Abstract
During meiotic prophase in the fission yeast Schizosaccharomyces pombe, the nucleus oscillates between the two ends of a cell. This oscillatory nuclear movement is important to promote accurate pairing of homologous chromosomes and requires cytoplasmic dynein. Dynein accumulates at the points where microtubule plus ends contact the cell cortex and generate a force to drive nuclear oscillation. However, it remains poorly understood how dynein associates with the cell cortex. Here we show that S. pombe Num1p functions as a cortical-anchoring factor for dynein. Num1p is expressed in a meiosis-specific manner and localized to the cell cortex through its C-terminal PH domain. The num1 deletion mutant shows microtubule dynamics comparable to that in the wild type. However, it lacks cortical accumulation of dynein and is defective in the nuclear oscillation as is the case for the dynein mutant. We also show that Num1p can recruit dynein independently of the CLIP-170 homolog Tip1p.
THE microtubular cytoskeleton has crucial roles in many cellular processes. Microtubules guide the motor-protein-mediated movement of organelles, including the nucleus. Precise regulation of the nuclear movement is essential in a number of contexts (Morris 2003).
In the fission yeast Schizosaccharomyces pombe, microtubules position the interphase nucleus at the middle of a cell (Drummond and Cross 2000; Tran et al. 2001). During meiotic prophase, striking nuclear migration occurs. The nucleus migrates back and forth between the two poles of a cell for some hours (Chikashige et al. 1994). This oscillatory nuclear movement, called “horse-tail” movement, is led by the spindle pole body (SPB; equivalent to the centrosome in higher organisms), to which telomeres are attached (Chikashige et al. 1994). The horse-tail movement in combination with telomere clustering is assumed to facilitate pairing of homologous chromosomes, which is a prerequisite for accurate chromosome segregation (Yamamoto et al. 1999; Niwa et al. 2000; Ding et al. 2004; Davis and Smith 2005). It has been shown that the horse-tail movement is driven by a cytoplasmic dynein–dynactin complex (Yamamoto et al. 1999; Miki et al. 2002; Niccoli et al. 2004). The dynein heavy chain (encoded by dhc1) and the Glued subunit of dynactin (encoded by ssm4) localize to the SPB and microtubules during the horse-tail period. Furthermore, dynein and dynactin are also detected on the point where microtubules contact the cell cortex (Yamamoto et al. 1999; Niccoli et al. 2004). It has been proposed that microtubules, formed from the SPB, generate a pulling force on the SPB by sliding along the cell cortex mediated by dynein anchored to it, which is likely also to regulate the disassembly of microtubules at the cell cortex (Yamamoto et al. 2001; Yamamoto and Hiraoka 2003). Thus, cortical accumulation of dynein appears crucial for the horse-tail movement. It has been reported that the dynein light chain (encoded by dlc1) and the Glued subunit of dynactin are indispensable for the accumulation of the dynein heavy chain at the cortex (Miki et al. 2002; Niccoli et al. 2004). However, it remains largely unknown how dynein associates the cell cortex.
We have shown previously that the Glued ortholog Ssm4p shares some function with the CLIP-170-like protein Tip1p in localizing Dhc1p (Niccoli et al. 2004). Tip1p, which regulates the stability of microtubules in interphase cells (Brunner and Nurse 2000), is not absolutely essential for the proper localization of Dhc1p. However, the tip1Δ ssm4Δ double mutant cannot localize Dhc1p along microtubules, although it can localize the protein to the SPB (Niccoli et al. 2004).
The dynein–dynactin complex is involved in nuclear migration in many organisms (Morris 2003). The nuclear positioning during mitosis in the budding yeast Saccharomyces cerevisiae may be one of the best-characterized examples. During mitosis in budding yeast, the nucleus migrates to the mother-bud neck and then into the neck (DeZwaan et al. 1997). Both movements require interaction of microtubules with the cortex, and the latter process is mediated by sliding of microtubules along the bud cortex, which requires dynein and dynactin (Stearns 1997; Adames and Cooper 2000; Yeh et al. 2000). Budding-yeast dynein heavy-chain Dyn1p shows discontinuous localization along microtubules with accumulation at the SPB and microtubule plus ends (Lee et al. 2003; Sheeman et al. 2003). It has been reported that the CLIP-170 ortholog Bik1p and the lissencephal protein LIS1 ortholog Pac1p are required to recruit Dyn1p onto microtubules (Lee et al. 2003; Sheeman et al. 2003). In addition, Num1p is known as a candidate for the cortical anchor of dynein (Bloom 2001). Num1p has a pleckstrin homology (PH) domain and localizes to the bud cortex (Farkasovsky and Kuntzel 1995; Heil-Chapdelaine et al. 2000; Farkasovsky and Kuntzel 2001). Loss of Num1p leads to a failure in dynein-dependent microtubule sliding and results in defective nuclear migration (Heil-Chapdelaine et al. 2000; Farkasovsky and Kuntzel 2001). In cells lacking Num1p, dynein localization at microtubule plus ends is enhanced, suggesting that Num1p may promote the transfer of dynein from microtubule plus ends to the cortical attachment site and anchor it there (Lee et al. 2003; Sheeman et al. 2003). Co-immunoprecipitation of Num1p with the dynein intermediate chain Pac11p (Farkasovsky and Kuntzel 2001) is consistent with this off-loading-anchoring model.
In the filamentous fungi Aspergillus nidulans, dynein is required for the distribution of nuclei within the syncytial hyphae (Xiang et al. 1994). The apsA mutants are defective in this distribution (Clutterbuck 1994). The apsA gene encodes a protein with structural similarities to S. cerevisiae Num1p (Fischer and Timberlake 1995). ApsA is a cortical protein and is involved in the regulation of dynein-dependent nuclear migration (Clutterbuck 1994; Suelmann et al. 1997; Efimov 2003).
We have identified a possible fission yeast homolog of Num1p and ApsA. The above results obtained in S. cerevisiae and A. nidulans led us to speculate that this protein might also participate in the regulation of dynein at the cell cortex. Thus, we set out to analyze this protein to elucidate the mechanism to anchor dynein on the cortex in fission yeast.
MATERIALS AND METHODS
Fission yeast strains, genetic procedures, and media:
Table 1 summarizes S. pombe strains used in this study. General genetic procedures for S. pombe were according to Gutz et al. (1974). Complete medium YE, minimal medium SD, minimal medium MM (Moreno et al. 1991), synthetic sporulation medium SSA (Egel and Egel-Mitani 1974), and sporulation medium SPA (Gutz et al. 1974) were used. Transformation of S. pombe was done by a lithium acetate method (Okazaki et al. 1990). To monitor subcellular localization of Num1p, we replaced the chromosomal num1 gene with the num1-GFP fusion gene according to Bahler et al. (1998). A strain carrying a fusion of the tip1 and the GFP ORFs was constructed similarly. The tagged strains behaved in the same manner as parental strains with no tag during both vegetative growth and meiosis, indicating that the tagging did not interfere with the function of the relevant gene products.
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype |
|---|---|
| JV421 | h90num1GFP<< kanrtip1∷ura4+ade6-M216 leu1 |
| JV422 | h90num1∷ kanrtip1∷ura4+ade6-M216 leu1 |
| JV471 | h90tip1GFP<< kanrade6-M216 leu1 |
| JV545 | h90num1∷ kanrtip1GFP<< kanrade6-M216 leu1 |
| JV626 | h90num1GFP<< kanrade6-M216 leu1 |
| JV627 | h90num1∷ kanrade6-M216 leu1 |
| JV650 | h90num1∷ kanrssm4∷ kanrade6-M216 leu1 |
| JV656 | h90num1∷ kanrkanr>> nmt1-GFP-dhc1 ade6-M210 leu1 |
| JV681 | h90num1∷ kanrtip1∷ura4+kanr>> nmt1-GFP-dhc1 ade6-M216 leu1 |
| JV897 | h90dhc1GFP<< LEU2 ade6-M216 leu1 |
| JV898 | h90dhc1GFP<< LEU2 num1∷ kanrade6-M216 leu1 |
| JW327 | h90dhc1∷ura4+ade6-M216 leu1 ura4-D18 |
| JW652 | h90ssm4∷ kanrade6-M216 leu1 |
| JW785 | h90kanr>> nmt1-GFP-dhc1 ade6-M216 leu1 |
| JX648 | h90tip1∷ura4+ade6-M216 leu1 ura4-D18 |
| JX650 | h90ssm4∷ura4+tip1∷ura4+ade6-M216 leu1 ura4-D18 |
| JY450 | h90ade6-M216 leu1 |
Construction of truncated num1 alleles:
The S. pombe expression vectors pREP1 and pREP41 carried the strong and the medial thiamine-repressible promoters, respectively (Basi et al. 1993). The num1 ORF was PCR amplified with a pair of primers, one carrying an NdeI site at the initiation codon and the other carrying a BamHI site at the stop codon. PCR products were digested with NdeI and BamHI and cloned in either pREP1 carrying the GFP ORF or pREP41 carrying three copies of the HA epitope, so that GFP or the HA epitope was fused to the C terminus of the num1 ORF. To create a derivative carrying a truncated num1 allele termed num1-ΔRU, two PstI sites were introduced immediately upstream and downstream of the conserved repeating unit by PCR and a short PstI fragment was deleted, which resulted in the replacement of 56 amino acids of the repeating unit (244–299) with 2 amino acids encoded by the inserted PstI site (L–Q). To create num1-ΔPH, which encoded truncated Num1p lacking the C-terminal PH domain (845–968), a BamHI site was introduced immediately upstream of the PH domain by PCR. The two truncated genes were cloned in expression vectors as described above.
Fluorescence microscopy:
To observe localization of Num1p-GFP and GFP-Dhc1p in living cells, strains JV626, JV656, JV681, JV897, JV898, and JW785 were cultured in MM medium at 30° up to the midlog phase, washed, and spotted onto SPA medium. After incubation for 6–8 hr at 30°, these cells were observed using a chilled Quantix CCD camera (Photometrics, Tucson, AZ) attached to a fluorescent microscope (Carl Zeiss, Axioplan 2) and the MetaMorph software (Universal Imaging, West Chester, PA). To visualize nuclei, cells were counterstained with Hoechst 33342. For live analysis of nuclear dynamics, cells were cultured in MM medium at 30° up to midlog phase, washed, shifted to MM–N medium, and incubated for 4–6 hr. These cells were then mounted on a thin layer of 1% agarose containing MM–N medium, which was attached to a glass slide. Live images were taken at room temperature (23°–25°).
Statistical analysis of the Num1p dot:
For live analysis of Num1p-GFP, JV626 was monitored using a microscope (Nikon, TE2000) equipped with a spinning disk confocal unit (Yokogawa, CSU22) and a chilled CCD camera (Andor, iXon). Z-stacks were taken at 0.5-min intervals. Each measurement was done for 10 min. Only those dots that were absent in the first and the last frames were subjected to analysis. We assumed that the lifetime of a Num1p dot was 0.5 min multiplied by the number of frames in which it was detected.
Live analysis of microtubular dynamics:
To visualize microtubules by GFP fluorescence, we constructed the plasmid pREP81-GFP-atb2, which expressed nonessential α-tubulin with GFP fused to its N terminus (Toda et al. 1984) from the weak thiamine-repressible promoter (Basi et al. 1993). Cells carrying this plasmid were processed as described above and imaged with confocal microscopes [Carl Zeiss (LSM5) and Nikon (TE2000) equipped with Yokogawa (CSU22)]. Z-stacks were taken at each time point with 10 focal planes spaced at 0.3- to 0.6-μm intervals. Tracking of microtubules was performed using ImageJ software (National Institutes of Health).
RESULTS
Fission yeast have a possible homolog of the budding yeast Num1p:
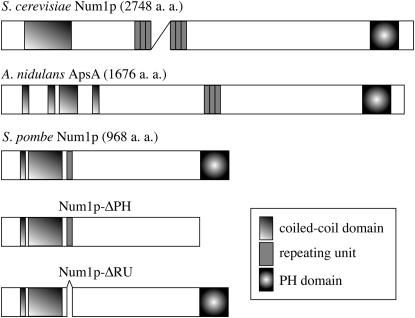
To investigate the mechanism for cortical anchoring of dynein at meiotic prophase, we searched the S. pombe genome database and found a putative gene (SPBC216.02) encoding a weak homolog of S. cerevisiae Num1p. We call this gene num1. The homology between S. pombe Num1p and S. cerevisiae Num1p was not high (27% identity). However, they had a similar domain structure (Figure 1), with a coiled-coil domain at the N terminus and a PH domain at the C terminus. S. cerevisiae Num1p has repeats of nearly identical 64-amino-acid residues at its central region (Kormanec et al. 1991). The number of repeats varies from 1 to 24 among strains (Revardel and Aigle 1993). S. pombe Num1p had a single copy of the repeat unit just after the coiled-coil domain (Figure 1). The putative Ca2+ site in S. cerevisiae Num1p was not conserved in S. pombe.
Figure 1.—
Schematic of the structure of S. pombe Num1p and its S. cerevisiae and A. nidulans homologs. Num1p-ΔPH lacks the PH domain and Num1p-ΔRU lacks the single conserved repeating unit.
Num1p is expressed in a meiosis-specific manner and localizes at the cell cortex:
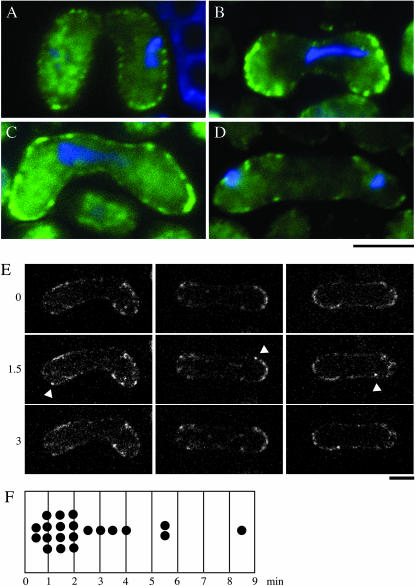
To analyze expression and localization of S. pombe Num1p, we connected the ORF for GFP to the end of the chromosomal num1 ORF in frame. This fusion gene did not express Num1p-GFP at a detectable level in mitotically growing cells (data not shown). In meiotic cells, however, green fluorescence of the protein could be observed (Figure 2). This was consistent with the expression profile of num1 shown previously by DNA microarray assays (Mata et al. 2002). Num1p-GFP was localized to the cell cortex in a punctate pattern during karyogamy and meiotic prophase (Figure 2, A–C). Num1p-GFP remained there until anaphase of the first meiotic division (Figure 2D), disappeared during the course of anaphase, and was never detected through the rest of meiosis (data not shown). This expression pattern resembled that of dhc1 (Yamamoto et al. 1999). Cortical spots of Num1p-GFP were not uniform in their behavior (Figure 2E), with some staying for only a short time as if blinking. We have measured the lifetime of the cortical Num1p dot as detailed in materials and methods. The results are shown in Figure 2F. A majority of the dots exited for an average lifetime of 2.5 min (91%; n = 23). However, there were some spots remaining for >10 min (9%). A correlation between this blink of Num1p-GFP fluorescence and the nuclear movement is currently uncertain.
Figure 2.—
Subcellular localization of Num1p in the course of meiosis. (A–D) Green fluorescence of Num1p-GFP in living cells. Homothallic haploid cells (JV626) carrying the num1-GFP fusion gene were starved for nitrogen to induce conjugation and subsequent meiosis. Nuclear DNA was stained with Hoechst 33342. GFP fluorescence is shown in green, and stained DNA in blue. Bar, 5 μm. Conjugating cells (A), prophase cells (B and C), and a cell at anaphase I (D) are shown. (E) Time-lapse recording of Num1p-GFP. Images were taken at 0.5-min intervals. Z-stacked images of three cells at 1.5-min intervals are shown. Arrowheads indicate GFP fluorescence, which went in and out during a filming period. Bar, 5 μm. (F) The lifetime of a blinking cortical Num1p dot.
Num1p is essential for the horse-tail nuclear movement:
We next examined whether Num1p was involved in the same cellular function as the dynein–dynactin complex. We deleted the num1 gene by replacing it with a kanamycin resistance gene. The num1Δ strain showed a phenotype highly similar to the dhc1Δ or the ssm4Δ mutant. Disruption of num1 had no obvious effect on cell growth and conjugation: The num1Δ strain showed a doubling time and a mating frequency that were indistinguishable from that of the wild type. However, the num1Δ strain frequently generated aberrant asci carrying fewer than four spores, as did the dhc1Δ or the ssm4Δ strain (Table 2) (Yamashita et al. 1997; Yamamoto et al. 1999; Niccoli et al. 2004). The frequency of aberrant asci formation was comparable among these strains. We constructed the num1Δ ssm4Δ double mutant and found that this strain produced aberrant asci at the same frequency as each single mutant (Table 2). This suggested strongly that Num1p might perform the same cellular function as the dynein–dynactin complex.
TABLE 2.
Number of spores per ascus in the num1 and other mutants
| % of asci carrying the following no. of spores:
|
||||
|---|---|---|---|---|
| Strain | One | Two | Three | Four |
| Wild type | <0.1 | <0.1 | 1.1 | 98.9 |
| dhc1Δ | 0.7 | 8.6 | 8.6 | 82.1 |
| ssm4Δ | 0.5 | 6.8 | 8.1 | 84.6 |
| num1Δ | 0.7 | 8.0 | 8.0 | 83.3 |
| num1Δ ssm4Δ | 0.3 | 7.9 | 8.4 | 83.4 |
| tip1Δ | <0.1 | 1.7 | 6.9 | 91.4 |
| ssm4Δ tip1Δ | 1.4 | 10.9 | 24.6 | 63.1 |
| num1Δ tip1Δ | 1.1 | 11.7 | 25.1 | 62.1 |
Sporulation was induced in each strain on SSA medium at 30°. Spores were scored after 3 days. More than 400 zygotes were examined for each strain.
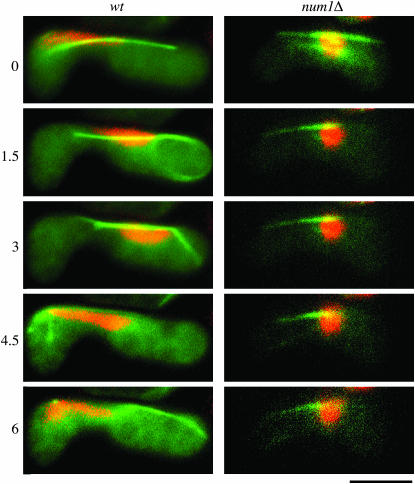
We then investigated if Num1p was essential for the horse-tail nuclear movement. In the num1Δ mutant, the nucleus did not show oscillatory movement and remained at the center of a cell (Figure 3), as was the case with the dynein–dynactin mutants (Yamamoto et al. 1999; Niccoli et al. 2004).
Figure 3.—
Nuclear behavior in wild-type and num1Δ zygotes. Chromosomal DNA in zygotes (JY450 or JV627) was stained with Hoechst 33342 and monitored. The numbers on the left indicate time in minutes. Microtubules were visualized simultaneously by GFP-tagged α-tubulin. Stained DNA is shown in red, and GFP fluorescence in green. Bar, 5 μm.
Microtubules fail to establish a lateral interaction with the cell cortex in the num1 mutant:
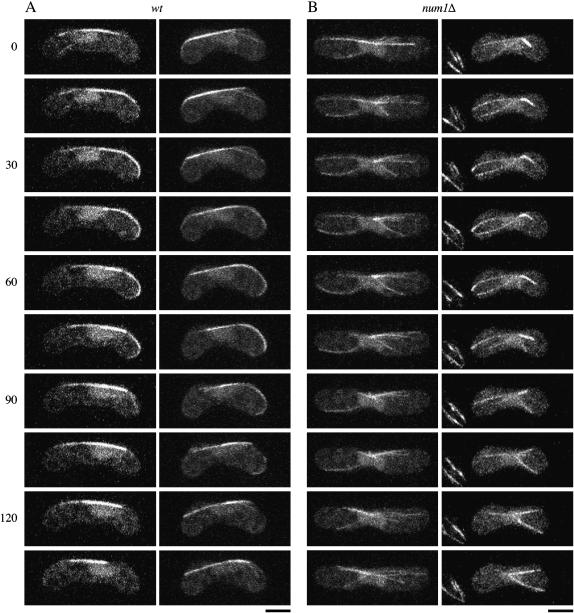
Microtubule dynamics at meiotic prophase is altered significantly in the dynein mutant (Yamamoto et al. 2001). We visualized microtubules in num1Δ cells using GFP-tagged α-tubulin. Figure 3 shows images of a cell in a single focal plane. In num1Δ cells, microtubules did not show a lateral interaction with the cell cortex, which was evident in wild-type cells (see also Figure 4). We then compared microtubule dynamics in meiotic prophase in wild-type and num1Δ cells, using an optical sectioning microscope (Figure 4). The growth and shrinkage rates of microtubules in num1Δ cells were comparable to those in wild type during the horse-tail period (Table 3). These observations appears to be distinct from those obtained with dhc1Δ cells, which have shown a faster shrinkage rate and a slower elongation rate than wild-type cells (Yamamoto et al. 2001), if we take into account that the rates for wild-type cells are similar in their measurements (3.4 ± 1.4 μm/min for elongation and 6.4 ± 3.4 μm/min for shrinkage) and in ours (3.4 ± 0.8 μm/min for elongation and 5.1 ± 2.0 μm/min for shrinkage). In num1Δ cells, microtubules grew until they reached the cell ends, but they stopped elongation without curving around and then shrank (Figure 4B), unlike in wild-type cells (Figure 4A). Ninety-two percent of microtubules in num1Δ cells underwent catastrophe within 2 min of contacting the cell cortex (n = 59). In contrast, 80% of microtubules that interacted with the cell cortex laterally in wild-type cells remained at the cell ends for >2 min (n = 15). Thus, Num1p appeared to be essential for the interaction between microtubules and the cell cortex during the horse-tail period.
Figure 4.—
Microtubule behavior in wild-type and num1Δ zygotes. (A) Wild type (JY450) and (B) num1Δ (JV627) homothallic haploid cells expressing GFP-tagged α-tubulin were starved for nitrogen to induce conjugation and meiosis. Confocal z-step series were recorded at 15-sec intervals and the projection images constructed are shown. The numbers indicate time in seconds. Bar, 5 μm.
TABLE 3.
Growth and shrinkage rates of microtubules during meiotic prophase
| Strain | Growth rate (μm/min) | Shrinkage rate (μm/min) |
|---|---|---|
| Wild type | 3.4 ± 0.8 (n = 12)a | 5.1 ± 2.0 (n = 12)a |
| num1Δ | 3.2 ± 0.8 (n = 18)a | 5.3 ± 1.7 (n = 13)a |
Rates are shown in mean ± SD. The number of microtubules observed is indicated by n.
t-Test showed no significant difference between wild-type and mutant values (P > 0.05).
Num1p is required to anchor dynein on the cortex:
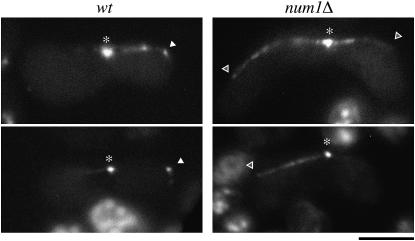
We examined localization of Dhc1p in num1Δ cells. Dhc1p, tagged with GFP and expressed from the authentic dhc1 promoter on the chromosome, was seen on the SPB and microtubules during meiotic prophase in num1Δ cells, as in wild-type cells (Yamamoto et al. 1999) (Figure 5). The same localization pattern was observed when expression of GFP-tagged Dhc1p was driven from the strong nmt1 promoter (data not shown). However, we never detected Dhc1p-GFP on the cell cortex of num1Δ. Dhc1p on the cortex has been supposed to generate a pulling force to drive nuclear oscillation (Yamamoto et al. 2001). Our observation suggested that Num1p was essential to anchor Dhc1p on the cortex.
Figure 5.—
Localization of Dhc1p in num1Δ cells. Wild-type (JV897) and num1Δ (JV898) homothallic haploid cells carrying dhc1-GFP were starved for nitrogen and observed under the fluorescence microscope. Asterisks and solid arrowheads indicate GFP fluorescence at SPB and on the cell cortex, respectively. Open arrowheads indicate possible microtubule contact sites on the cell cortex of num1Δ cells, where no accumulation of Dhc1p-GFP was detected. Bar, 5 μm.
The C-terminal PH domain and the central repeating unit are essential for the function of Num1p:
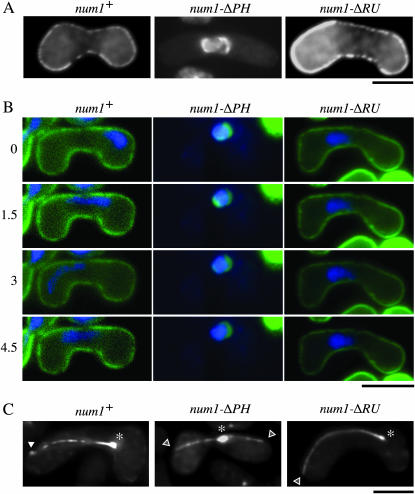
To dissect regions that are important for the function of Num1p, we created two truncated versions of this protein, namely Num1p-ΔPH, which lacked the PH domain at the C terminus, and Num1p-ΔRU, which lacked the conserved repeating unit (Figure 1). We transformed num1Δ cells with plasmids expressing these Num1p variants tagged with GFP. Num1p-ΔRU could localize to the cell cortex, whereas cortical localization of Num1p was abolished by the deletion of the PH domain (Figure 6, A and B). To see if the function of Num1p was affected by these deletions, we observed behavior of the nucleus at meiotic prophase in num1Δ cells expressing either Num1p-ΔPH or Num1p-ΔRU (Figure 6B). Unlike cells expressing wild-type Num1p, cells expressing Num1p-ΔPH showed no nuclear movement, which appeared to be consistent with the delocalization of Num1p-ΔPH from the cell cortex. Num1p-ΔRU localized to the cell cortex properly, but this variant also could not drive the horse-tail movement. Aberrant spore formation of num1Δ cells was rescued by neither Num1p-ΔPH nor Num1p-ΔRU (Table 4), suggesting that these variants were not functional.
Figure 6.—
Domain analysis of Num1p. (A) num1Δ cells (JV627) were transformed with a plasmid expressing Num1p-GFP, Num1p-ΔPH-GFP, or Num1p-ΔRU-GFP from the nmt1 promoter. Transformants were subjected to nitrogen starvation and observed under the fluorescence microscope. Bar, 5 μm. (B) Nuclear movement during meiotic prophase in the same set of transformants as in A. Chromosomal DNA of each transformant stained with Hoechst 33342 is shown in blue. Fluorescence of GFP-tagged Num1p and its variants is shown in green. The numbers on the left indicate time in minutes. Bar, 5 μm. (C) Localization of Dhc1p in cells expressing Num1p-ΔPH or Num1p-ΔRU. num1Δ homothallic haploid cells (JV656) carrying GFP-dhc1 were transformed with a plasmid expressing Num1p, Num1p-ΔPH, or Num1p-ΔRU from the nmt1 promoter and analyzed as described in Figure 5. Transformants were starved for nitrogen and observed under the fluorescence microscope. Asterisks and solid arrowheads indicate GFP fluorescence at SPB and on the cell cortex, respectively. Open arrowheads indicate possible microtubule contact sites on the cell cortex of num1Δ cells. Bar, 5 μm.
TABLE 4.
Number of spores per ascus in the num1Δ strain carrying a mutant num1 allele
| % of asci carrying the following no. of spores:
|
||||
|---|---|---|---|---|
| Plasmid | One | Two | Three | Four |
| num1+ | <0.1 | 0.7 | 2.1 | 97.2 |
| num1-ΔPH | 0.9 | 7.1 | 8.7 | 83.3 |
| num1-ΔRU | 0.6 | 6.6 | 7.2 | 85.6 |
| None | 0.9 | 6.4 | 7.0 | 85.7 |
A num1Δ homothallic haploid strain was transformed with a plasmid carrying each gene indicated. Transformants were examined for their ability to produce spores, as described in Table 2.
Although Num1p-ΔRU could localize to the cell cortex, it was not functional. We tested the possibility that Num1p-ΔRU could not anchor dynein on the cortex. In num1Δ cells expressing Num1p-ΔRU, GFP-Dhc1p, expressed from the inducible nmt1 promoter, was found to localize to the SPB and microtubules. However, we could not detect accumulation of Dhc1p on the cortex among cells carrying microtubules reaching the cell surface (0/7), even though Dhc1p was expressed from the strong nmt1 promoter (Figure 6C). We also failed to detect a cortical GFP-Dhc1p signal in num1Δ cells expressing either Num1p-ΔPH (0/10) or no Num1p derivative (0/12), as expected, whereas cells expressing intact Num1p showed a cortical Dhc1 dot at a considerable frequency (4/10) (Figure 6C).
Num1p functions independently of the CLIP-170-like protein Tip1p:
In addition to the Glued ortholog Ssm4p, the CLIP-170-like protein Tip1p is involved in localization of Dhc1p in fission yeast (Niccoli et al. 2004). In the tip1Δ ssm4Δ double mutant, Dhc1p totally loses its microtubular localization, although it can localize to the SPB (Niccoli et al. 2004).
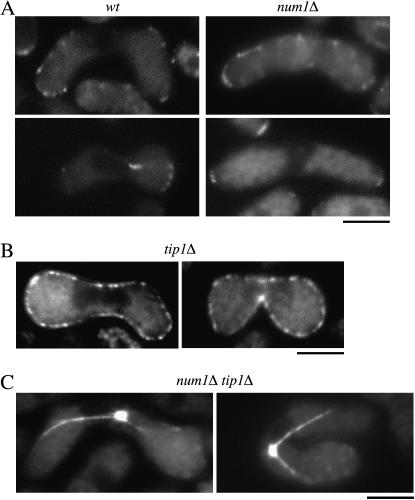
Tip1p has been shown to localize to microtubule tips and cell ends in interphase cells (Brunner and Nurse 2000). During shmooing growth, Tip1p localizes to the nongrowing cell end and in the cytoplasm as dots in a row (Niccoli and Nurse 2002; Niccoli et al. 2004). We thus investigated how Tip1p was involved in meiosis and whether it might cooperate with Num1p during the horse-tail movement. Tip1p was observed evidently at the cortex of cell ends during meiotic prophase (Figure 7A), although fluorescence of Tip1p-GFP in meiotic cells was weaker than that in mitotic interphase cells (data not shown). Cytoplasmic dots in a row were also observed in some zygotes. However, the localization of Tip1p in num1Δ cells appeared to be the same as that in wild-type cells (Figure 7A). Conversely, Num1p appeared to localize properly to the cell cortex in the absence of Tip1p (Figure 7B).
Figure 7.—
Functional independency of Num1p and Tip1p. (A) Localization of Tip1p during meiotic prophase in wild-type and num1Δ cells. Homothallic haploid wild-type (JV471) and num1Δ cells (JV545) carrying the tip1-GFP fusion gene were starved for nitrogen to induce conjugation and subsequent meiosis and observed under the fluorescence microscope. Bar, 5 μm. (B) Localization of Num1p in tip1Δ cells. Homothallic haploid tip1Δ cells (JV421) carrying the num1-GFP fusion gene were starved for nitrogen to induce conjugation and meiosis and observed under the fluorescence microscope. Bar, 5 μm. (C) Localization of Dhc1p in num1Δ tip1Δ cells. Homothallic haploid num1Δ tip1Δ cells (JV681) carrying GFP-dhc1 were starved for nitrogen and observed under the fluorescence microscope. Bar, 5 μm.
To further examine the relationship between Tip1p and Num1p, we constructed the num1Δ tip1Δ double mutant. During mitotic growth, the num1Δ tip1Δ mutant showed a phenotype similar to that of the tip1Δ mutant. The tip1Δ mutant produced aberrant asci having fewer than four spores, as did the dhc1Δ or ssm4Δ mutants, but at a lower frequency. The ssm4Δ tip1Δ double mutant showed an exaggerated phenotype compared to each single mutant (Niccoli et al. 2004) (Table 2). The num1Δ tip1Δ mutant produced aberrant asci at nearly the same frequency as the ssm4Δ tip1Δ mutant (Table 2). We then observed localization of Dhc1p in the num1Δ tip1Δ mutant. It was similar to that in the num1Δ single mutant; i.e., Dhc1p localized to microtubules and the SPB but not to the cell cortex (Figure 7C). This contrasts with the results in the ssm4Δ tip1Δ mutant, in which Dhc1p lost its microtubular localization (Niccoli et al. 2004). These observations altogether suggest that Num1p and Ssm4p are likely to contribute differently to the localization of Dhc1p. Ssm4p, in collaboration with Tip1p, may load Dhc1p onto microtubules independently of Num1p. At the cell cortex, Num1p anchors Dhc1p, and Ssm4p is also required for this anchoring (Niccoli et al. 2004).
DISCUSSION
In this study we have characterized a novel cortical protein, Num1p, in fission yeast. Num1p is required for the horse-tail nuclear movement. The driving force of this movement is thought to be generated by the dynein–dynactin complex localized to the site where microtubule plus ends contact the cell cortex (Yamamoto et al. 2001; Niccoli et al. 2004). As shown in this study, the dynein heavy chain Dhc1p cannot accumulate at this site in the absence of Num1p. This suggests strongly that Num1p may be a cortical dynein anchor. S. cerevisiae Num1p has been suggested to be a cortical factor anchoring dynein (Heil-Chapdelaine et al. 2000; Farkasovsky and Kuntzel 2001). In S. cerevisiae num1Δ cells, dynein heavy-chain Dyn1p concentrates at microtubule plus ends, suggesting that S. cerevisiae Num1p may unload Dyn1p from the end of microtubules when they reach the cortex (Lee et al. 2003; Sheeman et al. 2003). However, in our observations we have never seen concentration of dynein at the microtubule ends in S. pombe cells lacking Num1p. Thus, although the function of Num1p as the cortical anchor for dynein seems to be conserved in the two yeast species, the mechanisms to recruit dynein may be different. Recently, it has been reported in S. cerevisiae that dynein may be targeted to the microtubule plus end by kinesin Kip2p (Carvalho et al. 2004). It remains to be seen whether a similar mechanism operates in localizing dynein in S. pombe.
Both the central conserved repeating unit and the C-terminal PH domain are found to be essential for the function of S. pombe Num1p. This is in clear contrast to S. cerevisiae Num1p and A. nidulans ApsA. S. cerevisiae Num1p lacking the repeating unit and A. nidulans ApsA lacking the PH domain are reported to retain some residual activity (Farkasovsky and Kuntzel 1995; Suelmann et al. 1997). However, S. pombe Num1p lacking either of these two domains appears to have no activity, even if expressed from a multi-copy plasmid. As demonstrated in this study, the central conserved repeating unit of S. pombe Num1p is not necessary for the cortical localization of the protein and hence is likely to be involved in the interaction with dynein. In S. cerevisiae, Num1p interacts with the dynein intermediate-chain Pac11p, depending on the presence of the dynein heavy chain (Farkasovsky and Kuntzel 2001). It is intriguing and remains to be clarified critically whether S. pombe Num1p associates with the dynein complex in a similar manner, although to date we have not been successful in detecting physical interaction of Num1p with the dynein intermediate-chain Dic1p by either two-hybrid assay or immunoprecipitation.
We have shown in this study that Num1p and a CLIP-170-like protein Tip1p have distinct roles in localizing dynein. We have shown previously that the Glued subunit of dynactin Ssm4p regulates dynein localization in cooperation with Tip1p (Niccoli et al. 2004). In the absence of Ssm4p, Dhc1p cannot accumulate at the cell cortex, as in num1Δ cells. Tip1p itself is not required for the localization of Dhc1p, but Dhc1p fails to localize on microtubules in the ssm4Δ tip1Δ mutant. In the num1Δ tip1Δ mutant, however, Dhc1p localizes on microtubules, only failing to accumulate at the cell cortex. These observations suggest that Ssm4p and Tip1p collaborate to recruit Dhc1p on microtubules, whereas at the cell cortex both Num1p and Ssm4p participate in anchoring Dhc1p.
Regulation of dynein localization, especially anchoring of dynein on cellular organelles, is crucial for many migratory processes, and a variety of regulators have been identified. Many regulators are common among different systems, although the role of each factor may vary from organism to organism. To date, however, we have been unable to find an ortholog of Num1p in organisms other than fungi. In metazoans, spectrin, which associates to membranous organelles through its PH domain, plays an essential role for dynein-dependent vesicle transport by linking the dynein–dynactin complex to the membrane (Holleran et al. 2001; Muresan et al. 2001). In Drosophila oocytes, an ortholog of the Lissencephaly protein DLis-1 localizes along the cortex and is required for the recruitment of dynein to the cortex (Swan et al. 1999). It remains to be examined whether these factors function in the same manner as Num1p.
Acknowledgments
We thank Kayoko Tanaka for helpful discussion, the Yeast Genetic Resource Center for the dhc1-gfp strain, and Ayumu Yamamoto and Yasushi Hiraoka for the dhc1 strain. This work was supported by a grant-in-aid for Scientific Research (A.Y.) and a grant-in-aid for Specially Promoted Research (M.Y.) from the Ministry of Education, Culture, Sports and Technology of Japan.
References
- Adames, N. R., and J. A. Cooper, 2000. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J. Cell Biol. 149: 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler, J., J. Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III et al., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951. [DOI] [PubMed] [Google Scholar]
- Basi, G., E. Schmid and K. Maundrell, 1993. TATA box mutations in the Schizosaccharomyces pombe nmt1 promotor affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123: 131–136. [DOI] [PubMed] [Google Scholar]
- Bloom, K., 2001. Nuclear migration: cortical anchors for cytoplasmic dynein. Curr. Biol. 11: R326–R329. [DOI] [PubMed] [Google Scholar]
- Brunner, D., and P. Nurse, 2000. CLIP170-like tip1p spatially organizes microtubular dynamics in fission yeast. Cell 102: 695–704. [DOI] [PubMed] [Google Scholar]
- Carvalho, P., M. L. Gupta, Jr., M. A. Hoyt and D. Pellman, 2004. Cell cycle control of kinesin-mediated transport of Bik1 (CLIP-170) regulates microtubule stability and dynein activation. Dev. Cell 6: 815–829. [DOI] [PubMed] [Google Scholar]
- Chikashige, Y., D. Q. Ding, H. Funabiki, T. Haraguchi, S. Mashiko et al., 1994. Telomere-led premeiotic chromosome movement in fission yeast. Science 264: 270–273. [DOI] [PubMed] [Google Scholar]
- Clutterbuck, A. J., 1994. Mutants of Aspergillus nidulans deficient in nuclear migration during hyphal growth and conidiation. Microbiology 140(Pt. 5): 1169–1174. [DOI] [PubMed] [Google Scholar]
- Davis, L., and G. R. Smith, 2005. Dynein promotes achiasmate segregation in Schizosaccharomyces pombe. Genetics 170: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZwaan, T. M., E. Ellingson, D. Pellman and D. M. Roof, 1997. Kinesin-related KIP3 of Saccharomyces cerevisiae is required for a distinct step in nuclear migration. J. Cell Biol. 138: 1023–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, D. Q., A. Yamamoto, T. Haraguchi and Y. Hiraoka, 2004. Dynamics of homologous chromosome pairing during meiotic prophase in fission yeast. Dev. Cell 6: 329–341. [DOI] [PubMed] [Google Scholar]
- Drummond, D. R., and R. A. Cross, 2000. Dynamics of interphase microtubules in Schizosaccharomyces pombe. Curr. Biol. 10: 766–775. [DOI] [PubMed] [Google Scholar]
- Efimov, V. P., 2003. Roles of NUDE and NUDF proteins of Aspergillus nidulans: insights from intracellular localization and overexpression effects. Mol. Biol. Cell 14: 871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel, R., and M. Egel-Mitani, 1974. Premeiotic DNA synthesis in fission yeast. Exp. Cell Res. 88: 127–134. [DOI] [PubMed] [Google Scholar]
- Farkasovsky, M., and H. Kuntzel, 1995. Yeast Num1p associates with the mother cell cortex during S/G2 phase and affects microtubular functions. J. Cell Biol. 131: 1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkasovsky, M., and H. Kuntzel, 2001. Cortical Num1p interacts with the dynein intermediate chain Pac11p and cytoplasmic microtubules in budding yeast. J. Cell Biol. 152: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, R., and W. E. Timberlake, 1995. Aspergillus nidulans apsA (anucleate primary sterigmata) encodes a coiled-coil protein required for nuclear positioning and completion of asexual development. J. Cell Biol. 128: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz, H., H. Heslot, U. Leupold and N. Loprieno, 1974. Schizosaccharomyces pombe, pp. 395–446 in Handbook of Genetics, Vol. 1, edited by R. C. King. Plenum, New York.
- Heil-Chapdelaine, R. A., J. R. Oberle and J. A. Cooper, 2000. The cortical protein Num1p is essential for dynein-dependent interactions of microtubules with the cortex. J. Cell Biol. 151: 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran, E. A., L. A. Ligon, M. Tokito, M. C. Stankewich, J. S. Morrow et al., 2001. beta III spectrin binds to the Arp1 subunit of dynactin. J. Biol. Chem. 276: 36598–36605. [DOI] [PubMed] [Google Scholar]
- Kormanec, J., I. Schaaff-Gerstenschlager, F. K. Zimmermann, D. Perecko and H. Kuntzel, 1991. Nuclear migration in Saccharomyces cerevisiae is controlled by the highly repetitive 313 kDa NUM1 protein. Mol. Gen. Genet. 230: 277–287. [DOI] [PubMed] [Google Scholar]
- Lee, W. L., J. R. Oberle and J. A. Cooper, 2003. The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast. J. Cell Biol. 160: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata, J., R. Lyne, G. Burns and J. Bahler, 2002. The transcriptional program of meiosis and sporulation in fission yeast. Nat. Genet. 32: 143–147. [DOI] [PubMed] [Google Scholar]
- Miki, F., K. Okazaki, M. Shimanuki, A. Yamamoto, Y. Hiraoka et al., 2002. The 14-kDa dynein light chain-family protein Dlc1 is required for regular oscillatory nuclear movement and efficient recombination during meiotic prophase in fission yeast. Mol. Biol. Cell 13: 930–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., A. Klar and P. Nurse, 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194: 795–823. [DOI] [PubMed] [Google Scholar]
- Morris, N. R., 2003. Nuclear positioning: the means is at the ends. Curr. Opin. Cell Biol. 15: 54–59. [DOI] [PubMed] [Google Scholar]
- Muresan, V., M. C. Stankewich, W. Steffen, J. S. Morrow, E. L. Holzbaur et al., 2001. Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids. Mol. Cell 7: 173–183. [DOI] [PubMed] [Google Scholar]
- Niccoli, T., and P. Nurse, 2002. Different mechanisms of cell polarisation in vegetative and shmooing growth in fission yeast. J. Cell Sci. 115: 1651–1662. [DOI] [PubMed] [Google Scholar]
- Niccoli, T., A. Yamashita, P. Nurse and M. Yamamoto, 2004. The p150-glued Ssm4p regulates microtubular dynamics and nuclear movement in fission yeast. J. Cell Sci. 117: 5543–5556. [DOI] [PubMed] [Google Scholar]
- Niwa, O., M. Shimanuki and F. Miki, 2000. Telomere-led bouquet formation facilitates homologous chromosome pairing and restricts ectopic interaction in fission yeast meiosis. EMBO J. 19: 3831–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki, K., N. Okazaki, K. Kume, S. Jinno, K. Tanaka et al., 1990. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 18: 6485–6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revardel, E., and M. Aigle, 1993. The NUM1 yeast gene: length polymorphism and physiological aspects of mutant phenotype. Yeast 9: 495–506. [DOI] [PubMed] [Google Scholar]
- Sheeman, B., P. Carvalho, I. Sagot, J. Geiser, D. Kho et al., 2003. Determinants of S. cerevisiae dynein localization and activation: implications for the mechanism of spindle positioning. Curr. Biol. 13: 364–372. [DOI] [PubMed] [Google Scholar]
- Stearns, T., 1997. Motoring to the finish: kinesin and dynein work together to orient the yeast mitotic spindle. J. Cell Biol. 138: 957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suelmann, R., N. Sievers and R. Fischer, 1997. Nuclear traffic in fungal hyphae: in vivo study of nuclear migration and positioning in Aspergillus nidulans. Mol. Microbiol. 25: 757–769. [DOI] [PubMed] [Google Scholar]
- Swan, A., T. Nguyen and B. Suter, 1999. Drosophila lissencephaly-1 functions with Bic-D and dynein in oocyte determination and nuclear positioning. Nat. Cell Biol. 1: 444–449. [DOI] [PubMed] [Google Scholar]
- Toda, T., Y. Adachi, Y. Hiraoka and M. Yanagida, 1984. Identification of the pleiotropic cell division cycle gene NDA2 as one of two different alpha-tubulin genes in Schizosaccharomyces pombe. Cell 37: 233–242. [DOI] [PubMed] [Google Scholar]
- Tran, P. T., L. Marsh, V. Doye, S. Inoue and F. Chang, 2001. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 153: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, X., S. M. Beckwith and N. R. Morris, 1994. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 91: 2100–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, A., and Y. Hiraoka, 2003. Cytoplasmic dynein in fungi: insights from nuclear migration. J. Cell Sci. 116: 4501–4512. [DOI] [PubMed] [Google Scholar]
- Yamamoto, A., R. R. West, J. R. McIntosh and Y. Hiraoka, 1999. A cytoplasmic dynein heavy chain is required for oscillatory nuclear movement of meiotic prophase and efficient meiotic recombination in fission yeast. J. Cell Biol. 145: 1233–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, A., C. Tsutsumi, H. Kojima, K. Oiwa and Y. Hiraoka, 2001. Dynamic behavior of microtubules during dynein-dependent nuclear migrations of meiotic prophase in fission yeast. Mol. Biol. Cell 12: 3933–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, A., Y. Watanabe and M. Yamamoto, 1997. Microtubule-associated coiled-coil protein Ssm4 is involved in the meiotic development in fission yeast. Genes Cells 2: 155–166. [DOI] [PubMed] [Google Scholar]
- Yeh, E., C. Yang, E. Chin, P. Maddox, E. D. Salmon et al., 2000. Dynamic positioning of mitotic spindles in yeast: role of microtubule motors and cortical determinants. Mol. Biol. Cell 11: 3949–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]