholar]
- Cheng, H., X. He and C. Moore, 2004. The essential WD repeat protein Swd2 has dual functions in RNA polymerase II transcription termination and lysine 4 methylation of histone H3. Mol. Cell. Biol. 24: 2932–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio, M. J., and D. J. Garfinkel, 1992. Posttranslational control of Ty1 retrotransposition occurs at the level of protein processing. Mol. Cell. Biol. 12: 2813–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichtl, B., R. Aasland and W. Keller, 2004. Functions for S. cerevisiae Swd2p in 3′ end formation of specific mRNAs and snoRNAs and global histone 3 lysine 4 methylation. RNA 10: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover, J., J. Schneider, M. A. Tawiah-Boateng, A. Wood, K. Dean et al., 2002. Methylation of histone H3 by COMPASS requires ubiquitination of histone H2B by Rad6. J. Biol. Chem. 277: 28368–28371. [DOI] [PubMed] [Google Scholar]
- Fingerman, I. M., C. L. Wu, B. D. Wilson and S. D. Briggs, 2005. Global loss of Set1-mediated H3 Lys4 trimethylation is associated with silencing defects in Saccharomyces cerevisiae. J. Biol. Chem. 280: 28761–28765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze, C. E., K. Verschueren, R. Strich and R. Easton Esposito, 1997. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 16: 6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling, D. E., O. M. Aparicio, B. L. Billington and V. A. Zakian, 1990. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63: 751–762. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane, 1999. Immunoprecipitation, pp. 221–265 in Using Antibodies: A Laboratory Manual, edited by J. Cuddihy, T. Kuhlman, I. Sialiano, M. Cozza and P. Barker. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Jenuwein, T., and C. D. Allis, 2001. Translating the histone code. Science 293: 1074–1080. [DOI] [PubMed] [Google Scholar]
- Krogan, N. J., J. Dover, S. Khorrami, J. F. Greenblatt, J. Schneider et al., 2002. COMPASS, a histone H3 (lysine 4) methyltransferase required for telomeric silencing of gene expression. J. Biol. Chem. 277: 10753–10755. [DOI] [PubMed] [Google Scholar]
- Krogan, N. J., J. Dover, A. Wood, J. Schneider, J. Heidt et al., 2003. The Paf1 complex is required for histone H3 methylation by COMPASS and Dot1p: linking transcriptional elongation to histone methylation. Mol. Cell 11: 721–729. [DOI] [PubMed] [Google Scholar]
- Miller, T., N. J. Krogan, J. Dover, H. Erdjument-Bromage, P. Tempst et al., 2001. COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proc. Natl. Acad. Sci. USA 98: 12902–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon, A., N. Karabetsou, A. Nair and J. Mellor, 2005. Dynamic lysine methylation on histone h3 defines the regulatory phase of gene transcription. Mol. Cell 18: 723–734. [DOI] [PubMed] [Google Scholar]
- Nagy, P. L., J. Griesenbeck, R. D. Kornberg and M. L. Cleary, 2002. A trithorax-group complex purified from Saccharomyces cerevisiae is required for methylation of histone H3. Proc. Natl. Acad. Sci. USA 99: 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, H. H., S. Dole and K. Struhl, 2003. a The Rtf1 component of the Paf1 transcriptional elongation complex is required for ubiquitination of histone H2B. J. Biol. Chem. 278: 33625–33628. [DOI] [PubMed] [Google Scholar]
- Ng, H. H., F. Robert, R. A. Young and K. Struhl, 2003. b Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell 11: 709–719. [DOI] [PubMed] [Google Scholar]
- Nislow, C., E. Ray and L. Pillus, 1997. SET1, a yeast member of the trithorax family, functions in transcriptional silencing and diverse cellular processes. Mol. Biol. Cell 8: 2421–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roguev, A., D. Schaft, A. Shevchenko, W. W. Pijnappel, M. Wilm et al., 2001. The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. EMBO J. 20: 7137–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, M. D., F. Winston and P. Hieter, 1990. Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Plainview, NY.
- Rusche, L. N., A. L. Kirchmaier and J. Rine, 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72: 481–516. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa, H., R. Schneider, A. J. Bannister, J. Sherriff, B. E. Bernstein et al., 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419: 407–411. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa, H., A. J. Bannister, P. M. Dehe, V. Geli and T. Kouzarides, 2004. Methylation of H3 lysine 4 at euchromatin promotes Sir3p association with heterochromatin. J. Biol. Chem. 279: 47506–47512. [DOI] [PubMed] [Google Scholar]
- Schlichter, A., and B. R. Cairns, 2005. Histone trimethylation by Set1 is coordinated by the RRM, autoinhibitory, and catalytic domains. EMBO J. 24: 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, B. L., W. Seufert, B. Steiner, Q. H. Yang and A. B. Futcher, 1995. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 11: 1265–1274. [DOI] [PubMed] [Google Scholar]
- Schneider, J., A. Wood, J.-S. Lee, R. Schuster, J. Dueker et al., 2005. Molecular regulation of histone H3 trimethylation by COMPASS and the regulation of gene expression. Mol. Cell 19: 849–856. [DOI] [PubMed] [Google Scholar]
- Smith, J. S., and J. D. Boeke, 1997. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 11: 241–254. [DOI] [PubMed] [Google Scholar]
- Strahl, B. D., and C. D. Allis, 2000. The language of covalent histone modifications. Nature 403: 41–45. [DOI] [PubMed] [Google Scholar]
- Straight, A. F., W. Shou, G. J. Dowd, C. W. Turck, R. J. Deshaies et al., 1999. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell 97: 245–256. [DOI] [PubMed] [Google Scholar]
- Sun, Z. W., and C. D. Allis, 2002. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418: 104–108. [DOI] [PubMed] [Google Scholar]
- Swanson, M. S., E. A. Malone and F. Winston, 1991. SPT5, an essential gene important for normal transcription in Saccharomyces cerevisiae, encodes an acidic nuclear protein with a carboxy-terminal repeat. Mol. Cell. Biol. 11: 3009–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke, C. M., E. Maimer and J. Adams, 1992. The population biology and evolutionary significance of Ty elements in Saccharomyces cerevisiae. Genetica 86: 155–173. [DOI] [PubMed] [Google Scholar]
- Wood, A., J. Schneider, J. Dover, M. Johnston and A. Shilatifard, 2003. The Paf1 complex is essential for histone monoubiquitination by the Rad6-Bre1 complex, which signals for histone methylation by COMPASS and Dot1p. J. Biol. Chem. 278: 34739–34742. [DOI] [PubMed] [Google Scholar]