Abstract
Parent-of-origin effects create differences in gene expression among genetically identical individuals. Using measurements of allele-specific expression, we demonstrate that previously reported parent-of-origin effects on standing mRNA levels in Drosophila melanogaster are not attributable to genomic imprinting. Offspring from reciprocal crosses exhibit differences in total expression without differences in allelic expression, indicating that other types of maternal and/or paternal effects alter expression.
MOST genes exhibit Mendelian inheritance. Both parental alleles contribute to the phenotype of offspring, and alleles have the same phenotypic effect regardless of whether they were inherited from the mother or the father. The expression of some genes, however, is affected by parental transmission. Parent-of-origin effects include genomic imprinting, in which either the maternal or the paternal allele is epigenetically modified during gametogenesis, as well as maternal and paternal effects, which are often attributable to the cytoplasmic contributions of the egg and sperm.
Genomic imprinting is essential for the proper development of many mammals and plants (reviewed in Gehring et al. 2004; Scott and Spielman 2004; Morison et al. 2005). Imprinted genes typically have one allele, either the maternal or the paternal allele, completely silenced by epigenetic modifications (Reik and Walter 2001; da Rocha and Ferguson-Smith 2004), but partial imprinting also occurs (Morison et al. 2005). Although present in some insects (Crouse 1960; Goday and Esteban 2001), the role of imprinting in Drosophila melanogaster has been unclear. D. melanogaster is capable of genomic imprinting under some circumstances (Golic et al. 1998; Lloyd et al. 1999; Haller and Woodruff 2000; Joanis and Lloyd 2002; Maggert and Golic 2002), but imprinting is not strictly required for viability. Gynogenetic and androgenetic flies, which inherit chromosomes from a single parent, appear normal (Fuyama 1984; Komma and Endow 1995). In contrast, maternal and paternal effects in D. melanogaster are often essential for embryonic survival (e.g., Perrimon et al. 1989; Nusslein-Volhard 1991; Perrimon et al. 1996; Fitch et al. 1998) and can be propagated through development to affect adult phenotypes (Fitch et al. 1998; Fox et al. 2004; Malmanche and Clark 2004).
Recently, Gibson et al. (2004) identified parent-of-origin effects on standing mRNA transcript levels in D. melanogaster using genomic microarrays. As described by the authors, the male parent-like and female parent-like expression they observed could be caused by either genomic imprinting or more general maternal/paternal effects. The primary difference between these two molecular mechanisms is that imprinting has allele-specific effects on gene expression, whereas other maternal and paternal effects are caused by changes in cellular composition that should affect expression of both alleles. Here, we use measurements of allele-specific expression in offspring from reciprocal crosses to determine whether parent-of-origin effects on gene expression are attributable to genomic imprinting or other mechanisms.
In the absence of imprinting, the same ratio of allelic expression is expected in reciprocal crosses. If a gene is imprinted, relative allelic expression should differ in progeny of reciprocal crosses. Expression of each allele (Y) is determined by the activity (A) of its associated cis-regulatory sequences as well as by any epigenetic imprinting (I) that may affect expression of the maternal or paternal allele. In a cross between females from line 1 and males from line 2, the relative expression of the two alleles (Y1/Y2) can be written as (A1 × Im)/(A2 × Ip) or (A1/A2) × (Im/Ip). In the reciprocal cross, Y1/Y2 = (A1/A2) × (Ip/Im). When allelic expression is measured under constant cellular conditions, A1 and A2 are fixed properties of each allele. The ratio of allelic expression (Y1/Y2) in reciprocal crosses will then be the same only if Im/Ip = Ip/Im. This is true when there is no genomic imprinting and thus no distinction between the maternal and paternal alleles (Im = Ip). If a gene is imprinted, Im ≠ Ip and the relative allelic expression (Y1/Y2) will differ between reciprocal crosses. Note that this is true for complete (I = 0) as well as partial imprinting (0 < I < 1).
Maternal and paternal effects can alter total gene expression levels without affecting the relative expression of the maternal and paternal alleles. For example, parental effects can alter the distribution of cell types among genetically identical individuals (e.g., Rice et al. 1979; Carmena et al. 1991). If cell number differs in offspring from reciprocal crosses, genes expressed in affected cell types will exhibit parent-of-origin effects on transcript level without altering the relative expression from the two alleles. Parental effects may also alter total gene expression by changing the number of transcription factors within a cell. Changing the abundance of transcription factors should not affect relative allelic expression unless the factor mediating the parental effect (i.e., differing between reciprocal crosses) interacts preferentially with either the maternal or the paternal allele. Although possible, we are unaware of any such interactions within a species.
Using DNA microarrays Gibson et al. (2004) measured expression levels for 12,559 genes in the highly inbred Oregon R (Ore) and Russian2b (2b) strains of D. melanogaster and in F1 heterozygotes, O2b and 2bO, produced by reciprocal crosses of these strains (i.e., Ore × 2b and 2b × Ore, respectively, where the maternal strain is listed first). Of the genes surveyed, 2% showed parent-of-origin effects on gene expression: expression in female F1 heterozygotes resembled paternal expression for 115 genes and maternal expression for 174 genes. A subset of these genes was selected using the following criteria: (1) magnitude of expression difference between parental strains, (2) similarity of hybrid expression to parental expression, and (3) presence of a sequence polymorphism between parental Ore and 2b strains that distinguishes mRNA transcripts produced by each allele. Genes with larger expression differences between parental strains were preferentially selected.
Ultimately, nine of the best imprinting candidate genes in the genome were chosen, including five genes with female parent-like expression in F1 flies (mod, lambdaTry, thetaTry, Myo61F, and CG8952) and four genes with male parent-like expression in F1 flies (Spat, CG4847, Mp20, and CG9641). CG8952 and Spat are X linked, whereas the remaining genes are autosomal. Fifteen control genes, which showed no evidence of parent-of-origin effects in the microarray study, were also examined. For each gene, expression was measured in 7- to 10-day-old mated females (Ore, 2b, O2b, 2bO) from each cross (Ore × Ore, 2b × 2b, Ore × 2b, 2b × Ore, respectively), as described in Gibson et al. (2004). The same inbred Ore and 2b strains of D. melanogaster studied by Gibson and colleagues were used for this work.
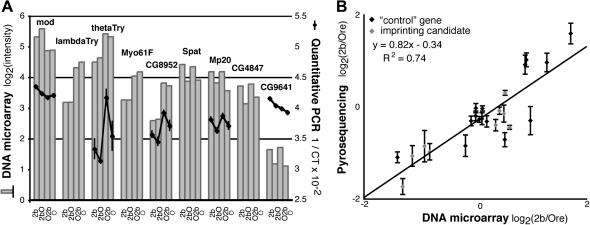
Microarray measurements of expression have inherently greater error than do liquid phase assays; thus we used quantitative PCR (qPCR) to investigate the validity of parent-of-origin effects observed in Gibson et al. (2004). Gene expression was measured in both parental (Ore, 2b) and hybrid (O2b, 2bO) flies for five of the nine imprinting candidate genes and for two housekeeping genes (srebp and pld). Patterns of expression observed among genotypes using qPCR were similar to those reported in the microarray study for all genes except CG9641 (Figure 1A). For CG9641, expression in O2b flies was lower, relative to the other three genotypes, than expression reported in Gibson et al. (2004).
Figure 1.—
DNA microarrays, quantitative PCR, and pyrosequencing produce similar measures of gene expression. (A) Expression of nine imprinting candidate genes selected from the Gibson et al. (2004) microarray study is shown in the bar chart. Quantitative PCR (qPCR) measurements of gene expression are shown by the black line graphs for five of the nine genes. For each genotype, two replicate cDNA synthesis reactions [using poly(T) primers] were performed with RNA extracted from three independent pools containing 12–14 flies each. Gene-specific Taqman probes (Heid et al. 1996) (Applied Biosystems) were used to measure expression in all six cDNA pools for each genotype, in duplicate. The inverse of the critical threshold (1/CT) was fitted to the following linear model using proc MIXED in SAS v. 8.02 (Cary, NC) for each gene, as described in (Fiumera et al. 2005): Yijk = μ + Genotypei + Poolij + cDNAijk + PLD + ε, where Y = 1/CT for the gene of interest, i is the genotype index (Ore, O2b, 2bO, 2b), j is the pool index (1, 2, 3), k is the cDNA sample index (1, 2), and PLD = 1/CT for the pld gene. Genotype was treated as a fixed effect, Pool and cDNA as random effects, and PLD as a covariate. Analyses using srebp expression as a covariate instead of pld expression gave similar results; expression of srebp and pld was strongly correlated (R2 = 0.97). LS means for each genotype are plotted with error bars indicating the standard error. (B) Relative expression between 2b and Ore (2b/Ore), measured by pyrosequencing, is plotted against the ratio of 2b/Ore expression reported in the Gibson et al. (2004) microarray data set. For pyrosequencing, total RNA and genomic DNA were sequentially extracted from four mixed pools, each containing seven Ore and seven 2b flies. RNA from each pool was used in three independent cDNA synthesis reactions with a poly(T) primer. After normalization using measurements from genomic DNA (Landry et al. 2005), the log2(2b/Ore) ratio from pyrosequencing (Y) was fitted to a gene-specific model including a random effect of replicate pools: Yi = μ + Pooli + ε, where i is the the pool index (1, 2, 3, 4). Estimates and standard errors of the intercept (μ) are shown. Supplemental Table 1 (http://www.genetics.org/supplemental/) summarizes expression levels measured using DNA microarrays, qPCR, and pyrosequencing.
Pyrosequencing, which uses a single nucleotide difference between two alleles to quantify their relative abundance in a combined sample, was used to measure allele-specific expression (Ahmadian et al. 2000). To compare pyrosequencing measurements of gene expression with measurements from microarrays, relative expression of all 24 genes in the 2b and Ore parental strains was examined. Pyrosequencing measurements of Ore and 2b transcripts in mRNA extracts from mixed pools of flies were used to directly quantify the relative expression in the two lines (Wittkopp et al. 2004). A comparable ratio of gene expression was calculated from the Gibson et al. (2004) microarray data by subtracting the log-transformed least-squares (LS) mean of fluorescence intensity for Ore from that for 2b. As shown in Figure 1B, both methods produced similar estimates of the relative expression between parental lines (R2 = 0.74).
The concordance of microarray, qPCR, and pyrosequencing measurements of gene expression (supplemental Table 1 at http://www.genetics.org/supplemental/) indicates that environmental differences (e.g., media composition, light, humidity) between our study (conducted at Cornell University) and that of Gibson et al. (2004) (conducted at North Carolina State University) had minimal effects on expression of the genes examined.
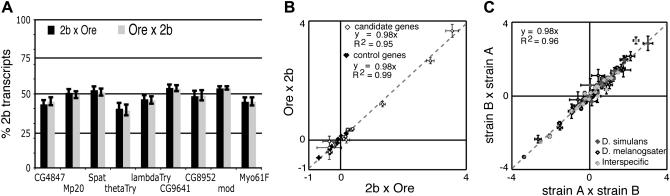
Relative expression of the 2b and Ore alleles was measured in genetically identical 2bO and O2b F1 females from reciprocal crosses. Pyrosequencing was used to measure allelic expression in two replicate cDNA samples from each of two pools containing 14 F1 flies. For each gene, the following general linear mixed model was fitted using “proc MIXED” in SAS v. 8.02 (Cary, NC): Yij = μ + Crossi + Poolij + ε, where Y is the relative expression log2(Y2b/YOre), i is the direction of cross O2b, 2bO (fixed effect), and j is the pool index 1, 2 (random effect). The ratio of allelic expression (Yij) was log2 transformed to meet the assumption of normality. The significance of the Cross term, comparing the least-squares means of the allelic ratio (Y2b/YOre) between the O2b and 2bO genotypes, was used to test the null hypothesis of no imprinting (H0: 2bO = O2b). The sensitivity of this test differs among genes and is determined by the variability among replicate samples and pyrosequencing measurements. The 95% confidence intervals indicate that, depending on the gene, this test would reject the null hypothesis at α = 0.05 if the abundance of either allele differed by >1–4% in reciprocal crosses (Figure 2A). These data provide sufficient power to detect complete (on/off) imprinting as well as partial imprinting that alters the relative expression of one allele >5%.
Figure 2.—
Allelic expression in reciprocal crosses indicates a lack of imprinting. (A) The percentage of total transcripts derived from the 2b allele in offspring from reciprocal crosses (2bO and O2b) is shown for imprinting candidate genes. The log2-transformed ratio of allelic expression, log2(Y2b/YOre), was fitted to the mixed model described in the main text. LS means derived from this model were used to calculate the percentage of the 2b allele in each sample. For example, if log2(Y2b/YOre) = 0, then Y2b/YOre = 2° = 1, and Y2b = 50%. Error bars indicate the 95% confidence interval surrounding each LS mean. Note that confidence intervals are not symmetric because the analysis of variance was performed on log-transformed data. LS means of relative allelic expression for 2bO and O2b flies were within 0.3 to 3 times the standard error of each other for all candidate genes. In all cases, this is <4.3 times the standard error (d.f. = 2) that defines the 95% confidence interval. (B) The LS mean and standard errors of log2(Y2b/YOre) are plotted for imprinting candidate genes (open diamonds) and control genes (solid diamonds). Expression in 2bO flies from a cross between 2b females and Ore males is shown on the x-axis, with expression in O2b flies from a cross of Ore females and 2b males shown on the y-axis. (C) Additional tests for imprinting from P. Wittkopp, B. Haerum and A. Clark (unpublished data) are shown. LS means are plotted in both panels with error bars indicating the standard error. Supplemental Table 2 (http://www.genetics.org/supplemental/) summarizes the genes, crosses, LS means, standard errors, and significance tests for all comparisons.
RESULTS AND DISCUSSION
Both parental alleles were expressed for all genes examined; neither the maternal nor the paternal allele was ever completely silenced (Figure 2A). There was also no evidence of partial imprinting: the 2b and Ore alleles maintained similar allelic expression in reciprocal crosses for all candidate genes (P > 0.05 for all tests, supplemental Table 2 at http://www.genetics.org/supplemental/). Asymmetrical expression between alleles was observed for some genes, which indicates differences in cis-regulatory activity between the 2b and Ore alleles (Cowles et al. 2002; Wittkopp et al. 2004), but the relative expression of the two alleles was similar regardless of which parent transmitted which allele (Figure 2A). This similarity in allelic expression exists despite parent-of-origin effects that alter total expression levels between 2bO and O2b flies (Figure 1A).
The same allelic expression in reciprocal crosses was observed not only for all imprinting candidate genes but also for all 15 control genes examined (Figure 2B). In addition, we have tested for allelic expression differences between reciprocal crosses in the context of other studies (P. Wittkopp, B. Haerum and A. Clark, unpublished data), including 24 additional genes examined in crosses among inbred strains of D. melanogaster (42 comparisons), among inbred strains of D. simulans (22 comparisons), and between these two species (24 comparisons) (Figure 2C). Again, we found no evidence of differential allelic expression between reciprocal crosses that would indicate genomic imprinting (P > 0.05 for all tests) (supplemental Table 2, http://www.genetics.org/supplemental/). In the absence of imprinting, parent-of-origin effects on gene expression are inferred to be caused by trans-acting maternal and paternal effects. Initiated during embryogenesis, these effects must be propagated during ontogeny to affect the physiology of adult flies (e.g., Rice et al. 1979; Forquignon 1981; Fox et al. 2004; Malmanche and Clark 2004;).
A total of 48 genes were tested for genomic imprinting in this study, including 9 of the best imprinting candidate genes identified in a genomewide survey of gene expression (Gibson et al. 2004). Using a sensitive allele-specific test, we found no evidence of imprinting, suggesting that this phenomenon may contribute little to normal gene expression in D. melanogaster. With methods for studying allele-specific transcription on a large scale now available, additional genes, developmental stages, and specific tissue types can be examined to determine whether imprinting affects D. melanogaster gene expression in other contexts. Genes located in heterochromatin will be particularly interesting to examine, since the transposition of euchromatic genes into or near heterochromatin often results in imprinting (Golic et al. 1998; Lloyd et al. 1999; Haller and Woodruff 2000; Joanis and Lloyd 2002; Maggert and Golic 2002).
Acknowledgments
We thank D. Barbash, H. Hollocher, C. Landry, S. Nuzhdin, and H. A. Orr for fly strains and K. Montooth and A. Fiumera for statistical advice. Funding for this work was provided by a National Institutes of Health grant GM-064590 to A.G.C. B.K.H. was supported by the Howard Hughes Undergraduate Scholars Program, and P.J.W. was a Damon Runyon Postdoctoral Fellow supported by the Damon Runyon Cancer Research Foundation.
References
- Ahmadian, A., B. Gharizadeh, A. C. Gustafsson, F. Sterky, P. Nyren et al., 2000. Single-nucleotide polymorphism analysis by pyrosequencing. Anal. Biochem. 280: 103–110. [DOI] [PubMed] [Google Scholar]
- Carmena, M., C. Gonzalez, J. Casal and P. Ripoll, 1991. Dosage dependence of maternal contribution to somatic cell division in Drosophila melanogaster. Development 113: 1357–1364. [DOI] [PubMed] [Google Scholar]
- Cowles, C. R., J. N. Hirschhorn, D. Altshuler and E. S. Lander, 2002. Detection of regulatory variation in mouse genes. Nat. Genet. 32: 432–437. [DOI] [PubMed] [Google Scholar]
- Crouse, H. V., 1960. The nature of the influence of X-translocation on sex of progeny in Sciara coprophila. Chromosoma 18: 230–235. [DOI] [PubMed] [Google Scholar]
- da Rocha, S. T., and A. C. Ferguson-Smith, 2004. Genomic imprinting. Curr. Biol. 14: R646–R649. [DOI] [PubMed] [Google Scholar]
- Fitch, K. R., G. K. Yasuda, K. N. Owens and B. T. Wakimoto, 1998. Paternal effects in Drosophila: implications for mechanisms of early development. Curr. Top. Dev. Biol. 38: 1–34. [DOI] [PubMed] [Google Scholar]
- Fiumera, A. C., B. L. Dumont and A. G. Clark, 2005. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics 169: 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forquignon, F., 1981. A maternal effect mutation leading to deficiencies of organs and homeotic transformations in the adults of Drosophila. Roux's Arch. Dev. Biol. 190: 132–138. [DOI] [PubMed] [Google Scholar]
- Fox, C. W., M. E. Czesak and W. G. Wallin, 2004. Complex genetic architecture of population differences in adult lifespan of a beetle: nonadditive inheritance, gender differences, body size and a large maternal effect. J. Evol. Biol. 17: 1007–1017. [DOI] [PubMed] [Google Scholar]
- Fuyama, Y., 1984. Gynogenesis in Drosophila melanogaster. Jpn. J. Genet. 59: 91–96. [Google Scholar]
- Gehring, M., Y. Choi and R. L. Fischer, 2004. Imprinting and seed development. Plant Cell 16(Suppl): S203–S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, G., R. Riley-Berger, L. Harshman, A. Kopp, S. Vacha et al., 2004. Extensive sex-specific nonadditivity of gene expression in Drosophila melanogaster. Genetics 167: 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goday, C., and M. R. Esteban, 2001. Chromosome elimination in sciarid flies. BioEssays 23: 242–250. [DOI] [PubMed] [Google Scholar]
- Golic, K. G., M. M. Golic and S. Pimpinelli, 1998. Imprinted control of gene activity in Drosophila. Curr. Biol. 8: 1273–1276. [DOI] [PubMed] [Google Scholar]
- Haller, B. S., and R. C. Woodruff, 2000. Varied expression of a Y-linked P[w+] insert due to imprinting in Drosophila melanogaster. Genome 43: 285–292. [DOI] [PubMed] [Google Scholar]
- Heid, C. A., J. Stevens, K. J. Livak and P. M. Williams, 1996. Real time quantitative PCR. Genome Res. 6: 986–994. [DOI] [PubMed] [Google Scholar]
- Joanis, V., and V. K. Lloyd, 2002. Genomic imprinting in Drosophila is maintained by the products of Suppressor of variegation and trithorax group, but not Polycomb group, genes. Mol. Genet. Genomics 268: 103–112. [DOI] [PubMed] [Google Scholar]
- Komma, D. J., and S. A. Endow, 1995. Haploidy and androgenesis in Drosophila. Proc. Natl. Acad. Sci. USA 92: 11884–11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry, C. R., P. J. Wittkopp, C. H. Taubes, J. M. Ranz, A. G. Clark et al., 2005. Compensatory cis-trans evolution and the dysregulation of gene expression in interspecific hybrids of Drosophila. Genetics 171: 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, V. K., D. A. Sinclair and T. A. Grigliatti, 1999. Genomic imprinting and position-effect variegation in Drosophila melanogaster. Genetics 151: 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggert, K. A., and K. G. Golic, 2002. The Y chromosome of Drosophila melanogaster exhibits chromosome-wide imprinting. Genetics 162: 1245–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmanche, N., and D. V. Clark, 2004. Drosophila melanogaster Prat, a purine de novo synthesis gene, has a pleiotropic maternal-effect phenotype. Genetics 168: 2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison, I. M., J. P. Ramsay and H. G. Spencer, 2005. A census of mammalian imprinting. Trends Genet. 21: 457–465. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard, C., 1991. Determination of the embryonic axes of Drosophila. Dev. Suppl. 1: 1–10. [PubMed] [Google Scholar]
- Perrimon, N., L. Engstrom and A. P. Mahowald, 1989. Zygotic lethals with specific maternal effect phenotypes in Drosophila melanogaster. I. Loci on the X chromosome. Genetics 121: 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrimon, N., A. Lanjuin, C. Arnold and E. Noll, 1996. Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics 144: 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik, W., and J. Walter, 2001. Genomic imprinting: parental influence on the genome. Nat. Rev. Genet. 2: 21–32. [DOI] [PubMed] [Google Scholar]
- Rice, T. B., F. A. Rice and A. Garen, 1979. Adult abnormalities resulting from a localized blastoderm defect in a maternal-effect mutant of Drosophila. Dev. Biol. 69: 194–201. [DOI] [PubMed] [Google Scholar]
- Scott, R. J., and M. Spielman, 2004. Epigenetics: imprinting in plants and mammals—the same but different? Curr. Biol. 14: R201–R203. [DOI] [PubMed] [Google Scholar]
- Wittkopp, P. J., B. K. Haerum and A. G. Clark, 2004. Evolutionary changes in cis and trans gene regulation. Nature 430: 85–88. [DOI] [PubMed] [Google Scholar]