Abstract
We have studied how a set of male-specific sensory neurons in Caenorhabditis elegans establish axonal connections during postembryonic development. In the adult male, 9 bilateral pairs of ray sensory neurons innervate an acellular fan that serves as a presumptive tactile and olfactory organ during copulation. We visualized ray axon commissures with a ray neuron-specific reporter gene and studied both known and new mutations that affect the establishment of connections to the pre-anal ganglion. We found that the UNC-6/netrin-UNC-40/DCC pathway provides the primary dorsoventral guidance cue to ray axon growth cones. Some axon growth cones also respond to an anteroposterior cue, following a segmented pathway, and most or all also have a tendency to fasciculate. Two newly identified genes, rax-1 and rax-4, are highly specific to the ray neurons and appear to be required for ray axon growth cones to respond to the dorsoventral cue. Among other genes we identified, rax-2 and rax-3 affect anteroposterior signaling or fate specification and rax-5 and rax-6 affect ray identities. We identified a mutation in sax-2 and show that the sax-2/Furry and sax-1/Tricornered pathway affects ectopic neurite outgrowth and establishment of normal axon synapses. Finally, we identified mutations in genes for muscle proteins that affect axon pathways by distorting the conformation of the body wall. Thus ray axon pathfinding relies on a variety of general and more ray neuron-specific genes and provides a potentially fruitful system for further studies of how migrating axon growth cones locate their targets. This system is applicable to the study of mechanisms underlying topographic mapping of sensory neurons into target circuitry where the next stage of information processing is carried out.
FORMATION of a functional nervous system requires that neurons generate appropriate circuits. Axon pathfinding, the mechanism by which neurons extend processes through the body to the region where they can locate and synapse with their targets, represents an important first step in circuit formation. Over the past decade, several families of attractive and repulsive guidance cues have been identified in Caenorhabditis elegans, Drosophila, and vertebrates, including the UNC-6/Netrins, Slits, Ephrins, and Semaphorins. Specific receptors on the growth cones of developing axons recognize these cues and transduce signals that ultimately lead to changes in the direction of axonal growth (reviewed by Tessier-Lavigne and Goodman 1996; Mueller 1999; Dickson 2002). Many cell adhesion molecules of the immunoglobulin superfamily are also key players in the control of axonal growth and guidance (Rougon and Hobert 2003). Recent studies reveal that secreted cell-signaling molecules best known for their roles as morphogens in controlling cell fate and early embryonic patterning can also act as axon guidance molecules, including Shh, BMP, and Wnts (Butler and Dodd 2003; Charron et al. 2003; Lyuksyutova et al. 2003; Yoshikawa et al. 2003). In addition to these broadly acting factors, axon guidance also involves cell-specific factors. For instance, odorant receptors provide the core determinant of identity for axons of olfactory sensory neurons to coalesce into glomeruli in the olfactory bulb (Feinstein et al. 2004). Localized cytosolic Ca2+ signals can control the direction of Ca2+-dependent growth cone turning, which requires a calcium–calmodulin-dependent protein kinase II (CaMKII) and calcineurin (CaN) phosphatase switch (Wen et al. 2004) and depends on myelin-associated glycoprotein (Henley et al. 2004).
We have analyzed axon pathfinding by a set of male sensory neurons in C. elegans to determine what general and cell-specific functions guide these axons to their target region. In C. elegans, most circuits in the nervous system are established during embryonic development and additional neurons are added during postembryonic development. Previous studies of axon pathfinding in C. elegans have focused on development of the ventral nerve cord (Durbin 1987; Wightman et al. 1997; Hutter et al. 2005), sensory neurons of the head (Zallen et al. 1999), pharyngeal neurons (Morck et al. 2003), and dorsoventral motor and sensory neuron commissures (Hedgecock et al. 1985, 1990; McIntire et al. 1992; Colavita and Culotti 1998). Here we report the results of an investigation of postembryonic axon pathfinding by a set of sensory neurons in the male tail. Differentiation of these neurons, known as ray sensory neurons, provides a particularly clear example of axon pathfinding amenable to genetic analysis.
Eighteen bilateral pairs of ray sensory neurons are born in the male tail hypodermis during the last larval stage (Sulston et al. 1980). In the adult, these neurons have sensory dendrites within the protruding, finger-like rays, which act as probable mechano- and chemosensors to detect proximity to or contact with the hermaphrodite during copulation (Liu and Sternberg 1995). Each ray contains the processes of two neurons of different morphological type, designated A type and B type. The postsynaptic targets of both types of ray neurons are multiple male specific as well as non-sex-specific neurons that contribute to the control of the male's movements during mating (Sulston et al. 1980). The A-type and B-type neurons in different rays are both specialized into subtypes with distinct molecular properties and postsynaptic targets (Troemel et al. 1995; Lints et al. 2004; M. Xu, D. H. Hall and S. W. Emmons, unpublished observations). Consistent with this observation, the rays function differentially to affect the male's behavior during copulation (Liu and Sternberg 1995). Therefore the fan and rays may be viewed as a compound sensory organ conveying topographically mapped information to the next stage of the processing circuitry. Analysis of ray axon pathfinding can help to understand the mechanisms underlying the mapping of sensory neurons into central target circuitry.
The targets of the ray neurons lie mostly within the pre-anal ganglion (PAG), a complex neuropil containing the processes of dozens of neurons lying at the posterior end of the ventral nerve cord. The PAG is located some distance away from where the ray neurons are born. During the final stages of maturation of the male sex-specific nervous system, which occurs during the last larval stage, the ray neurons send axons out of the dorsolateral lumbar ganglia into which their cell bodies have migrated and into the PAG. Depending on the ray, the growth cones of these axons navigate various routes around the basal surface of the body-wall epidermis to reach their destination. After they arrive at the PAG they insinuate into the preexisting and probably evolving neuropil, sending out branches that eventually locate appropriate synaptic targets (M. Xu, D. H. Hall and S. W. Emmons, unpublished data).
Here we describe the results of a genetic screen to identify genes required for the outgrowth and pathfinding of the ray axons. We have used a GFP reporter gene that labels the processes of the B-type neurons to identify mutants with abnormal ray neuron processes in the adult. We identified seven mutations in six known genes, several of which were previously known to be involved in axon guidance, and nine mutations that define at least six potentially new genes. We also tested the effects of mutations in additional genes known to act in axon guidance. Our studies provide insight into the mechanism of pathfinding by the ray axon growth cones and show that ray axons depend on a combination of widely acting axon guidance genes as well as genes that appear to function more specifically in the rays. The new mutations have additional pleiotropic defects that help to provide insights into the possible functions of the corresponding genes.
MATERIALS AND METHODS
Strains:
All strains used in this study contain the him-5(e1490)V mutation, which gives rise to a high percentage of males in self-fertilizing populations (Broverman and Meneely 1994). We refer to him-5(e1490) as wild type. Strains were maintained using standard culture conditions (Brenner 1974). All experiments were performed at 20° unless otherwise mentioned. The following additional alleles were used:
LGI: dpy-5(e61), unc-40(e271), lev-11(x12), unc-15(e73), unc-87(e1216), unc-54(e190)
LGII: dpy-2(e8), unc-130(ev505), unc-104(e1265)
LGIII: dpy-17(e164), sax-2(ky216), lon-1(e185), unc-36(e251), unc-32(e189), unc-93(e1500), sma-3(e491), mab-5(e1239), egl-5(u202), pha-1(e2123ts), dpy-18(e364), unc-25(e156)
LGIV: dpy-13(e184), unc-17(e245), mab-26(bx80), unc-129(ev554), unc-22 (e66)
LGV: unc-51(e369)
LGX: sax-3(ky123), unc-6 (e78, bx123, ev400), lon-2(e678), dpy-6(e14), unc-27(su195, su142, e155), unc-58(e665), unc-18(e81), sax-1(ky211), unc-115(e2225), egl-15(n484), dpy-7(e88), mup-2 (e2346ts)
Visualization of neuronal morphology:
The following fluorescent reporter genes were used to visualize neurons: pkd-2∷GFP (bxIs14, see below) (strain EM733), cat-2∷GFP (bxEx38, Lints and Emmons 1999) (strain EM284), unc-47∷GFP (oxIs12, McIntire et al. 1997), glr-1∷GFP (nuIs1, Zheng et al. 1999), and unc-119∷GFP (edIs16, Maduro and Pilgrim 1995). Animals with extrachromosomal or integrated fluorescent reporter genes were scored using a Zeiss Axioplan microscope fitted with a digital camera (Spot Diagnostic 3.0) and processed using Adobe Photoshop 5.0.
Genetic screen and mapping of mutants:
bxIs14 him-5 young hermaphrodites (EM733), which carry integrated pkd-2∷gfp, were mutagenized with 0.05 m EMS for 4 hr at room temperature (Brenner 1974). Mutagenized animals were grown at 20°. Individual F1 progeny were picked to separate plates and allowed to self. We identified plates in which F2 males and hermaphrodites had generally wild-type behavior and some males had abnormal ray axon morphology scored by the expression of pkd-2∷gfp. Hermaphrodite siblings were picked from candidate plates to isolate homozygous lines. Mutants were backcrossed twice to bxIs14 him-5 and once to him-5. All of the obtained mutants were recessive. Mapping was performed by linkage analysis, three-factor cross, deficiency mapping, SNP mapping, and complementation testing (Table 1). In all cases, ray axon defects were scored for mapping. The phenotype of bx141 was too weak for linkage analysis.
TABLE 1.
Mapping results of newly isolated mutants
| Genes (alleles) | Genetic location | Mapping data |
|---|---|---|
| rax-1(bx126, bx132) | V(−20–−18) | rax-1 is covered by deficiency sDf 56 (−20–−18) |
| rax-2(bx131) | III (−0.59–−0.64) | dpy-17rax-2 (6/7) non-unc-36; non-dpy-17 rax-2 unc-36 (1/6) |
| rax-3(bx133, bx138) | I | ND |
| rax-4(bx139) | I (0.27–0.92) | dpy-5rax-4 (2/23) non-unc-29; non-dpy-5 rax-4 unc-29 (15/21) |
| rax-5(bx137) | II | Complements mab-20(ev574)II |
| rax-6(bx140) | IV | Complements mab-26(bx80)IV |
| unc-27(bx124, bx127) | X (0.44–0.48) | dpy-6 unc-27 (37/148) non-unc-115; non-dpy-6 unc-27 unc-115 (83/114); fail to complement unc-27(e155) |
| sax-2(bx130) | III (−2.14) | dpy-17sax-2 (2/56) non-unc-36; non-dpy-17 sax-2 unc-36 (51/51); fail to complement sax-2(ky216) |
ND, not determined.
Mating behavior assays:
Mating efficiency tests:
The mating efficiency of males was measured by the method of Hodgkin (1983): six tester males and six unc-51(e369) hermaphrodites at the young adult stage were placed on mating plates for 24 hr, then males were removed. The number of cross progeny (nonUnc) was determined and divided by the total number of progeny.
Observation of copulation:
Males were isolated at the L4 stage and kept on same-sex plates until used for mating behavior assays. A single tester male and four unc-51(e369) hermaphrodites were put onto a mating plate with a small (1 cm) patch of food and observed for 10 min under a dissecting microscope. A non-mater was a male that did not copulate within the 10 min observation time. The latency for male response to contact with the hermaphrodite was measured as the time interval between putting the male onto the plate to the first mating attempt as indicated by arrest of the male's forward locomotion and the pressing of the ventral side of his tail against the surface of a hermaphrodite. The ventral turning behavior performed during copulation was divided into three categories: good, sloppy, and missed, following Loer and Kenyon (1993).
Reporter constructs, transformation, and integration:
Plasmid EM#310 (pPKD-2∷SNB-1∷GFP) was constructed by fusing snb-1 coding sequence (Nonet 1999) with 1.8-kb pkd-2 5′ upstream sequence (Barr and Sternberg 1999). Extrachromosomal array bxEx94 was generated by co-injecting EM#310 and pha-1(+) plasmid pBX-1 into pha-1(e2123ts), him-5(e1490) (Granato et al. 1994). Plasmid EM#311 is a translational fusion of unc-27∷GFP, containing 3.6 kb upstream and 2.3-kb full genomic sequence of unc-27 fused with the GFP coding sequence and the unc-54 3′-UTR by overlapping PCR (Hobert 2002). Co-injection of EM#311 and pBX-1 into pha-1(e2123ts), him-5(e1490) generated extrachromosomal array bxEx97. To generate the integrated line bxIs14 used for the genetic screen, extrachromosomal syEx313 (pkd-2∷GFP) (Barr and Sternberg 1999) was integrated into chromosome V by γ-irradiation as described by Mello and Fire (1995).
Molecular identification of unc-27:
Mutations bx124 and bx127 were mapped between 0.44 and 0.48 cM on linkage group X and failed to complement unc-27(e155). A total of 16 cosmids from the unc-27 genetic region were purified following standard methods. Cosmids, pkd-2∷GFP reporter plasmid (Barr and Sternberg 1999), and pha-1 rescue plasmid pBX-1 (Granato et al. 1994) were co-injected into unc-27(bx124); pha-1(e2123ts); him-5(e1490) hermaphrodites at concentrations of 8.5 ng/μl, 30–80 ng/μl, and 100 ng/μl, respectively (Mello and Fire 1995). The cosmid ZK721 and a 6.4-kb PCR fragment within ZK721 (nucleotide 13786–20279) rescued both Unc and axon wandering phenotypes of unc-27(bx124). Predicted gene ZK721.2 in the 6.4-kb region encodes an isoform of troponin I. Mutations in bx124 and bx127 were identified by sequencing PCR-generated templates amplified from genomic DNA.
Molecular identification of sax-2:
Mutation bx130 was mapped between dpy-17 (on cosmid F54D8) and unc-36 (on cosmid C50C3), closely linked to dpy-17, and failed to complement sax-2(ky216). Five overlapping cosmids, including F54D8, were co-injected pairwise with pkd-2∷GFP and pBX-1 into sax-2(bx130) pha-1(e2123ts); him-5(e1490) hermaphrodites. Co-injection of cosmids F54D8 and F21H11 rescued the ectopic neurite defects. An 11.8-kb PCR fragment (nucleotide F54D8 30987–F21H11 9396) and a 13-kb PCR fragment within F21H11 (nucleotide 8969–21990) rescued the ectopic outgrowth phenotype of bx130. These two overlapping fragments contain a single predicted gene encoding a novel protein, F21H11.2. Mutation in bx130 was identified within F21H11.2 by sequencing PCR-generated templates amplified from bx130 genomic DNA.
Troponin I RNAi:
RNA interference (RNAi) was carried out by micro-injection of the gonad as described by Fire et al. (1998). RNA was synthesized using MEGAscript T3 and T7 kit (Ambion). Approximately 200 ng/μl dsRNA was injected into bxIs14 (pkd-2∷GFP) him-5 or bxEx97 (unc-27∷GFP), him-5 hermaphrodites. The F1 progeny of injected animals were collected from 4 to 24 hr after micro-injection and scored for phenotypes at adult stage.
RESULTS
Ray neuron development in wild type:
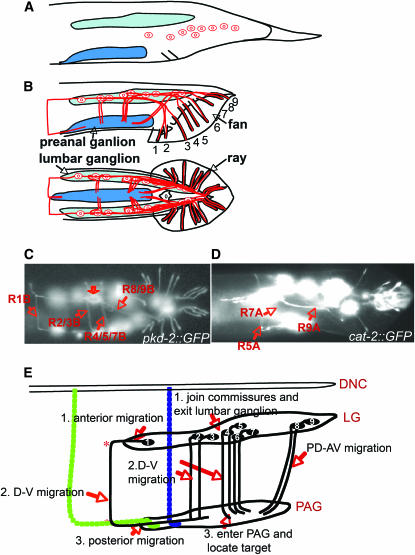
We visualized ray sensory neurons using a reporter gene for pkd-2, a C. elegans homolog of a mammalian polycystic kidney disease gene (Barr and Sternberg 1999). PKD-2∷GFP labels the B-type ray neurons (except R6B) and their processes. During the final stages of differentiation of the male tail sexual structures in mid to late L4, the ray neuron cell bodies migrate away from the posterior tail hypodermis where they are born and enter the lumbar ganglia (Figure 1, A and B). They then extend axons out of the lumbar ganglia that migrate around the body wall between the basal surface of the hypodermis and the hypodermal basal lamina, forming four or five circumferential commissures (Sulston et al. 1980) (Figure 1). On reaching the ventral side, the axon terminals enter the PAG and terminate within a defined subregion, where a diffuse halo of GFP fluorescence can be seen (Figure 1C). The axons of the A-type neurons, visualized by cat-2∷GFP, a tyrosine hydroxylase reporter (Lints and Emmons 1999), follow similar, if not identical, pathways (Figure 1D). Electron microscopy reveals that axons of both A- and B-type neurons can be found within the same commissures (M. Xu, D. A. Hall and S. W. Emmons, unpublished data).
Figure 1.—
Ray axon pathways in wild type. (A) Lateral view of the L4 larval male tail, showing the positions of the nine neurons of one type before they migrate into the lumbar ganglion during remodeling of the tail. (B) Lateral (top) and ventral (bottom) views of the adult male tail, showing the fan and rays and one of the two sets of ray sensory neurons. Ray neuron sensory dendrites extend to the tips of the rays, cell bodies are in the lumbar ganglia, and axonal output is in the pre-anal ganglion. (C and D) Fluorescence photomicrographs of strains carrying pkd-2∷GFP and cat-2∷GFP. pkd-2∷GFP labels all RnB neurons except R6B; cat-2∷GFP labels R5A, R7A, and R9A. In C, commissural pathways are indicated; the open arrow indicates a region of diffuse fluorescence within the pre-anal ganglion where there is a concentration of ray synapses. (E) The ray axons follow various pathways between the lumbar ganglia and the pre-anal ganglion. The axon of R1B follows a segmented pathway defined by apparent choice points (labeled *), first migrating anteriorly out of the pre-anal ganglion, then turning to traverse around the body wall to the ventral side, then progressing ventrally into the pre-anal ganglion. On the right side, there are two ventrodorsal commissures in the vicinity of the ray 1 commissure, one containing AS11 (blue) and a second containing motor neurons VD12 and VD13 (green). R1BR axon could follow one of these or pioneer its own route. The axon of R2B sometimes follows a similar pathway, but more often fasciculates with the axon of R3B in a dorsoventral commissure. The axons of R4B, 5B, 7B, and presumably 6B follow a similar dorsoventral commissure. The axons of R8B and R9B follow the preexisting lumbar commissure. RnA neurons follow similar pathways and can fasciculate with the RnB neurons. For a diagram of these pathways in transverse section showing the pathway of the ray axon commissures between the basal surface of the hypodermis and the hypodermal basal lamina, see Figure 7.
For most of the ray neurons, the axons follow a more or less direct dorsoventral pathway to the PAG (Figure 1B). In the case of R1B and sometimes R2B, however, the axon first migrates anteriorly out of the lumbar ganglion for some distance before turning abruptly to progress around the body. On reaching the ventral side (where the ventral cord is situated), it turns again toward the posterior and enters the PAG from the anterior. In each lumbar ganglion, the cell bodies of the 18 ray neurons are intermingled with the cell bodies of 12 non-sex-specific neurons (White et al. 1986; Hall and Russell 1991). The final positions of the ray neurons among these additional neurons and with respect to one another varies from animal to animal (Sulston et al. 1980). The position a cell takes appears to influence the pathway that its axon follows. The variability in choice of pathway is most pronounced for the axon of R2B. In 75% of sides (n = 38), the R2B cell body is close to the R3B cell body and the R2B axon fasciculates with the R3B axon. In 25% of sides, the R2B cell body is located more anteriorly and follows a pathway like that of the R1B axon, first migrating for a distance anteriorly before turning ventrally and progressing to the PAG. Thus, in 75% of sides there are four commissures: R1, R2/3, R4/5/7 (this commissure may also include R6), R8/9; whereas 25% of sides have five commissures: R1, R2, R3/4, R5/7, R8/9.
From the nature of these pathways a number of conclusions may be drawn regarding the various signals and decision points that guide the ray growth cones to their target region. For ray neurons in the midregion of the lumbar ganglion, the axon pathways consist of a single dorsoventral segment. Since no preexisting commissures are present in this region, one growth cone must pioneer each route, apparently following a dorsoventral gradient or an attractive signal from the PAG. The growth cones of the remaining axons in the same commissure may follow this pioneer. For neurons in the anterior region of the lumbar ganglion, the axon pathways consist of three segments, the axonal growth cones appearing to respond first to an anteroposterior gradient or other polarity cue, then a dorsoventral one, and finally an anteroposterior one in the opposite direction, possibly an attractive signal from the PAG itself. For all three segments, the growth cone could either pioneer the route or fasciculate with one of several preexisting commissures in this region. On the right side, there are two in the vicinity of the ray 1 commissures, one containing interneuron AS11 and a second containing the posterior motorneuron commissures of VD12 and VD13 (Figure 1E). The first change in direction occurs at different positions in different animals and when both R1B and R2B migrate anteriorly, they change direction at different positions. If the change in direction is caused by encountering an existing dorsoventral commissural neuron, variable position could be because these commissures vary in their locations or because the choice among the preexisting commissures is not fixed. If the growth cone is pioneering the route, then the change in direction could be triggered by a broadly localized positional cue or a graded change in the physical environment or by a timing mechanism that changes the responsiveness of the cell to orthogonal cues. However the change in direction occurs, it apparently involves a switch, as the axon terminal changes direction abruptly, suggesting it responds to only one cue at a time.
Rays 8 and 9 enter the PAG following the lumbar commissures previously established by the non-sex-specific lumbar neurons (White et al. 1986; Hall and Russell 1991; M. Xu, D. H. Hall and S. W. Emmons, unpublished observations). For these neurons, the tendency to fasciculate may be the most important determinant of pathway.
A genetic screen for ray axon defects identified alleles of new and previously described axon guidance genes:
To identify genes that are required for ray axons to grow along these pathways, we mutagenized the pkd-2∷ GFP strain (bxIs14) and screened for abnormal axon trajectories. From 4800 mutagenized genomes we isolated 16 recessive mutations (Table 2). Seven of these mutations are new alleles of previously identified genes: unc-27(bx124 and bx127), sax-2(bx130), unc-6(bx123), unc-40(bx134), unc-51(bx135), and egl-35(bx129). The properties of egl-35(bx129) will be described in a future article. According to the results of mapping and complementation tests, 8 of the remaining 9 mutations define six tentatively new genetic loci named rax for ray axon abnormal. These are rax-1(bx126 and bx132), rax-2(bx131), rax-3(bx133 and bx138), rax-4(bx139), rax-5(bx137), and rax-6(bx140). A ninth mutation, bx141, could not be mapped because of a weakly penetrant phenotype.
TABLE 2.
Phenotypes of ray axon guidance mutants
| Genes (alleles) | Ray axon defect | Defects in other axons | Major pleiotropic defect | Additional phenotypes | Mating efficiencya |
|---|---|---|---|---|---|
| rax-1(bx126, bx132) | Lateral to ventral | No | Ray axon specific | No | ME3 |
| rax-4(bx139) | Lateral to ventral | No | No | ME1 | |
| rax-3(bx133, bx138) | Multiple defectsb | ND | Cell migration | Slightly loopy movement, anterior cell migration | ME2 |
| rax-2 (bx131) | Multiple defects | Ectopic neurons with pkd-2∷gfp | Slightly long, anterior cell migration | ND | |
| rax-5(bx137) | Multiple defects | No | Ray differentiation | Ray fusion | ME2 |
| rax-6(bx140) | Multiple defects | No | Ray fusion | ME3 | |
| bx141 | Ectopic neurites | ND | Control of outgrowth | ND | ND |
| sax-2(bx130) | Ectopic neurites | Sensory neurons | Gonad dysmorphology | ME1 | |
| unc-27(bx124,bx127) | Wandering | Many | Muscle attachment | Unc | ME1 |
| unc-6(bx123) | Lateral to ventral | Many | Global guidance molecules | Unc | ND |
| unc-40(bx134) | Lateral to ventral | Many | Unc | ||
| unc-51(bx135) | Lateral to ventral | Many | Unc |
Mutations are grouped according to their common axonal and pleiotropic defects.
Mating efficiency is estimated by the percentage of cross progeny from mutant males relative to the wild-type males. ME4 = 40–100% of wild-type male mating efficiency; ME2 = 20–40%; ME3 = 10–20%, ME = 1–10%.
Multiple defects include outgrowth, guidance, and fasciculation.
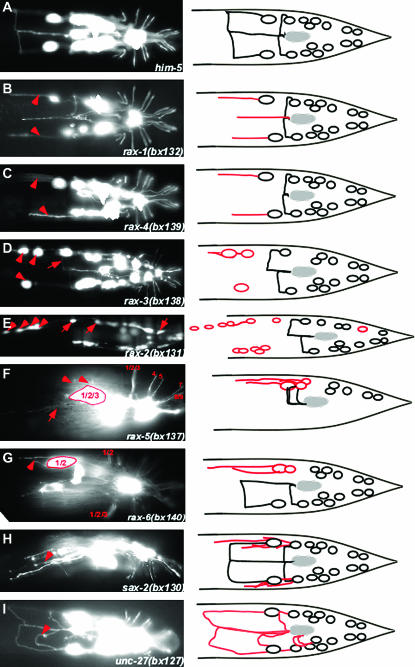
All mutations except unc-27(bx124, bx127), unc-6(bx123), unc-40(bx134), and unc-51(bx135) have wild-type or near wild-type body morphology and locomotion. They affect a variety of RnB axonal properties, including axon outgrowth, extension, guidance, and fasciculation (Figure 2, B–I). Defects were generally more penetrant and more easily scored for the axon of R1B than for the axons of the other ray neurons (Table 3). Some RnA axons exhibit similar defects (data not shown). The mutations affected non-ray neurons to varying degrees and had a variety of other pleiotropic effects on the ray neurons or rays, including abnormal positions of the ray cell bodies, number of ray cells, and fusion of rays (Table 2). No mutations were found that appeared to affect the formation of the ray dendrites. Dendritogenesis, which occurs by the reeling out of the dendritic process as the cell body migrates away from the site of ray attachment to the body wall, may be more redundantly determined and occur somewhat independently of axon outgrowth, or possibly neurons defective in the earlier steps of cell differentiation involving dendrite attachment and cell body migration may fail to reach the stage of pkd-2 expression.
Figure 2.—
Abnormal ray neuron axon trajectories in mutants. Fluorescence photomicrographs and diagrams of wild-type and mutant axon trajectories. RnB neurons are labeled with pkd-2∷GFP (bxIs14). All diagrams and most photomicrographs are ventral views, whereas in the photomicrographs rax-5(bx137) is shown in ventrolateral view and sax-2(bx130) is shown in lateral view. Arrowheads and arrows point out specific defects as follows: (B and C) R1B axons fail to turn to the ventral side. (D) Abnormal anterior positions of R1B and R2B cell bodies and commissural pathway of R3B following the normal path of R1B (arrow). (E) Ectopic anterior GFP-positive cells and anterior displacement of R1B, R2B, and R8B cell bodies (arrows). (F) Despite abnormal clustering of their cell bodies, R1B, R2B, and R3B have normal commissures and also send out ectopic neurites that do not turn (arrow). (G) Ray 1 and 2 are fused; R1B and R2B cell bodies are clustered and send out axons that fail to turn. (H) Ray neurons extend multiple neurites. (I) Axons appear to take wandering pathways.
TABLE 3.
Ray 1 axon defects
| Ray axon morphology
|
||||||||
|---|---|---|---|---|---|---|---|---|
 |
 |
 |
 |
 |
 |
 |
||
| Genotype | Wild type (%) | Wandering (%) | Ectopic outgrowth (%) | No ventral turning (%) | Anteriorly ventral turn (%) | Bifurcating (%) | Cell migration (%) | N |
| him-5(e1490) | 97 | 2 | 0.4 | 228 | ||||
| rax-1(bx126) | 51 | 11 | 26 | 3.3 | 8.5 | 274 | ||
| rax-1(bx132) | 20 | 16 | 49 | 3.9 | 8.1 | 405 | ||
| rax-4(bx139) | 39 | 2 | 48 | 5 | 6 | 288 | ||
| rax-2(bx131) | 82 | 16 | 2 | 100a | 84 | |||
| rax-3(bx133) | 73 | 27b | 78 | 101 | ||||
| rax-3(bx138) | 81 | 19b | 77 | 170 | ||||
| rax-5(bx137) | 70 | 7 | 13 | 10 | 17c | 225 | ||
| rax-6(bx140) | 77 | 7 | 16 | 48c | 109 | |||
| bx141 | 73 | 27 | 68 | |||||
| sax-2(bx130) | 100 | 200 | ||||||
| unc-27(bx124) | 9 | 79 | 12 | 153 | ||||
The percentage of sides with ray 1 defect is shown. Defects in the ray 1 process were the most easily scored and most likely to be abnormal. With the exception of rax-1 and rax-4, ray commissures of the posterior rays were usually present in mutants.
80% of males have more than 8 pkd-2∷GFP positive neurons in the tail, in addition to the anterior mispositioned ray neuons.
Ray axons from 27% and 19%of sides in bx133 and bx138 mutant, respectively, fail to make the ventral turn when the neurons are mispositioned anteriorly. The rest of the neurons extend posteriorly-directed axons.
The neurons of fused rays form cluster in the lumbar ganglion. Axon defects and cell migration defect are counted separately.
The UNC-6/netrin-UNC-40/DCC pathway provides an essential dorsoventral guidance cue for formation of ray commissures:
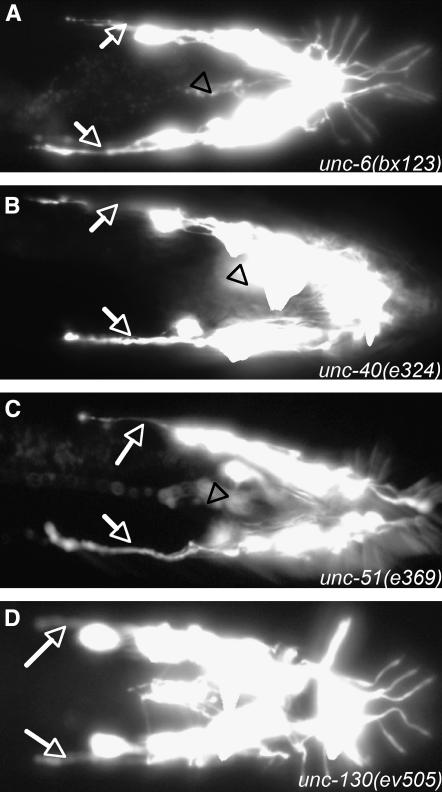
As for other commissures in the C. elegans body that traverse around the body wall from the dorsal to the ventral side, UNC-6/netrin and its cell surface receptor UNC-40/DCC appear to provide the primary dorsoventral guidance system for formation of the ray commissures (Hedgecock et al. 1990; Wadsworth 2002). Two of our ray axon mutations fell respectively in these two genes. In these mutants, the axon of ray 1 grows out from the lumbar ganglion anteriorly as usual and stops around the normal position but fails to make a turn toward the ventral side. The axons of rays 2–5 grow out but migrate anteriorly instead of ventrally, stopping short of the position where the ray 1 axon stops. The axons of rays 8 and 9 are unaffected. The ray axons form a dorsolaterally positioned bundle running anteriorly out of the lumbar ganglion and all the ray commissures except the most posterior commissure are absent (Figure 3, A and B). Prompted by the isolation of new alleles of these two genes, we examined existing alleles and confirmed that they had similar defects in ray axon outgrowth (Table 4).
Figure 3.—
RnB axon defects in unc-6, unc-40, unc-51, and unc-130. pkd-2∷GFP (bxIs14) fluorescence is shown in the respective mutants (ventral views). In unc-6, unc-40, and unc-51, most or all axons extend anteriorly in bundles (arrows). Dorsoventral commissures are absent and there is very little fluorescence in the pre-anal ganglion (arrowhead; compare Figure 2A and Figure 3D). In unc-130, the phenotype is similar but less severe.
TABLE 4.
Ray commissure defects in mutants
| % of ray commissure absence by ray
|
|||
|---|---|---|---|
| Genotype | R1 | R2/3 | R4/5 |
| him-5(e1490) | 0 (28) | 0 (28) | 0 (28) |
| unc-6(bx123) | 86 (44) | 74 (40) | 70 (39) |
| unc-6(ev400) | 88 (124) | 72 (112) | 88 (92) |
| unc-40(bx134) | 82 (56) | 72 (52) | 72 (53) |
| unc-40(e324) | 90 (109) | 73 (104) | 84 (95) |
| unc-51(bx135) | 71 (104) | 70 (98) | 43 (87) |
| unc-51(e369) | 80 (68) | 61 (68) | 57 (60) |
| unc-130(ev505) | 66 (102) | 26 (94) | 11 (87) |
| unc-129(ev557) | 0 (114) | 0 (114) | 0 (114) |
| sax-3(ky123) | 14 (41) | 0 (41) | 0 (41) |
| mab-26(bx80) | 0 (46) | 0 (46) | 0 (46) |
| mab-20(ev574) | 6 (58) | 0 (58) | 0 (52) |
| rax-1(bx126) | 41 (134) | 24 (119) | 8 (106) |
| rax-1(bx132) | 54 (122) | 18 (101) | 9 (92) |
| rax-4(bx139) | 58 (118) | 10 (111) | 1 (108) |
| rax-1(bx132), unc-6(ev400) | 85 (78) | 81 (78) | 80 (75) |
| rax-4(bx139), unc-6(ev400) | 87 (52) | 77 (52) | 80 (52) |
An additional mutation fell in the gene unc-51. The ray axon phenotype of unc-51(bx135), as well as that of the prior existing allele unc-51(e369), was similar to that of unc-6 and unc-40 mutants (Figure 3C, Table 4). unc-51 encodes a serine/threonine kinase demonstrated previously to play a general role in axon outgrowth and cell migration along the anteroposterior body axis (Ogura et al. 1994; Lai and Garriga 2004). A role in dorsoventral guidance has not been reported previously. The similarity of the ray phenotype of unc-51 to those of unc-6 and unc-40 suggests that unc-51 may be a downstream component of the UNC-6 guidance pathway.
We examined the functions of several additional genes shown previously to play roles in axon guidance. We found that unc-130(ev505) causes a mild ray axon defect similar to that seen in unc-6 and unc-40 mutants (Figure 3D, Table 4). For dorsally orientated migrations of gonadal distal tip cells and for dorsoventral guidance of growth cones in formation of the motor neuron commissures, unc-130 acts in a pathway parallel to the UNC-6/netrin guidance pathway (Nash et al. 2000). It encodes a forkhead transcription factor that acts by repressing unc-129 expression in ventral body muscles (Nash et al. 2000). We examined the effect of mutation in unc-129, which encodes a C. elegans TGF-β involved in motor axon guidance (Colavita et al. 1998). Although unc-129(ev554) enhances the ray fusion defect caused by mutation in mab-20/semaphorin and unc-129 is expressed in Rn ray precursor cells and ray neurons (Ikegami et al. 2004), it had no effect on formation of the ray axon commissures. Mutations in several additional genes known to affect guidance also had little or no effect. These genes included slt-1, a repulsive cue, and its receptor, sax-3, mab-26/ephrin, and mab-20/semaphorin (Table 4).
rax-1 and rax-4 are required for the change from anteroposterior to dorsoventral migration:
Among the mutations in putative new genes, mutations in rax-1 and rax-4 were the most specific to the rays and their phenotypes most resembled the phenotypes of unc-6 and unc-40 mutants. The effects of mutations in these two genes were similar, disrupting the dorsoventral migration of some ray axons. The axon of ray 1 was most strongly affected, followed by the axons of rays 2 and 3. More posterior rays were affected weakly (Figure 2, B and C, Tables 3 and 4). Anteriorly directed outgrowth and axon extension appear normal, since the axons of these neurons extend along the dorsolateral side out of the lumbar ganglia and stop around the correct position. There was in addition a low level of ectopic axon outgrowth evident in the adult. In ∼10% of sides, one or two extra neurites extended anteriorly, stopping around the normal position of the ventral turn. The axons of the remaining ray neurons appear to make normal trajectories into the PAG. The phenotype of rax-1(bx132)/sDf56 was similar to that of the rax-1(bx132) homozygote, indicating this is the strong loss-of-function or a null phenotype.
The similarity of the rax-1 and rax-4 mutant phenotypes to the phenotypes of unc-6 and unc-40 mutations raises the question of whether these genes act in the same or in parallel pathways. We found that the already high penetrance (80%) of unc-6 and unc-40 mutations was not increased in double mutants with rax-1 and rax-4 (Table 4). This suggests that the functions of these genes may not be independent and that dorsoventral axon guidance promoted by rax-1 and rax-4 requires the products of unc-6 and unc-40. The gross axonal morphology of non-ray neurons, by contrast, appeared to be normal. Many commissures requiring the unc-6/unc-40 pathway we examined by scoring unc-119∷GFP, including the 19 D-type GABAergic motor neurons, had no defects (data not shown). Therefore, rax-1 and rax-4 appear to be required by ray neuron but not by other neuron growth cones to access or follow the UNC-6/netrin cue.
To determine whether other aspects of ray neuron differentiation in addition to axon guidance were disrupted, we asked whether other differentiated properties of the ray neurons were also affected in rax-1 and rax-4 mutants. Rays have normal morphology and, apart from their axons, neurons appear properly differentiated. The B-type neurons of rays 1, 3, and 9 expressed serotonin normally and the A-type neurons of rays 5, 7, and 9 expressed dopamine correctly (data not shown). These observations indicate that at least some differentiated properties of ray neurons are normal and thus rax-1 and rax-4 could be specific for guidance of ray axons.
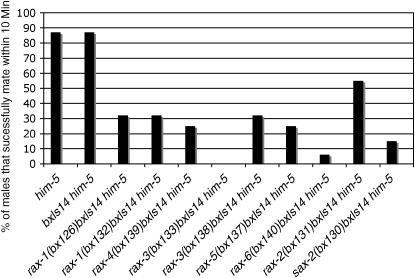
However, examination of male copulatory behaviors suggested that rax-1 and rax-4 mutations had a more severe defect than suggested by the only moderately penetrant aberrant axon pathfinding. While rax-1 males had normal fertility in a mating efficiency test (Hodgkin 1983) (Table 2), close observation revealed at least two defects in their copulatory behavior. Only 31% of rax-1 males successfully mated with hermaphrodites within a 10-min observation period, compared with 87% of wild-type males (Figure 4). Most rax-1 mutant males required more time to stop locomotion and initiate mating (Table 5). rax-4(bx139) males had reduced mating efficiency (Table 2) and only 28% of rax-4(bx139) males successfully mated within a 10-min observation period (Figure 4). In wild-type males, response to contact with the hermaphrodite is mediated through the sensory rays, the V-derived rays (rays 1–6) appearing to be most important (Liu and Sternberg 1995). The delayed response seen in rax-1 and rax-4 mutants thus suggests a functional defect in the V-rays. Rays also have a role in the execution of a turn that males make upon reaching the end of the hermaphrodite during their search for the vulva (Liu and Sternberg 1995). The males of rax-1 and rax-4 mutants display an increased percentage of sloppy or missed turns compared with wild-type males (Table 5). Abnormal turns suggest a defect in the function of rays 7–9, which are specifically required for this sub-behavior (Liu and Sternberg 1995), yet we observed no defects in ray 7–9 neuron morphology. Thus ray neuron function may be compromised in additional ways not suggested by the visible axon defects or possibly other types of neurons are also affected.
Figure 4.—
Mating behavior of rax mutant males. For each genotype, a single male was placed with four paralyzed hermaphrodites and his mating attempts scored (n = 16). A mater was a male that made at least one attempt at mating in a 10-min observation period.
TABLE 5.
Defects in the mating steps of mutant males
| Latencya (min)
|
Ventral turningb
|
|||||
|---|---|---|---|---|---|---|
| Genotype | ≥10 | 5–10 | ≤5 | Good | Sloppy | Missed |
| him-5 | 1 | 1 | 14 | 232 | 0 | 2 |
| bxIs14him-5 | 2 | 2 | 12 | 299 | 5 | 2 |
| rax-1(bx126), bxIs14him-5 | 6 | 4 | 6 | 130 | 10 | 46 |
| rax-1(bx132), bxIs14him-5 | 7 | 5 | 4 | 20 | 0 | 21 |
| rax-4(bx139), bxIs14him-5 | 6 | 5 | 5 | 37 | 2 | 18 |
| rax-3(bx133), bxIs14him-5 | 12 | 4 | 0 | 44 | 0 | 10 |
| rax-3(bx138), bxIs14him-5 | 5 | 8 | 3 | 73 | 2 | 22 |
| rax-5(bx137), bxIs14him-5 | 4 | 8 | 4 | 74 | 11 | 42 |
| rax-5(bx140), bxIs14him-5 | 12 | 2 | 2 | 16 | 0 | 2 |
| sax-2(bx130), bxIs14him-5 | 8 | 4 | 2 | 32 | 12 | 0 |
Latency is a measure of the time required for a tester male to find a hermaphrodite and pause to press its ventral side against the surface of hermaphrodite and initiate backing up to search for the vulva. >10 means that animal was not observed to stop during 10-min observation period.
The total number of turns executed by all tester males (n = 16 for each genotype) during the 10-min observation period is shown.
rax-2 and rax-3 affect specification of anteroposterior positions of ray neuron cell bodies and axons:
In rax-2 and rax-3 mutants, ray axons failed to turn toward the ventral side ∼20% of the time (Figure 2D, Table 3). In addition, ray neuron cell bodies were located at more anterior positions compared to wild type (Figure 2, D and E). In rax-2(bx131) there are additional developmental defects: ray 6 is transformed to ray 4 by morphology and expresses pkd-2∷GFP in the B-type neuron (50%, N = 40), and longitudinal cuticular ridges known as alae are absent in the posterior body region (not shown), where instead there are three or four ectopic pkd-2∷GFP-positive neurons (Figure 2E). This phenotype could come about as a result of transformation of an alae-forming hypodermal cell into a neuroblast cell. Thus, rax-2 may regulate the lineage of seam cells and affect axon development due to cell fate specification errors. In both rax-2 and rax-3 mutants, cell positions or cell fates are shifted toward the anterior, suggesting these mutations may affect an anteroposterior cue along the body or the interpretation of such a cue by ray and hypodermal cells.
Despite the anterior displacement of their cell bodies, most ray neurons successfully extend axons through commissures into the PAG. Commissure formation by misplaced ray neurons provides an opportunity to examine the relationship between the position of a cell and the route that its axon follows. We observed a change in axon pathways in rax-2 and rax-3 mutants. For example, if an R4B neuron is in the normal position of R1B, its axon will follow a trajectory similar to that of R1B in wild-type animals, first migrating anteriorly out of the lumbar ganglion for some distance before turning to go circumferentially around the body and returning into the PAG from the anterior (Figure 5). One possible reason for the change of pathway is that the axon growth cone followed local cues that depended on the position of the cell. However, it is also possible that ray identity is altered in these mutants, explaining both misposition of the cell body and alteration in axon pathway.
Figure 5.—
Axon trajectory is influenced by the position of the cell body. In rax-3(bx138), axons of neurons with cell bodies at more anterior positions (R1B-3B) send their axons anteriorly (arrowhead), sometimes turning abnormally (e.g., in the animal shown there is a turn to the dorsal side). The cell body of R4B is located at the normal position of R1B (compare to wild type in bottom image) and its axon follows the expected pathway of the R1B axon in the wild type (arrow).
rax-5 and rax-6 are required for both axon guidance and ray morphology:
The mutations rax-5(bx137) and rax-6(bx140) affect ray morphology in addition to axon guidance. Both mutations have multiple defects in axonal morphology, including ectopic outgrowth, dorsoventral guidance errors, and anterior migration defects (Figure 2, F and G, Table 3). In rax-5(bx137), the ray morphology defects correspond to fusion of rays scored as ray1/2/3, ray1/2, ray 2/3/4, or ray 3/4 (28%, N = 225 sides). rax-6(bx140) animals also have fusions involving ray 1, 2, 3, and 4 (70%, N = 124 sides) (data not shown). Ray fusion indicates rax-5 and rax-6 mutations may affect differentiated properties of the structural cell as well as the sensory neurons (Zhang and Emmons 1995). Hence these mutations may be ray identity mutations in which the properties of multiple cells of the ray sublineage are affected. Interestingly, in rax-5 and rax-6 mutants the ray cell bodies are positioned in the lumbar ganglia differently from wild type, the cell bodies of neurons of fused rays often forming clusters (Figure 2, F and G). Similar to other rax genes, these mutations dramatically reduced male mating ability (Figure 4, Table 5).
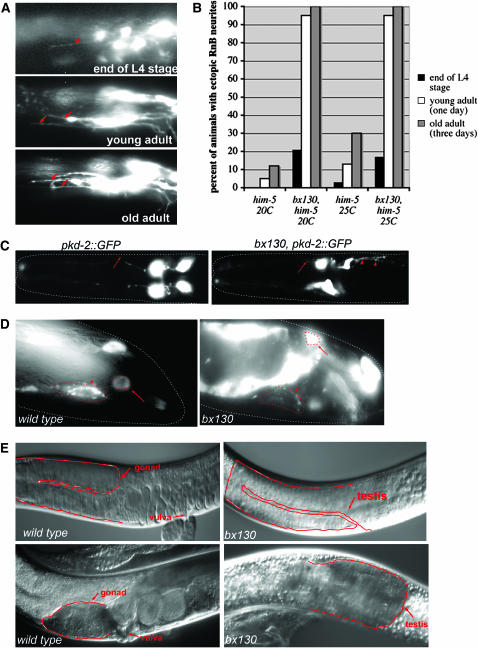
sax-2 and sax-1 are required for inhibiting persistent outgrowth of ray axons during adulthood:
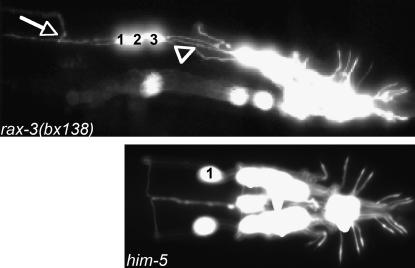
In sax-2(bx130), ray neurons send out axons that grow normally and form commissures to the PAG. In addition, they grow ectopic neurites that extend for varying distances. These aberrant outgrowths continue to form during the adult stage (Figure 2H, Table 2), becoming continuously more abundant as the animals age (Figure 6, A and B). Ectopic extensions behave like axons in growing anteriorly, but they fail to fasciculate or turn toward the ventral side. We examined additional neurons and found that the four male-specific CEM neurons of the head, visualized with pkd-2∷GFP, also have several abnormal posterior processes (Figure 6C). The mutation bx130 mapped to and failed to complement the previously described gene sax-2. We showed that bx130 was rescued by the predicted gene F21H11.2, consistent with the results reported at the same time by Gallegos and Bargmann (2004) (materials and methods). F21H11.2 encodes a large conserved protein with HEAT/Armadillo repeats.
Figure 6.—
sax-2(bx130) mutant phenotypes. (A and B) Ectopic neurites continue to grow out from ray neuron cell bodies as the animals age. (C) CEM neurons in the head have normal dendritic processes (arrows) but extend ectopic neurites (arrowheads). (D) Fluorescence of SNB-1∷GFP is decreased in the pre-anal ganglion (arrow) and increased in the ray neuron cell body (arrow). (E) Gonad morphology in sax-2(bx130) mutants, Nomarski photomicrographs. The head is to the left. In both sexes, in sax-2(bx130) the gonad may fail to extend and instead forms a bulb-like structure.
sax-2 functions with sax-1, an NDR ser/thr kinase, in the maintenance of amphid neuronal morphology and mechanosensory neurite termination and tiling (Zallen et al. 1999; Gallegos and Bargmann 2004). Their Drosophila homologs, trc and fry, regulate the dendritic branching and tiling of Drosophila sensory neurons (Emoto et al. 2004). We found that sax-1(ky211) causes ectopic processes of ray axons similar to those in sax-2 mutants (data not shown), suggesting that sax-1 and sax-2 function together to regulate ray axon morphology as they do for other sensory neurons. Interestingly, the unassigned mutation bx141 also causes the production of ectopic processes at late stage, albeit weakly (Table 3). The similarity of sax-2(bx130) and bx141 mutant phenotypes suggests that bx141 either is a weak allele of sax-1 or sax-2 or may define an additional gene in this pathway.
Previous studies found little or no apparent disruption of neuron function in sax-2 and sax-1 mutants, consistent with a role in a maintenance pathway with little consequence for neuron activity (Zallen et al. 1999). However, we found that sax-2(bx130) males were defective in mating, suggesting ray neuron function was compromised (Table 2, Figure 4). We therefore examined the density of presynaptic vesicles of ray neurons in the PAG by scoring the expression of a PKD-2 promoter-driven SNB-1∷GFP fusion protein (bxEx94) (Nonet 1999). SNB-1 encodes C. elegans synaptobrevin, a synaptic vesicle protein expressed at synaptic sites (Nonet 1999). The presence of the sax-2(bx130) mutation dramatically reduced the density of GFP puncta in the PAG in the adult, suggesting a reduced density of synaptic vesicles (Figure 6D). Thus sax-2 gene function may be necessary for normal synaptogenesis of the ray neurons.
Interestingly, a significant percentage of sax-2 animals also exhibit abnormal gonad morphology in both males and hermaphrodites. This phenotype was present for both alleles, bx130 (22%, n = 148) and ky216 (21%, N = 86, 25°). In affected animals, the normally two-armed hermaphrodite gonad has either only the anterior arm or only the posterior arm. In the male, the testis fails to extend and forms a large bulb in the middle of the body (Figure 6E). These observations suggest that sax-2 may also play a role in gonad morphogenesis.
Correct muscle attachment is necessary for formation of normal axon pathways:
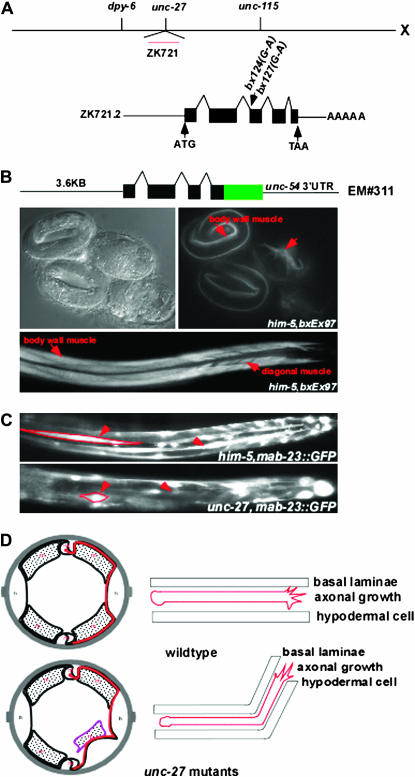
Two mutations, bx124 and bx127, were mapped to the gene unc-27. These mutations result in the wandering of a large number of axons in addition to ray neuron axons (Figure 2I, Table 2). A single predicted ORF (ZK721.2) was identified and shown to be capable of rescuing both unc-27(bx124) and unc-27(bx127) (materials and methods). Analysis of the rescuing ORF indicated that unc-27 encodes an isoform of troponin I, TnI-2. bx124 and bx127 have an identical missense mutation, which changes the intron 4 (18341 on ZK721) 3′-splice acceptor ttttcaG to ttttcaA (Figure 7A). Three other mutant alleles (e155, su142, and su195) were crossed to bxIs14 and found to exhibit a similar axon-wandering defect (data not shown).
Figure 7.—
Muscle and axon defects in troponin I mutants. (A) bx124 and bx127 failed to complement unc-27(e155) and were rescued by cosmid ZK721. Both mutations consist of the same G-to-A mutation in a splice acceptor sequence of the predicted gene ZK721.2, which encodes troponin I and is thereby identified as unc-27. (B) Expression of an unc-27∷GFP reporter gene in embryonic and adult body-wall muscles and in adult male diagonal muscles. (C) Mutant muscle structure in unc-27. Normally elongated body-wall muscles have shortened, abnormal structures (arrowheads). (D) The ray neuron and other axons follow commissural pathways between the basal surface of the hypodermis and the underlying basement membrane. Distortion of these pathways in muscle mutants results in apparent wandering axon pathways.
In unc-27 mutants, axons grew out, but most did not appear to follow their correct pathways to the ventral PAG (Figure 2I). We found that all circumferential axons in the body labeled by the pan-neuronal marker unc-119∷GFP and the motor neurons labeled by unc-47∷GFP and glr-1∷GFP also exhibit wandering axons (data not shown). These observations indicate that unc-27 is likely to affect the migration of a wide variety of axons.
Troponin I is an inhibitory subunit of the troponin complex, which binds to tropomyosin fibrils and regulates calcium-dependent muscle contraction. In view of the unexpected finding of a role of a contractile protein in axon migration from the screening, we further investigated unc-27/TnI-2 function in C. elegans. In the adult, unc-27∷gfp (bxEx97), a rescuing reporter, is expressed exclusively in body-wall muscles, inner and outer longitudinal muscles, anal depressor, sphincter, and male-specific diagonal muscles. GFP expression is first detected in the threefold embryo in embryonic body-wall muscles and is maintained after hatching into the adult stage (Figure 7B). Mosaic analysis with unc-27(bx124) carrying both unc-27∷gfp (bxEx97) and pkd-2∷gfp in an extrachromosomal array showed that non-Unc animals containing unc-27∷GFP-positive muscles and neurons had wild-type axon trajectories (N = 106), whereas Unc animals that lost GFP in the muscles but retained it in the neurons had abnormal axon trajectories (N = 9). Thus unc-27 function in muscle is necessary to rescue the axon guidance defect. These observations prompted us to examine muscle structure in unc-27 mutants. By using reporters mab-23∷gfp (Figure 7C) (Lints and Emmons 2002) and ser-2∷gfp (Tsalik et al. 2003), as well as polarized light microscopy (data not shown), we found that both body-wall muscles and male-specific diagonal muscles are malformed in unc-27 mutants. Sarcomeric structure is abnormal and the position of dense bodies is often distorted (Mup phenotype) (T. Allen, personal communication). We conclude that the apparent wandering of axons is due to a defect in muscle, in particular, muscle misattachment that results in a physical deformation of the basal surface of the epidermis along which axons extend (Figure 7D).
We were interested in whether inactivation of other muscle genes might also disrupt axon pathways and cause apparent wandering of ray axons. We first performed RNAi to knock down the other TnI isoforms in the C. elegans genome. RNA interference of TnI-1(F42E11.4) but not TnI-3 (T20B3.2) or TnI-4 (W03F8.1) led to 36% of ray axon defects (N = 139). Similar to UNC-27/TnI-2, TnI-1 is expressed in body-wall muscles and male diagonal muscles, while TnI-3 and TnI-4 mRNA are found in vulva muscles and pharyngeal muscles, respectively (T. Allen, personal communication). We found that mup-2(e2346ts) and pat-10(st568), which encode the other two subunits, TnT and TnC, of the troponin complex, respectively, cause muscle mispositioning and axon wandering (data not shown). However, ray axons are normal in lev-12 (tropomyosin), unc-22 (twitchin), unc-15 (paramyosin), myo-3 (myosin A), unc-60 (destin), unc-87 (calponin), and unc-54 (myosin B) mutants, which were previously demonstrated to disrupt the assembly of sarcomeres but not muscle positioning or attachment (Moerman and Williams 2005). These results, together with other studies of mutations in mua (muscle attachment) and mup (muscle positioning) genes (Myers et al. 1996; McArdle et al. 1998; Plenefisch et al. 2000; Shioi et al. 2001), suggest that establishing muscle attachment to the correct hypodermal locations during development is critical for formation of the pathways that axon growth cones follow in their migrations.
DISCUSSION
Axon pathfinding by the ray neurons:
In projecting axons along stereotyped pathways into a central neuropil, the C. elegans male ray sensory neurons resemble the receptor neurons of sensory organs in other animals, such as retinal photoreceptor neurons or olfactory neurons of vertebrates or olfactory neurons of Drosophila (e.g., Wang et al. 1998; O'Leary et al. 1999; Vosshall et al. 2000). A number of lines of evidence suggest that the male's copulatory fan, which contains the rays, acts as a tactile and olfactory organ. In this organ, the nine bilateral pairs of rays function collectively to convey topographic information about the male's contact with the hermaphrodite. Each ray is situated at a specific, genetically specified location within the fan and contributes differentially to the multiple steps of copulation according to its position (Sulston et al. 1980; Baird et al. 1991; Liu and Sternberg 1995). Although the ray neurons are essentially of only two ultrastructural types (Sulston et al. 1980), ray neurons of a single type in different rays are molecularly differentiated and synapse onto different postsynaptic target cells (Chow and Emmons 1994; Troemel et al. 1995; Zhang and Emmons 1995; Salser and Kenyon 1996; Ferreira et al. 1999; Lints and Emmons 1999; Portman and Emmons 2000; Lints et al. 2004; M. Xu, D. H. Hall and S. W. Emmons, unpublished results). With these properties, the ray neurons provide an opportunity within a relatively simplified system to study the problem of topographic mapping of sensory neurons into the next level of the circuitry where information processing is carried out (Udin and Fawcett 1988).
We initiated a study of the problem of ray topographic mapping by identifying mutants in which the axon pathways of the ray neurons were disrupted. We identified and characterized 16 mutations defining at least 12 genes that affect the ability of the ray neurons to establish normal axon trajectories into the PAG. Some of the genes we identified are already known to have a role in axon guidance, indicating that ray axon growth cones utilize some of the same cues followed by other neurons. We showed that ray axon pathway was independent of some other known cues. Mutations in the presumptively new genes we identified have effects confined more specifically to the ray neurons, possibly explaining why they have not been identified previously. Two of them, rax-1 and rax-4, caused no detectable defects in other neurons. The remaining four new genes affect ray development as well as ray neuron pathfinding.
The ray axon growth cones follow multiple pathways from the lumbar ganglia to the PAG. They can either pioneer a route or fasciculate with a previously established nerve or commissure. The routes navigated consist of a series of segments punctuated by choice points (Tessier-Lavigne and Goodman 1996). These segments reveal the presence of at least two global signals, a dorsoventral signal and an anteroposterior signal. The evidence is against the presence of labeled pathways specific for each ray axon, because the precise routes navigated vary somewhat from animal to animal. In a mutant in which cell bodies were shifted anteriorly, the route navigated shifted accordingly, suggesting that growth cones could respond to and follow different local cues, although a change in this mutant in the ray neurons themselves affecting pathway choice cannot be ruled out. While the set of ray neurons of each type appears to consist of nine subtypes differing from each other with respect to neurotransmitters, receptors, and postsynaptic targets, the response to cues guiding growth cones to the PAG could be the same for all subtypes (Troemel et al. 1995; Lints et al. 2004; M. Xu, D. H. Hall and S. W. Emmons, unpublished results).
Growth cones of ray neurons with cell bodies lying in the midregion of the lumbar ganglion respond immediately to the dorsoventral cue, whereas those of ray neurons with cell bodies lying in the anterior region of the lumbar ganglion first respond to the anteroposterior signal, then to the dorsoventral signal, before fasciculating with the ventral cord. The pathways of the axons originating from ray neurons in the posterior region of the lumbar ganglion (rays 8 and 9) appear to be determined by their fasciculation with an existing nerve, the lumbar commissure. For those growth cones that respond first to the anteroposterior signal and then to the dorsoventral signal, the change in responsiveness is abrupt and apparently complete. This suggests that there is a switching mechanism that acts at the choice point to alter cell behavior. The switch might occur when the migrating growth cone encounters an existing dorsoventral commissure or there could be a broadly localized physical cue. Alternatively, cell behavior might be changed by an external or internal timing mechanism, such as that which underlies the turns made by the migrating gonadal distal tip cell (Antebi et al. 1998).
These properties are similar to those of other neurons that have processes following circumferential pathways around the C. elegans body wall. The axons of a number of motor and sensory neurons pioneer commissures that are located at variable positions according to cell body location (Hedgecock et al. 1990). If the dorsoventral cue (the UNC-6/netrin pathway) is lost, these axons may instead migrate along the anteroposterior axis, revealing an alternative response to an anteroposterior cue. In addition, many of these neurons fasciculate with preexisting nerves. Availability of alternative guidance mechanisms, including fasciculation, means that loss of a particular guidance cue affects different neurons to varying extents.
The UNC-6/netrin-UNC-40/DCC guidance pathway provides the primary dorsoventral guidance cue for the ray axons:
The primary dorsoventral guidance cue the ray neurons appear to follow is the UNC-6/netrin-UNC-40/DCC signaling pathway. Thus the ray neurons are similar to other embryonic and postembryonic motor and sensory neurons that follow commissures around the body wall between the dorsal and ventral sides (Hedgecock et al. 1990; Wadsworth, 2002). In unc-6 or unc-40 mutants, ray axons that first migrate anteriorly continue to do so, stopping at or near the normal position but failing to turn toward the ventral side. Further, ray axons that normally migrate directly around the dorsoventral axis instead migrate anteriorly. The latter observation indicates that the anteroposterior cue extends through the lumbar ganglia but normally may be ignored by the growth cones of the more posterior rays, possibly because its strength relative to the dorsoventral cue is weaker there. We investigated whether one or more male-specific neurons in the PAG contributed an important attractive cue to the ray axons. We ablated the precursors to these neurons (blast cells B, Y, U, F, and P10–12 individually or in combination) but found no effects on the ray axon trajectories (Jia 2006).
Among the other known genes we tested, the involvement of unc-51 was unexpected since this gene had previously been implicated only in navigation along the anteroposterior body axis (Lai and Garriga 2004). UNC-51, the conserved serine/threonine kinase, generally is involved in the axon outgrowth and CAN axon migration along the anteroposterior axis. Two other genes, vab-8 and unc-14, act as substrates of unc-51 to direct CAN axon guidance posteriorly. It will be of interest to examine the role of unc-14 and vab-8 in ray axon guidance to determine whether the same pathway functions in the dorsoventral migration of ray axons. unc-130 had been shown to act in a pathway parallel to UNC-6/UNC-40 by repressing expression of the TGF-β family growth factor gene unc-129. Its role in guidance of the ray neurons may be through a different mechanism, because mutation in unc-129 had no effect on the ray axons, although it is required for normal formation of the rays (Colavita et al. 1998; Nash et al. 2000; Ikegami et al. 2004). However, UNC-129 might be redundant with another gene in ray axon guidance, the unc-130 phenotype being due to ectopic unc-129 expression. Likewise, mab-20/semaphorin and efn-1/ephrin are required for normal morphology of the rays (Ikegami et al. 2004) but mutations in these genes had no effect on the ray axons.
rax-1 and rax-4 are required for dorsoventral migration of ray axons:
Among the putative new genes we identified, mutations in rax-1 and rax-4 were most similar to mutations in unc-6 and unc-40 in having an effect on the ray axons confined to their dorsoventral migration. Outgrowth of the axon terminals stops at or near the correct position, suggesting the defect is not in sensing a physical or temporal signal to turn but is in following the dorsoventral cue. Since the phenotype of an unc-6 mutation was not enhanced in double mutant combination with rax-1 or rax-4 (admittedly a weak test given the already high penetrance of the unc-6 phenotype), axon guidance promoted by rax-1 and rax-4 appears to require the function of the UNC-6/UNC-40 pathway. Since mutations in these genes had no effects on other neurons, they may act cell autonomously within the ray neurons, possibly allowing the ray neurons to access the UNC-6/UNC-40 pathway. Mutations in these genes affected several aspects of male mating behavior related to ray function in a way suggesting that ray neuron function is more severely compromised than expected from the overt axon defects. rax-1 and rax-4 may therefore be regulatory genes required for expression of several differentiated characteristics of the ray neurons, including axon guidance. A low but significant level of ectopic neurite outgrowth in these mutants suggests there might be a defect in synaptogenesis.
rax-2 and rax-3 affect anteroposterior signaling or specification:
In rax-2 and rax-3 mutants, in addition to abnormalities in the ray commissures, cell positions and cell fates were shifted along the anteroposterior body axis. Previous studies have implicated the Wnt pathway, through its effects on expression of Hox genes, in specification of anteroposterior cell migrations and cell fates along the a/p axis (see Eisenmann 2005 for review). For example, migrations of the progeny of the Q neuroblast are directed to the anterior in Wnt pathway loss-of-function mutants and toward the posterior in Wnt pathway gain-of-function mutants through loss or gain of activation of the Hox gene mab-5. Similarly through activation of mab-5 expression, Wnt pathway gain-of-function mutation or global gene repressor sop-2 loss-of-function mutation results in an anterior-to-posterior fate transformation of seam hypodermal cells to ray neuroblasts, similar to that seen in rax-2(bx131). Indeed, we have found that a reporter gene for the Hox gene egl-5 fails to be expressed in the ray lineages in rax-2(bx131), implicating a role of rax-2 in regulation of Hox gene expression (H. Zhang and S. W. Emmons, unpublished observations). In an egl-5 loss-of-function mutation, ray axons fail to turn toward the ventral side similar to that seen in rax-2(bx131), so this phenotype might be a consequence of loss of egl-5 expression (L. Jia and S. W. Emmons, unpublished observations). However, anterior displacement of cell bodies and anterior ectopic ray neurons are not seen in egl-5 loss-of-function mutants, but rather suggest a mab-5 gain-of-function phenotype. These novel properties suggest that rax-2 and rax-3 may define new functions in regulation of Hox gene expression.
rax-5 and rax-6 affect ray identity:
In mutations of rax-5 and rax-6, along with axon defects there were fusions of rays. Ray formation requires the ray structural cell. The ray fusion phenotype is presumed to arise when the structural cells of adjacent rays lose specificity determinants required to keep the rays separated (Sulston et al. 1980; Zhang and Emmons 1995). Prior genes identified with ray fusion mutant phenotypes include genes encoding effector proteins, such as semaphorin (mab-20) and ephrin (mab-26/efn-1), that are involved directly in cell–cell interactions (Roy et al. 2000; Chin-Sang et al. 2002; Ikegami et al. 2004). A second class encodes regulatory proteins, including transcription factors and cell-signaling pathways, that define distinct ray identities and specify differential characteristics of all three ray cell types (Chow and Emmons 1994; Zhang and Emmons 1995; Lints and Emmons 1999). rax-5 and rax-6 could be genes of either type. Our finding that not only are rays fused in rax-5 and rax-6 mutants but also cell bodies appear to cluster in the lumbar ganglion indicates that despite the variability of their arrangement from animal to animal, the positions of the ray cell bodies in the lumbar ganglion are determined somewhat by an active mechanism involving attraction or repulsion between cells.
sax-2 and sax-1 are required for both inhibition of neurite outgrowth and formation of synapses:
The SAX-1 kinase and the HEAT/Armadillo repeat protein SAX-2 are conserved proteins that function in multiple cellular pathways with a variety of effects on cellular architecture. These include pathways involved in asymmetric cell division in budding yeast (Colman-Lerner et al. 2001), branching of hyphae in Neurospora (Yarden et al. 1992), growth and morphology of bristles and wing hairs in Drosophila (Geng et al. 2000; Cong et al. 2001), branching and outgrowth of neurites in C. elegans and Drosophila (Zallen et al. 2000; Emoto et al. 2004), and tiling of sensory dendrites in C. elegans and Drosophila (Emoto et al. 2004; Gallegos and Bargmann 2004). The ectopic neurite outgrowth phenotype we observed for ray neurons is very similar to that observed for amphid sensory neurons in the C. elegans head (Zallen et al. 2000). Ectopic neurite outgrowth suggests that sax-2 and sax-1 may participate in a pathway that stops nerve cell outgrowth once correct synapses are formed. Our observation of incomplete SNB-1∷GFP localization to the PAG suggests that aspects of synaptogenesis itself may lie downstream of the sax-2/sax-1 pathway. Synaptobrevin is a component of synaptic vesicles localized to the axon terminal in processes that are independent of other aspects of synaptogenesis, such as formation of the presynaptic density (Jin 2005). This suggests a model in which a retrograde signal initiated by early steps of a pathway of synapse formation signals through sax-2/sax-1 to both stop neurite outgrowth and promote later steps of synaptogenesis, such as assembly of synaptic vesicles at the axon terminal. Ray or possibly other male-specific neurons are apparently more dependent on this process than other types of neurons because, in contrast to other aspects of worm behavior, male mating was disrupted in sax-2 and sax-1 mutants (this work; Zallen et al. 2000). An alternative hypothesis to explain the loss of synaptobrevin localization to the PAG in sax-2(bx130) is that sax-2 might be necessary for axon branching. Upon entering the PAG, ray axons branch repeatedly in their apparent search for appropriate synaptic targets (M. Xu, D. H. Hall and S. W. Emmons, unpublished results). If unable to branch, ray axons might fail to reach their targets and hence form fewer synapses.
Muscle attachment mutations distort the pathways that axons travel to their targets:
Finally, we identified a class of mutations that cause apparent axon misguidance by distorting the substrate along which axonal growth cones make their way to their targets. Previous studies have reached a similar conclusion (Shioi et al. 2001). In C. elegans, as in other organisms, axons migrate along the surface of the basal lamina underlying the hypodermis. The shape of the body wall, influenced by the attachment between body-wall muscles and hypodermis, thus determines the geometry of the axon pathways. In wild-type animals, the myofilaments of body-wall muscles attach to the hypodermis through multiple dense bodies distributed along the boundary between the muscle cells and hypodermis and are then anchored to the cuticle (Moerman and Fire 1997). Other studies have shown that mutants with defects in muscle attachment (mua) or muscle positioning (mup) also exhibit a displacement or detachment of nerves (Shioi et al. 2001). For example, the ventral nerve cord detachment in mup-2/TnT mutant appears to be a secondary consequence of muscle defects, which arise during development (Myers et al. 1996; McArdle et al. 1998; Shioi et al. 2001).
Along with TnI/UNC-27, other troponin components (TnC and TnT) are necessary for the normal development of muscle and hypodermis (Myers et al. 1996; McArdle et al. 1998). Defects in all these proteins cause abnormal positioning of muscles and the detachment of muscle from the basal lamina and hypodermis, which leads to the displacement of basal lamina. Particularly in the male tail, this attachment between body-wall muscles, diagonal muscles, and hypodermis may be crucial for setting up the pathways of axons, including ray axons. However, muscle disruption might also have an effect by altering the distribution of secreted or substrate-associated attractive and repulsive guidance cues that regulate axon attachment and migration.
Acknowledgments
We thank T. Allen, O. Hobert, E. M. Jorgensen, and M. Barr for sharing reporters and unpublished data; Sanger Center (Cambridge, UK) for cosmids; Caenorhabditis Genetics Center (funded by National Institutes of Health National Center for Research Resources) for strains; members of the Emmons laboratory for assistance and guidance; D. H. Hall, R. Lints, and Z. Kaprielian for their comments on the manuscript. S.W.E. is the Siegfried Ullmann Professor of Molecular Genetics. This work was funded by National Institute of Health grants R01 GM-39353, R01 NS-30986 and R01 GM-066897 to S.W.E.
References
- Antebi, A., J. G. Culotti and E. M. Hedgecock, 1998. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development 125: 1191–1205. [DOI] [PubMed] [Google Scholar]
- Baird, S. E., D. A. Fitch, I. A. A. Kassem and S. W. Emmons, 1991. Pattern formation in the nematode epidermis: determination of the arrangement of peripheral sense organs in the C. elegans male tail. Development 113: 515–526. [DOI] [PubMed] [Google Scholar]
- Barr, M. M., and P. W. Sternberg, 1999. A polycystic kidney disease gene homologue required for male mating behavior in C. elegans. Nature 401: 339–340. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broverman, S. A., and P. M. Meneely, 1994. Meiotic mutants that cause a polar decrease in recombination on the X chromosome in Caenorhabditis elegans. Genetics 136: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, S. J., and J. Dodd, 2003. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron 38: 389–401. [DOI] [PubMed] [Google Scholar]
- Charron, F., E. Stein, J. Jeong, A. P. McMahon and M. Tessier-Lavigne, 2003. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113: 11–23. [DOI] [PubMed] [Google Scholar]
- Chin-Sang, I. D., S. L. Mosley, M. Ding, R. J. Harrington, S. E. George et al., 2002. The divergent C. elegans ephrin EFN-4 functions inembryonic morphogenesis in a pathway independent of the VAB-1 Eph receptor. Development 129: 5499–5510. [DOI] [PubMed] [Google Scholar]
- Chow, K. L., and S. W. Emmons, 1994. HOM-C/Hox genes and four interacting loci determine the morphogenetic properties of single cells in the nematode male tail. Development 120: 2579–2592. [DOI] [PubMed] [Google Scholar]
- Colavita, A., and J. G. Culotti, 1998. Suppressors of ectopic UNC-5 growth cone steering identify eight genes involved in axon guidance in Caenorhabditis elegans. Dev. Biol. 194: 72–85. [DOI] [PubMed] [Google Scholar]
- Colavita, A., S. Krishna, H. Zheng, R. W. Padgett and J. G. Culotti, 1998. Pioneer axon guidance by UNC-129, a C. elegans TGF-β. Science 281: 706–709. [DOI] [PubMed] [Google Scholar]
- Colman-Lerner, A., T. E. Chin and R. Brent, 2001. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 107: 739–750. [DOI] [PubMed] [Google Scholar]
- Cong, J., W. Geng, B. He, J. Liu, J. Charlton et al., 2001. The furry gene of Drosophila is important for maintaining the integrity of cellular extensions during morphogenesis. Development 128: 2793–2802. [DOI] [PubMed] [Google Scholar]
- Dickson, B. J., 2002. Molecular mechanisms of axon guidance. Science 298: 1959–1964. [DOI] [PubMed] [Google Scholar]
- Durbin, R. M., 1987. Studies on the development and organization of the nervous system of Caenorhabditis elegans. Ph.D. Thesis, Cambridge, UK.
- Eisenmann, D. M., 2005. Wnt signaling, in WormBook, edited by The C. elegans Research Community (doi/10.1895/wormbook.1.7.1; http://www.wormbook.org).
- Emoto, K., Y He, B. Ye, W. B. Gruober, P. N. Adler et al., 2004. Control of dentritic branching and tiling by the tricornered-kinase/furry signaling pathway in Drosophila sensory neurons. Cell 119: 245–256. [DOI] [PubMed] [Google Scholar]
- Feinstein, P., T. Bozza, I. Rodriguez, A. Vassalli and P. Mombaerts, 2004. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell 117: 833–846. [DOI] [PubMed] [Google Scholar]
- Ferreira, H. B., Y. Zhang, C. Zhao and S. W. Emmons, 1999. Patterning of Caenorhabditis elegans posterior structures by the Abdominal-B homolog, egl-5. Dev. Biol. 207: 215–228. [DOI] [PubMed] [Google Scholar]
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver et al., 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811. [DOI] [PubMed] [Google Scholar]
- Gallegos, M. E., and C. I. Bargmann, 2004. Mechanosensory neurite termination and tiling depend on SAX-2 and the SAX-1 kinase. Neuron 44: 239–249. [DOI] [PubMed] [Google Scholar]
- Geng, W., B. He, M. Wang and P. N. Adler, 2000. The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics 156: 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato, M., H. Schnabel and R. Schnabel, 1994. pha-1, a selectable marker for gene transfer in C. elegans. Nuleic Acids Res. 22: 1762–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, D. H., and R. L. Russell, 1991. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J. Neurosci. 11: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock, E. M., J. G. Culotti, J. N. Thomson and L. A. Perkins, 1985. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111: 158–170. [DOI] [PubMed] [Google Scholar]
- Hedgecock, E. M., J. G. Culotti and D. H. Hall, 1990. The unc-5, unc-6, unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C.elegans. Neuron 2: 61–85. [DOI] [PubMed] [Google Scholar]
- Henley, J. R., K. H. Huang, D. Wang and M. M. Poo, 2004. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron 44: 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert, O., 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730. [DOI] [PubMed] [Google Scholar]
- Hodgkin, J., 1983. Male phenotypes and mating efficiency in Caenorhabditis elegans. Genetics 103: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter, H., I. Wacker, C. Schmid and E. M. Hedgecock, 2005. Novel genes controlling ventral cord asymmetry and navigation of pioneer axons in C. elegans. Dev. Biol. 284: 260–272. [DOI] [PubMed] [Google Scholar]
- Ikegami, R., H. Zheng, S. Ong and J. G. Culotti, 2004. Integration of Semaphorin-2A/MAB-20, epgrin-4 and UNC-129 TGF-β signaling pathways regulates sorting of distinct sensory rays in C. elegans. Dev. Cell 6: 383–395. [DOI] [PubMed] [Google Scholar]
- Jia, L., 2006. Genetic mechanisms for axonal development of ray sensory neurons and behaviors in the C. elegans male. Ph.D. Thesis, Albert Einstein College of Medicine, Yeshiva University, New York.
- Jin, Y., 2005. Synaptogenesis, in WormBook, edited by The C. elegans Research Community (http://www.wormbook.org).
- Lai, T., and G. Garriga, 2004. The conserved kinase UNC-51 acts with VAB-8 and UNC-14 to regulate axon outgrowth in C. elegans. Development 131: 5991–6000. [DOI] [PubMed] [Google Scholar]
- Lints, R., and S. W. Emmons, 1999. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFβ family signaling pathway and a Hox gene. Development 126: 5819–5831. [DOI] [PubMed] [Google Scholar]
- Lints, R., and S. W. Emmons, 2002. Regulation of sex-specific differentiation and mating behavior in C. elegans by a new member of the DM domain transcription factor family. Genes Dev. 16: 2390–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints, R., L. Jia, K. Kim, C. Li and S. W. Emmons, 2004. Axial patterning of C. elegans male sensilla identities by selector genes. Dev. Biol. 269: 137–151. [DOI] [PubMed] [Google Scholar]
- Liu, K. S., and P. W. Sternberg, 1995. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14: 1–20. [DOI] [PubMed] [Google Scholar]
- Loer, C. M., and C. J. Kenyon, 1993. Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans. J. Neurosci. 13: 5407–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyuksyutova, A. I., C. C. Lu, N. Milanesio, L. A. King, N. Guo et al., 2003. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302: 1903–1904. [DOI] [PubMed] [Google Scholar]
- Maduro, M., and D. Pilgrim, 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle, K., T. S. Allen and E. A. Bucher, 1998. Ca2+-dependent muscle dysfunction caused by mutation of the Caenorhabditis elegans troponin T-1 gene. J. Cell Biol. 143: 1201–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire, S. L., G. Garriga, J. White, D. Jacobson and H. R. Horvitz, 1992. Genes necessary for directed axonal elongation or fasciculation in C. elegans. Neuron 8: 307–322. [DOI] [PubMed] [Google Scholar]
- McIntire, S. L., R. J. Reimer, K. Schuske, R. H. Edwards and E. M. Jorgensen, 1997. Identification and characterization of the vesicular GABA transporter. Nature 389: 870–876. [DOI] [PubMed] [Google Scholar]
- Mello, C., and A. Fire, 1995. DNA transformation. Methods Cell Biol. 48: 451–482. [PubMed] [Google Scholar]
- Moerman, D. G., and B. Williams, 2005. Sarcomere assembly in C. elegans muscle, in WormBook, edited by The C. elegans Research Community (http://www.wormbook.org). [DOI] [PMC free article] [PubMed]
- Moerman, D. G., and A. Fire, 1997. Muscle: structure, function and development, pp. 417–470 in C. elegans II, edited by W. B. Wood and The C. elegans Research Community. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Morck, C., C. Axang and M. Pilon, 2003. A genetic analysis of axon guidance in the C. elegans pharynx. Dev. Biol. 260: 158–175. [DOI] [PubMed] [Google Scholar]
- Mueller, B. E., 1999. Growth cone guidance: first steps towards a deeper understanding. Annu. Rev. Neurosci. 22: 351–388. [DOI] [PubMed] [Google Scholar]
- Myers, C. D., P. Y. Goh, T. S. Allen, E. A. Bucher and T. Bogaert, 1996. Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J. Cell Biol. 132: 1061–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash, B., A. Colavita, H. Zheng, P. J. Roy and J. G. Culotti, 2000. The forkhead transcription factor UNC-130 is required for the graded spatial expression of the UNC-129 TGF-beta guidance factor in C. elegans. Genes Dev. 14: 2486–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet, M. L., 1999. Visualization of synaptic specifications in live C. elegans with synaptic vesicle protein-GFP fusions. J. Neurosci. Methods 89: 33–40. [DOI] [PubMed] [Google Scholar]
- Ogura, K., C. Wicky, L. Magnenat, H. Tobler, I. Mori et al., 1994. Caenorhabditis elegans unc-51 gene required for axonal elongation encodes a novel serine/threonine kinase. Genes Dev. 8: 2389–2400. [DOI] [PubMed] [Google Scholar]
- O'Leary, D. D. M., P. A. Yates and T. McLaughlin, 1999. Molecular development of sensory maps: representing sights and smells in the brain. Cell 96: 255–269. [DOI] [PubMed] [Google Scholar]
- Plenefisch, J., X. Zhu and E. M. Hedgecock, 2000. Fragile skeletal muscle attachments in dystrophic mutants of Caenorhabditis elegans: isolation and characterization of the mua genes. Development 127: 1197–1207. [DOI] [PubMed] [Google Scholar]
- Portman, D. S., and S. W. Emmons, 2000. The basic helix-loop-helix transcription factors LIN-32 and HLH-2 function together in multiple steps of a C. elegans neuronal sublineage. Development 127: 5415–5426. [DOI] [PubMed] [Google Scholar]
- Rougon, G., and O. Hobert, 2003. New insights to the diversity and function of neuronal immunoglobulin superfamily molecules. Annu. Rev. Neurosci. 26: 207–238. [DOI] [PubMed] [Google Scholar]
- Roy, P. J., H. Zheng, C. E. Warren and J. G. Culotti, 2000. mab-20 encodes Semaphorin-2a and is required to prevent ectopic cell contacts during epidermal morphogenesis in Caenorhabditis elegans. Development 127: 755–767. [DOI] [PubMed] [Google Scholar]
- Salser, S. J., and C. Kenyon, 1996. A C. elegans Hox gene switches on, off, on and off again to regulate proliferation, differentiation and morphogenesis. Development 122: 1651–1661. [DOI] [PubMed] [Google Scholar]
- Shioi, G., M. Shoji, M. Nakamura, T. Ishihara, I. Katsura et al., 2001. Mutations affecting nerve attachment of Caenorhabditis elegans. Genetics 157: 1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J. E., D. G. Albertson and J. N. Thomson, 1980. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev. Biol. 78: 542–576. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne, M., and C. S. Goodman, 1996. The molecular biology of axon guidance. Science 274: 1123–1133. [DOI] [PubMed] [Google Scholar]
- Troemel, E.R., J. H. Chou, N. D. Dwyer, H. A. Colbert and C. I. Bargmann, 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83: 207–218. [DOI] [PubMed] [Google Scholar]
- Tsalik, E. L., T. Niacaris, A. S. Wenick, K. Pau, L. Avery et al., 2003. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev. Biol. 263: 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udin, S. B., and J. W. Fawcett, 1988. Formation of topographic maps. Annu. Rev. Neurosci. 11: 289–327. [DOI] [PubMed] [Google Scholar]
- Vosshall, L. B., A. M. Wong and R. Axel, 2000. An olfactory sensory map in the fly brain. Cell 102: 147–159. [DOI] [PubMed] [Google Scholar]
- Wadsworth, W. G., 2002. Moving around in a worm: netrin UNC-6 and circumferential axon guidance in C. elegans. Trends Neurosci. 25: 423–429. [DOI] [PubMed] [Google Scholar]
- Wang, F., A. Nemes, M. Mendelsohn and R. Axel, 1998. Odorant receptors govern the formation of a precise topographic map. Cell 93: 47–60. [DOI] [PubMed] [Google Scholar]
- Wen, Z., C. Guirland, G. L. Ming and J. Q. Zheng, 2004. A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron 43: 760–772. [DOI] [PubMed] [Google Scholar]
- White, J. G., E. Southgate, J. N. Thomson and S. Brenner, 1986. The structures of the nervous system of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Wightman, B., R. Baran and G. Garriga, 1997. Genes that guide growth cones along the C. elegans ventral nerve cord. Development 124: 2571–2580. [DOI] [PubMed] [Google Scholar]
- Yarden, O., M. Plamann, D. J. Ebbole and C. Yanofsky, 1992. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J. 11: 2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa, S., R. D. Mckinnon, M. Kokel and J. B. Thomas, 2003. Wnt-mediated axon guidance via the Drosophila derailed receptor. Nature 422: 583–588. [DOI] [PubMed] [Google Scholar]
- Zallen, J. A., S. A. Kirch and C. I. Bargmann, 1999. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development 216: 3679–3692. [DOI] [PubMed] [Google Scholar]
- Zallen, J. A., E. Pockel, D. M. Tobin and C. I. Bargmann, 2000. Neuronal cell shape and neurite initiation are regulated by the Ndr Kinase SAX-1, a member of the Orb-6/COT-1/warts serine/threonin kinase family. Mol. Biol. Cell 11: 3177–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., and S. W. Emmons, 1995. Specification of sense-organ identity by a Caenorhabditis elegans Pax-6 homologue. Nature 373: 74–78. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., P. J. Brockie, J. E. Mellem, D. M. Madsen and A. V. Maricq, 1999. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron 24: 347–361. [DOI] [PubMed] [Google Scholar]