Organization of leukotriene and prostaglandin synthesis
As described in the introduction to this Perspective series (1), signaling by arachidonic acid represents a paradigm for the use of oxygen in the transmission of information. At the same time, arachidonic acid signaling can also contribute to the propagation of cellular damage. This duality is typified by signaling cascade that (a) prevents the activation of 5-lipoxygenase (5-LO) in resting cells and (b) results in the formation and release of leukotrienes (LTs), which requires the sequential activation and interaction of at least eight different proteins. In fact, all lipoxygenases require membrane translocation to exert activity. In the case of the formation of COX products, particularly prostaglandin E2 (PGE2) and PGD2, humans have evolved two sets of biosynthetic enzymes that differ not only in their cell- and tissue-specific localization, but also in their subcellular localization and requirement for reduced glutathione, a cellular defense against oxidative damage. This review will focus on three aspects of arachidonic acid biology. First, the compartmentalization and organization of eicosanoid synthesis, specifically LTs and PGs, will be discussed. This will illustrate the elaborate mechanisms that keep unwanted lipoxygenation at arm’s length and also show that the enzymes such as glutathione-S-transferases, epoxide hydrolases, and carrier proteins that are commonly thought of as biosynthetic also belong to families that are generally considered to play a role in detoxification. Second, the potential cellular oxidative damage that is produced as a by-product of the use of oxygen and lipid substrates is examined. Finally, mechanisms that are used to amplify signaling diversity from a core of LTs and PGs are discussed.
The role of leukotrienes C4 and D4 in disease
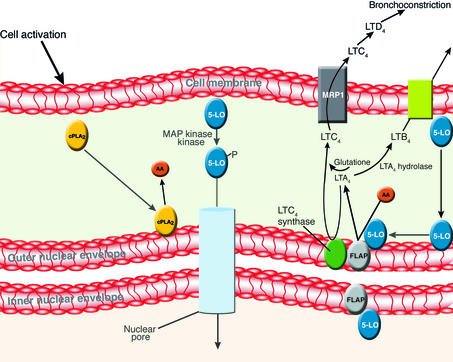
LTs are the products of the 5-LO pathway of arachidonic acid metabolism (Figure 1). The initial interest in LTs followed largely from their association with the pathogenesis of asthma (2). LTC4, identified as the parent molecule of the sulfidopeptide LTs, is generated from eosinophils and mast cells in large amounts, and also from monocytes and macrophages (2–7). However, it is not formed by polymorphonuclear leukocytes (2–5). When released from cells, LTC4 is converted to LTD4 (2–7), and both exert their biological effects via G protein–coupled receptors (8, 9). LTD4 and LTC4 cause the constriction of smooth muscle, and the clinical correlate is bronchial smooth muscle constriction in asthma (2, 10, 11). The role of LTD4 as a major contributor to asthmatic bronchospasm has been firmly established, and aerosolized LTD4 and LTC4 cause bronchospasm when taken by inhaler (10, 11). The metabolic product of LTD4, 5(S)-hydroxy,6(R)-cysteinyl-7,9,11-trans,14-cis-eicosatetraenoic acid, (LTE4), has been found at high levels in the serum and urine of patients with asthma and allergic rhinitis (12). When cold-induced bronchoconstriction, allergen-induced asthma, and exercise-induced asthma were analyzed, antagonists of the 5-LO pathway were able to alleviate bronchospasm (13–17). Pretreatment with the LTD4 receptor antagonist MK-0476 has been shown to block the bronchoconstrictive response to all forms of challenge and alleviate ongoing brochoconstriction (16). The use of LTD4 receptor and 5-LO antagonists has also blocked the severity and occurrence of asthmatic attacks in clinical trials, and these agents are particularly effective in chronic mild asthma, for which they are recommended for clinical use (18–23). LTs are also considered to be central to the pathogenesis of allergic rhinitis, in which cells expressing the cysteinyl LT1 receptor are highly expressed in CD45+ nasal leukocytes, and recent studies have shown that cysteinyl LTs regulate the trafficking of T cells in vivo (24, 25). Subsequently, LTD4 receptor antagonists have proved to constitute an effective therapeutic modality for this disorder. Knockout animals that lack LTC4 synthase have markedly attenuated vascular responses that are dependent on IgE-mediated LTC4 release (26).
Figure 1.
LT biosynthesis and assembly. Upon cellular activation of a mast cell or macrophage by IgE-antigen complexes or other stimuli, a cascade of cell activation events results in LT biosynthesis. A concomitant rise in free calcium induces translocation of cPLA2 to intracellular membranes, where it releases arachidonic acid. In parallel, 5-LO is phosphorylated by MAP kinase kinase and traffics through the nuclear pore to the nucleus (possibly in association with NF-κB) or directly to the outer nuclear envelope. 5-LO then associates with the nuclear membrane, and possibly with FLAP. FLAP facilitates arachidonic acid presentation to 5-LO and subsequent conversion of arachidonic acid to LTA4. LTA4 interacts with LTA4 hydrolase to form LTB4, or with LTC4 synthase to form LTC4. The synthesis of LTs A4, B4, and C4probably takes place within the lumen of, or in close proximity to, the nuclear membranes. However, for clarity they are shown here throughout the cytosol. FLAP is present on both the inner and the outer nuclear envelope, but LTC4 synthase is exclusively expressed on the outer nuclear membrane and ER.
As described above, LTD4 and LTC4 have been shown to function via both high- and low-affinity G protein–coupled receptors (8, 9, 27, 28), and these receptors are present on human eosinophils and monocytes, where they function in chemotaxis and cell activation. Thus, insight into the cellular mechanisms that regulate the enzymatic interactions during LT biosynthesis represents a critical step in our understanding of the formation and activity of these molecules in both health and disease.
Cellular and molecular biology of LT biosynthesis
The formation of LTs is initiated when eosinophils, mast cells, polymorphonuclear leukocytes, or monocytes are activated to release arachidonic acid. This occurs after the translocation of the 86-kDa calcium-dependent cytoplasmic phospholipase A2 (cPLA2) to the nuclear envelope, ER, or Golgi apparatus (Figure 1) (29). 5-LO is also translocated to the nuclear envelope (30–32) and acts on arachidonic acid in sequential steps to generate 5(S)-hydroxy,6-trans-8,11,14-cis-eicosatetraenoic acid (5-HPETE) and then 5(S),6(R)-oxo-7,9,11-trans-14-cis-eicosatetraenoic acid, (LTA4) (33). Within cells, these enzymatic steps require the expression of the 17-kDa nuclear envelope protein 5-LO–activating protein (FLAP). FLAP is critical to cellular 5-LO activity and to its membrane interactions, but not to 5-LO translocation (34, 35). It has been postulated that FLAP presents arachidonic acid to 5-LO (36) but may also restrict the diffusion of arachidonic acid through cellular membranes. FLAP may also “dock” 5-LO to its membrane target after translocation. LTA4 is then converted to LTB4 by the action of the enzyme LTA4 hydrolase (37) (Figure 1). Once formed, LTB4 is exported from cells by an active process (38) and exerts its effects on other phagocytic cells via high- or low-affinity G protein–coupled receptors (39, 40). Alternatively, in eosinophils, monocytes, and mast cells, LTA4 is conjugated with reduced glutathione to form LTC4. This reaction is catalyzed by the enzyme LTC4 synthase, a 17-kDa protein also located in the ER and outer nuclear membrane (41–44). LTC4 is exported from cells by the multidrug-resistance protein 1 (MRP1) and then metabolized by LTD4, which plays a subsequent role in the induction of bronchoconstriction and edema (45–47). MRP1 knockout animals are characterized by intracellular retention of LTC4 and significant asthmatic responses to allergen challenge (48), and dendritic cells from these animals are deficient in trafficking under certain circumstances (49). As described above, the fact that the enzymes commonly thought of as biosynthetic belong to families that are generally considered to play a role in detoxification raises the intriguing possibility that LT signaling may have evolved as an adaptive response to oxidative stress.
LT formation is regulated by compartmentalization
The generation of LTs and PGs is under a complex set of controls. As exemplified in mast cells, the synthesis of LTs and PGs is initiated by cPLA2, which is specific for phospholipids that contain arachidonic acid in the SN2 position (50). The enzyme is calcium-dependent and is translocated to the nuclear membrane in rat basophilic leukemia cells stimulated with IgE or calcium ionophore A23187 (29). This translocation is required for cellular activity and is mediated by a phospholipid-binding domain (51). Phosphorylation by MAPK at S505 can augment activity three- to fourfold but is not required for membrane association (52–55). The central role of cPLA2 in LT production has been confirmed by studies using peritoneal macrophages prepared from cPLA2 knockout mice (56). These cells produce no LTs in response to cell activation with calcium ionophore A23187. The enzyme is also found to localize within the cytosol of resting cells in this system. However, in other systems, detailed studies have shown that cPLA2 traffics to the Golgi apparatus (57). This implies that arachidonic acid can be rapidly moved to the ER and nuclear envelope, where it can subsequently interact with FLAP and 5-LO.
5-LO is phosphorylated concomitantly with translocation to the nucleus and nuclear envelope (58–60). Phosphorylation via MAPK-activated protein kinase-2 is critical in controlling targeting and activation of this enzyme (61). A second critical aspect of 5-LO control may be its interaction with the p65 subunit of NF-κB (62). Whether 5-LO activation can modify the activity of NF-κB remains to be determined, though this has been suggested to occur via 5-LO–mediated generation of reactive oxygen species (ROS) (63). 5-LO has a functional C2-like domain, which is important in mediating its membrane interactions, specifically with phosphatidyl choline (64). Recent studies have also shown that LTA4 hydrolase is translocated to the nucleus upon stimulation of rat basophilic leukemia cells (65), but not in human peripheral blood leukocytes. This would place the site of LTB4 synthesis within the nucleus of rat basophilic leukemia cells, a site where LTB4 has been suggested to function as a ligand for PPARα (66).
FLAP, LTC4 synthase, and LTC4 generation: the compartmentalization of LTC4 and LTB4 biosynthesis
Both sides of the nuclear envelope are potential sites of LT formation, and the interactions between the enzymes mediating LT biosynthesis at the membrane interface ultimately determine the fate of LTA4 and the regulation of LTC4 synthesis (30–32, 34–36, 67–71). As described above, the two proteins that regulate the synthesis of LTC4 in bone marrow–derived cells are FLAP and LTC4 synthase. These proteins are members of the superfamily known as membrane-associated proteins in eicosanoid and glutathione metabolism (MAPEG). This family is composed of six members that are central to the synthesis of LTs and PGs. MAPEG proteins are 17-kDa integral membrane proteins with three transmembrane domains and high structural identity. Three MAPEG proteins, FLAP, LTC4 synthase, and microsomal glutathione-S-transferase 2 (GST2) (72), control the synthesis of LTC4 and LTB4. A fourth member of the MAPEG family, inducible microsomal prostaglandin E2 synthase (mPGES-1), regulates the formation of PGE2 in inflammatory settings (73–76). Very little is known about the regulation of these MAPEG proteins, but it is becoming increasingly clear that their membrane interactions may play a critical role in their regulation.
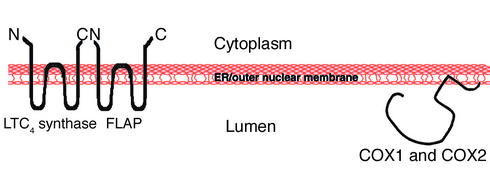
Figure 2 illustrates the topological relationships of the membrane domains of LTC4 synthase as recently determined by our group (44), in addition to the likely orientation of FLAP and the topology of both COX1 and COX2 (77). Within transmembrane domains 1 and 3, LTC4 synthase and FLAP share 52% identity between amino acids 41 and 97 of FLAP and between amino acids 45 and 101 of LTC4 synthase (32–36, 42). LTC4 synthase and microsomal GST2 share an even higher identity within this region. FLAP, characterized by three hydrophobic regions, has been localized to the nuclear envelope in polymorphonuclear leukocytes and monocytes, and the lipid-binding moiety is contained within the first hydrophilic loop (36). Immunoelectron microscopy of FLAP, using antibody to the first hydrophilic loop, has shown that FLAP is preferentially localized to the inner nuclear membrane, with the lipid-binding site oriented to the lumen (32).
Figure 2.
Membrane topology of proteins involved in eicosanoid synthesis. The active sites of LTC4 synthase, COX1, COX2, and the lipid-binding domain of FLAP are all oriented to the lumen of the ER and the nuclear envelope.
LTC4 synthase has recently been found to localize to the ER and outer nuclear membrane (44). However, in contrast to FLAP, it is excluded from the inner nuclear membrane. Similar to FLAP, LTC4 has its active site localized to the ER lumen.
These results, combined with the observation that LTA4 hydrolase translocates to the nucleus, indicate that in certain settings, the synthesis of LTB4 and LTC4 is compartmentalized. The molecular mechanism by which LTC4 synthase and FLAP are segregated between the inner and outer nuclear membrane is one basis for the compartmentalized synthesis of LTB4 and LTC4, but the molecular basis for the compartmentalization of the latter two proteins remains unknown.
Recent studies of PGE2 biosynthesis emphasize that an understanding of the membrane interactions of MAPEG proteins is central to an understanding of their biological role and suggest that regulated protein interactions may be important to their biological activity. PGES2 is coinduced and functionally coupled with COX2 in inflammatory settings, whereas cytosolic PGE2 synthase 1 is coupled to COX1 (73–77). How COX2 and mPGES-1 interact is not known, but three possible mechanisms have been proposed (73–77). First, direct passage of PGH2 between COX2 and mPGES-1 may occur, indicating that specific interactions between proteins of eicosanoid biosynthesis are critical to the efficient formation of PGE2. Alternatively, the association of COX2 and mPGES-1 may simply be kinetically favored. Finally, differential membrane compartmentalization has also been postulated to play a role.
Because the formation of LTC4 is controlled by two members of the MAPEG family (FLAP and LTC4 synthase), a critical question in understanding the intracellular biology of LT formation should be raised: Do the biosynthetic enzymes of LT formation interact with each other? Two possible models can be proposed. In one, a multiprotein complex is formed that mediates the efficient transfer of LTA4 to LTC4 synthase. This would have the advantage of preventing oxidative damage secondary to the formation of LTA4 adducts. In a second model, all the proteins are independent, and LTA4 passes between them and is captured by individual molecules. Evidence supports the existence of at least some protein interactions. As described above, 5-LO is targeted to the nuclear envelope. This interaction is partly dependent on FLAP, and cells that lack FLAP or are treated with the FLAP inhibitor MK-886 do not synthesize LTs and have deficient targeting of 5-LO to nuclear membranes (34, 78). Direct interactions between 5-LO and FLAP have been postulated, but not demonstrated; however, this may be secondary to the technology employed. An ability of FLAP and LTC4 synthase to form heterodimers would imply that interactions between MAPEG proteins are critical in controlling the formation of LTs. The situation is made more complex by the fact that both COXs and LTC4 synthase are known to form homodimers (77).
An additional important protein interaction occurs between PKC and LTC4 synthase. Pretreatment of cells with the activator of PKC, phorbol myristate acetate, inhibits subsequent LTC4 formation by up to 70% (79–82), suggesting another potential interaction on the outer nuclear envelope. This interaction has not been directly demonstrated, and the specific isoform of PKC that interacts with LTC4 synthase are unknown. Whether LTC4 synthase is phosphorylated during cell activation has not been demonstrated. Two intriguing possibilities are that phosphorylation disrupts protein interactions and/or that it causes membrane redistribution of LTC4 synthase.
Consequences of eicosanoid signaling
Why have cells evolved such complex mechanisms to control the initiation of signaling by 5-LO, other lipoxygenases, and COXs? Increasing evidence indicates that the initiation of lipid signaling comes at a potential cost to cells, particularly with respect to the generation of ROS and lipid hydroperoxides. In several model systems, the 5-LO enzyme contributes to the generation of ROS that activate NF-κB (62). Secondly, LTA4 has the potential to form adducts with DNA bases, suggesting that it may potentially serve as a modulator of transcription or as a mutagen (83). In the case of terminally differentiated myeloid cells, the adduction of LTA4 may function as a modulator of gene expression similar to methylation. More dire consequences may occur in conditions of chronic inflammation. Recent studies have shown that transcellular LTA4 conversion of LTA4 to LTB4 occurs in in vivo models of inflammation (84). This finding suggests that LTA4, generated by phagocytes, has the potential to form adducts in tissue DNA. In addition, the initial product of 5-LO, 5-HPETE, is a reactive hydroperoxide. The potential for the products of lipoxygenase reactions to generate cellular damage is typified by 15-LO, which has been implicated in the pathogenesis of atherosclerosis secondarily to its ability to utilize esterified phospholipids as a substrate (85, 86).
A second series of studies has suggested a mechanism by which the generation of PGs and electrophilic lipid hydroperoxides might couple inflammation and cancer but may also downregulate inflammation by interacting with NF-κB (87–95). Cyclopentanone PGs are generated late in the inflammatory process. PGs of the J and A series can inactivate wild-type p53 tumor-suppressor protein. This is based on the chemical reactivity of molecules that contain α,β-unsaturated ketones. The J series of PGs are more potent antiproliferative molecules than the A series and are more stable, and last longer. They are derived from PGD2, and their addition to cells results in the inactivation of wild-type tumor suppressor p53. It is becoming clear that these molecules have the ability to modify multiple redox-sensitive transcription factors. The addition of PGJ2 to cells triggers a series of events that are dependent on the generation of ROS and that can be prevented by the addition of the radical quencher N-acetyl-L-cysteine. These effects suggest that cyclopentanone PGs, 15-deoxy-Δ12,14-PGJ2 in particular, are either a source of markedly increased ROS generation or modulators of ROS sensitivity. Recently, a mechanism has been proposed that integrates these observations. As a consequence of its unique chemical reactivity properties, PGA1 and a PGA analog can react with and covalently modify selenium-containing enzymes and proteins, including thioredoxin reductase. This impairs the reduction of redox-sensitive proteins by thioredoxin, indicating that electrophilic PGs and lipids can function as amplifiers of oxidative stress. This would include the activation of apoptosis signal–related kinase-1, which would be released during the oxidation of thioredoxin. Δ12-PGJ2 can also inactivate ubiquitin isopeptidase activity of the proteasome pathway. A series PGs antagonize p53-dependent apoptosis but not cell-cycle arrest. These effects are consistent with inhibition of thioredoxin reductase. Moos et al. (87, 90) have suggested that inhibition of thioredoxin reductase–thioredoxin cycling would prevent the assembly of p53 into a transcriptionally competent form, blocking apoptosis. Inhibition of thioredoxin reductase–thioredoxin cycling would disrupt ribonucleotide reductase activity, resulting in cell-cycle arrest during G1, because ribonucleotide reductase is the rate-limiting enzyme in DNA synthesis.
The generation of lipid hydroperoxides within or near the cell nucleus must confer a benefit. One possibility is that the generation of PGs and LTs on the nuclear envelope is important for the signaling or regulation of transcription. For example, LTB4 has been suggested as an endogenous ligand for PPARα (66), though the significance of this observation is unclear. An intriguing, though untested, possibility is that the generation of PGs and LTs on the nuclear envelope could provide a mechanism for altering nuclear redox tone as a result of the “controlled” lipoxygenase or COX reactions. This would require a series of redox-sensitive transcriptional events. The physical association of 5-LO with other proteins suggests a direct role in transcription. The role of redox signaling and oxygen-dependent transcription in regulating the inflammatory response has recently been supported by the observation that hypoxia-inducible factor 1α is required for an inflammatory response by myeloid cells. The potential for 5-LO to generate sufficient ROS to affect NF-κB (62) provides a direct and feasible link between 5-LO–generated redox changes and transcription.
The topology of the biosynthetic enzymes involved in PG and LT synthesis may also be an adaption to the generation of lipid hydroperoxides. The active sites of COX1 and COX2 (96), FLAP (39), and LTC4 synthase (42) (as shown in Figure 2) are all oriented toward the ER lumen, a location with high glutathione levels. Whether mPGES-1 has the same topology as these other MAPEG enzymes is unknown, but it would not be surprising if they were similarly oriented.
Signaling by LTs and PGs
Although signaling that is mediated by a combination of oxygen and lipid interaction has potentially disastrous consequences for cells, it provides one great advantage: diversity. Whereas a large portion of the genome is devoted to coding for kinases and phosphatases, the multiple signaling pathways of arachidonic acid are governed by only three classes of enzymes that initially add oxygen to the substrate. These are (a) COXs, which initiate the synthesis of PGs; (b) lipoxygenases such as 5-LO, which initiate the synthesis of LTs, 12-, 15-, and 8-LO; and (c) cytochrome P450s, which catalyze the formation of epoxyeicosatrienoic acids (EETs) or the formation of20-hydroxyeicosatetraenoic acid. These enzymes generate products of differing biological activity by inserting oxygen at different positions in arachidonic acid. The use of enzymes of different classes to initiate oxygenation has a distinct advantage in that, because of their different mechanisms, the initial oxygenations result in molecules of different properties, limiting the next series of oxygenations, isomerizations, or reductions that can be performed. Thus, cytochrome P450 epoxygenases can generate 5,6-EET, but the biology of this molecule is completely distinct from that of LTA4, another 5,6-epoxide. Similarly, the products of COX, but not of lipoxygenases, can be converted to thromboxane and prostacyclin in subsequent reactions.
The second mechanism that generates functional diversity within any individual class of enzymes is regiospecificity for the substrate. The 5-, 12-, and 15-LOs each generate products with different potential biological function. In addition, 12- and 15-LO can utilize esterified fatty acids as substrates. This positional specificity and the ability to utilize esterified phospholipids as substrates provide a basis for the proposed role of 15-LO in the early phases of atherosclerosis with the generation of reactive lipid aldehydes and free radicals (85, 86).
It was initially presumed that the presence of two COX genes was a unique property of the COX enzyme class, but it now appears to be more the rule than the exception in eicosanoid biochemistry. As described above, there are two PGE2 synthase enzymes. There are also two enzymes that catalyze the synthesis of PGD2 (one in hematopoietic cells and one in the brain), two 15-LOs, two 12-LOs, and multiple cytochrome P450 epoxygenases. Each of these variants may function differently by being coupled to different stimuli in different tissues, or by being differentially compartmentalized within cells. The advantage of this is that different pools of eicosanoids can potentially be coupled to different functions (for examples see refs. 73–76, 96). In addition, the recent discovery of a G protein–coupled receptor for arachidonic acid indicates that even the lack of enzymatic oxygen can import biological information mediated by arachidonic acid (97).
As recently described by Narumiya and Fitzgerald (98), this theme is echoed by PG receptors. Eight types and subtypes of membrane prostanoid receptors are conserved in mammals from mice to humans: the PGD receptor, DP; four subtypes of the PGE receptor, EP1, EP2, EP3, and EP4; the PGF receptor, FP; the PGI receptor, IP; and the thromboxane A receptor, TP. They have different cell- and tissue-specific functions, determined by selective coupling to G proteins and by the expression of splicing isoforms. Their role as inhibitory versus constrictive is determined by coupling and splicing. The theme of multiple receptors has been expanded to include the LT receptors, whose different functions have been found to be mediated by two LTB4 receptors, two cysteinyl LT receptors, and two PGD2 receptors. Thus, the temporal, cell-specific, and intracellular distribution of biosynthetic enzymes controls the generation of eicosanoids within inflammation, whereas the cellular distribution, temporal induction, and coupling of their G protein–coupled receptors can diversify responses and control whether the responses are proinflammatory. Gs, Gi, or Gq proteins can couple signaling by a single molecule to multiple responses in different tissues or cells, and in a given cell.
In summary, the combination of 20 carbons and four unsaturated double bonds has proved to be one of the most flexible molecular combinations yet described and has provided the stimulus for over 50 years of intense investigation. As more roles and interactions for arachidonic acid products are identified, it is clear that this molecule will remain an integral component of biomedical research for at least another 50 years.
Acknowledgments
This work was supported by NIH grants R01GM-061823 (to R.J. Soberman), K01DK-59991 (to P. Christmas), and an unrestricted gift from the Jewish Communal Fund (to R.J. Soberman). The authors would like to thank Lawrence Marnett, K. Frank Austen, Robert C. Murphy, and Frank Fitzpatrick for helpful discussions.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: 5-lipoxygenase (5-LO); leukotriene (LT); cytoplasmic phospholipase A2 (cPLA2); 5(S)-hydroxy,6-trans-8,11,14-cis-eicosatetraenoic acid (5-HPETE); 5-lipoxygenase–activating protein (FLAP); multidrug-resistance protein 1 (MRP1); reactive oxygen species (ROS); membrane-associated proteins in eicosanoid and glutathione metabolism (MAPEG); glutathione-S-transferase 2 (GST2); microsomal prostaglandin E2 synthase–1 (mPGES-1); epoxyeicosatrienoic acid (EET).
References
- 1.Soberman RJ. The expanding network of redox signaling: new observations, complexities, and perspectives. J. Clin. Invest. 2003;111:571–574. doi:10.1172/JCI200318099. doi: 10.1172/JCI18099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N. Engl. J. Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson B, et al. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1177. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 4.Weller PF, et al. Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C. Proc. Natl. Acad. Sci. U. S. A. 1983;80:7626–7630. doi: 10.1073/pnas.80.24.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen WF, Jr, et al. Synthesis and release of leukotriene C4, by human eosinophils. J. Immunol. 1987;138:532–538. [PubMed] [Google Scholar]
- 6.Tripp CS, Mahoney M, Needleman P. Calcium ionophore enables soluble agonists to stimulate macrophage 5-lipoxygenase. J. Biol. Chem. 1985;260:5895–5898. [PubMed] [Google Scholar]
- 7.Goldyne ME, Burrish GF, Poubelle P, Borgeat P. Arachidonic acid metabolism among human mononuclear leukocytes. Lipoxygenase-related pathways. J. Biol. Chem. 1984;259:8815–8819. [PubMed] [Google Scholar]
- 8.Lynch KR, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:78–93. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 9.Heise CE, et al. Characterization of the human cysteinyl leukotriene 2 receptor. J. Biol. Chem. 2002;275:30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- 10.Griffin M, et al. Effects of leukotriene D on the airways in asthma. N. Engl. J. Med. 1983;308:436–439. doi: 10.1056/NEJM198302243080807. [DOI] [PubMed] [Google Scholar]
- 11.Weiss JW, et al. Bronchoconstrictor effects of leukotriene C in humans. Science. 1982;216:196–198. doi: 10.1126/science.7063880. [DOI] [PubMed] [Google Scholar]
- 12.Taylor GW, et al. Urinary LTE4 after antigen challenge in acute asthma and allergic rhinitis. Lancet. 1989;1:584–588. doi: 10.1016/s0140-6736(89)91611-5. [DOI] [PubMed] [Google Scholar]
- 13.Manning PJ, et al. Inhibition of excercised induced bronchonstriction by MK-571, a potent leukotriene D4-receptor antagonist. N. Engl. J. Med. 1990;323:1736–1769. doi: 10.1056/NEJM199012203232504. [DOI] [PubMed] [Google Scholar]
- 14.Finnerty JP, Wood-Baker R, Thompson H, Holgate ST. Role of leukotrienes in exercise induced asthma. Inhibitory effect of ICI 204,219, a potent LTD4 receptor antagonist. Am. Rev. Respir. Dis. 1993;145:746–749. doi: 10.1164/ajrccm/145.4_Pt_1.746. [DOI] [PubMed] [Google Scholar]
- 15.Taylor IK, O’Shaughnessy KM, Fuller RW, Dollery CT. Effect of cysteinyl-leukotriene receptor antagonist ICI 204.219 on allergen induced bronchoconstriction and airway hyperreactivity in atopic subjects. Lancet. 1991;337:690–694. doi: 10.1016/0140-6736(91)90277-v. [DOI] [PubMed] [Google Scholar]
- 16.Reiss TF, et al. Effects of monteleukast (MK-0476), a new potent cysteinyl leukotriene (LTD4) receptor antagonist, in patients with chronic asthma. J. Allergy Clin. Immunol. 1996;98:528–534. doi: 10.1016/s0091-6749(96)70086-6. [DOI] [PubMed] [Google Scholar]
- 17.Israel E, Cohn J, Dube L, Drazen JM. Effect of treatment with zileuton, a 5-lipoxygenase inhibitor, in patients with asthma. A randomized controlled trial. Zileuton Clinical Trial Group. JAMA. 1996;275:931–936. [PubMed] [Google Scholar]
- 18.Bonnin AJ, et al. Mild asthma. N. Engl. J. Med. 2002;346:1335–1336. doi: 10.1056/NEJM200204253461720. [DOI] [PubMed] [Google Scholar]
- 19.Kay AB. Advances in immunology: allergy and allergic diseases. First of two parts. N. Engl. J. Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 20.Busse WW, Lemanske RF. Advances in immunology: asthma. N. Engl. J. Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 21.Israel E, et al. The effect of inhibition on 5-lipoxygenase by zileuton in mild to moderate asthma. Ann. Intern. Med. 1993;119:1059–1066. doi: 10.7326/0003-4819-119-11-199312010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Naureckas ET, Solway J. Mild asthma. N. Engl. J. Med. 2001;345:1257–1262. doi: 10.1056/NEJMcp010434. [DOI] [PubMed] [Google Scholar]
- 23.Drazen JM, Israel E, O’Byrne PM. Drug therapy. Treatment of asthma with drugs modifying the leukotriene pathway. N. Engl. J. Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 24.Sousa AR, et al. Leukotriene-receptor expression on nasal mucosa in aspririn-sensitive rhinosinusitis. N. Engl. J. Med. 2002;347:1524–1526. doi: 10.1056/NEJMoa013508. [DOI] [PubMed] [Google Scholar]
- 25.Honig SM, et al. FTY720 stimulates multidrug transporter– and cysteinyl leukotriene–dependent T cell chemotaxis to lymph nodes. J. Clin. Invest. 2003;111:627–637. doi:10.1172/JCI200316200. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanaoka Y, et al. Attenuated zymosan-induced peritoneal vascular permeability and IgE-dependent passive cutaneous anaphylaxis in mice lacking leukotriene C4 synthase. J. Biol. Chem. 2001;276:22608–22613. doi: 10.1074/jbc.M103562200. [DOI] [PubMed] [Google Scholar]
- 27.Hui Y, et al. The murine cysteinyl leukotriene 2 (CysLT2) receptor. cDNA and genomic cloning, alternative splicing, and in vitro characterization. J. Biol. Chem. 2001;276:47489–47495. doi: 10.1074/jbc.M107556200. [DOI] [PubMed] [Google Scholar]
- 28.Maekawa A, Kanaoka Y, Lam BK, Austen KF. Identification in mice of two isoforms of the cysteinyl leukotriene 1 receptor that result from alternative splicing. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2256–2261. doi: 10.1073/pnas.041624398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glover S, et al. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J. Biol. Chem. 1995;270:15399–15407. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 30.Kargman S, Vickers PJ, Evans JF. A23187-induced translocation of 5-lipoxygenase in osteosarcoma cells. J. Cell Biol. 1992;119:1701–1709. doi: 10.1083/jcb.119.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouzer CA, Kargman S. Translocation of 5-lipoxygenase to the membrane in human leukocytes challenged with ionophore A23187. J. Biol. Chem. 1988;263:10980–10988. [PubMed] [Google Scholar]
- 32.Woods JW, et al. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J. Exp. Med. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouzer CA, Matsumoto T, Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc. Natl. Acad. Sci. U. S. A. 1986;83:857–861. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller DK, et al. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 1990;343:278–281. doi: 10.1038/343278a0. [DOI] [PubMed] [Google Scholar]
- 35.Dixon RA, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 36.Mancini JA, et al. 5-Lipoxygenase-activating protein is an arachidonic acid binding protein. FEBS Lett. 1993;318:277–281. doi: 10.1016/0014-5793(93)80528-3. [DOI] [PubMed] [Google Scholar]
- 37.Radmark O, Shimizu T, Jornvall H, Samuelsson B. Leukotriene A4, hydrolase in human leukocytes. Purification and properties. J. Biol. Chem. 1988;259:12339–12345. [PubMed] [Google Scholar]
- 38.Lam BK, Gagnon L, Austen KF, Soberman RJ. The mechanism of leukotriene B4 export from human polymorphonuclear leukocytes. J. Biol. Chem. 1990;265:13438–13441. [PubMed] [Google Scholar]
- 39.Yokomizo T, et al. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 40.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B4 receptor, BLT2: a new therapeutic target in inflammation and immunological disorders. J. Exp. Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penrose JF, et al. Purification of human leukotriene C4 synthase. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11603–11606. doi: 10.1073/pnas.89.23.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam BK, Penrose JF, Freeman GJ, Austen KF. Expression cloning of a cDNA for human leukotriene C4 synthase, an integral membrane protein conjugating reduced glutathione to leukotriene A4. Proc. Natl. Acad. Sci. U. S. A. 1994;91:7663–7667. doi: 10.1073/pnas.91.16.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsch DJ, et al. Molecular cloning and expression of human leukotriene-C4 synthase. Proc. Natl. Acad. Sci. U. S. A. 1994;91:9745–9749. doi: 10.1073/pnas.91.21.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christmas P, et al. Membrane localization and topology of leukotriene C4 synthase. J. Biol. Chem. 2002;277:28902–28908. doi: 10.1074/jbc.M203074200. [DOI] [PubMed] [Google Scholar]
- 45.Lam BK, Owen WF, Jr, Austen KF, Soberman RJ. The identification of a distinct export step following the biosynthesis of leukotriene C4 by human eosinophils. J. Biol. Chem. 1989;264:12885–12889. [PubMed] [Google Scholar]
- 46.Jedlitschky G, Buchholz U, Keppler D. Characterization of the ATP-dependent leukotriene C4 export carrier in mastocytoma cells. J. Biochem. 1994;220:599–563. doi: 10.1111/j.1432-1033.1994.tb18661.x. [DOI] [PubMed] [Google Scholar]
- 47.Loe DW, Almquist KC, Deeley RG, Cole SPC. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. J. Biol. Chem. 1996;271:9675–9683. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- 48.Wijnholds J, et al. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat. Med. 1997;3:1275–1279. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]
- 49.Robbiani DF, et al. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 50.Clark JD, et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 51.Nalekski EA, et al. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca2+-dependent lipid binding domain and a Ca2+-independent catalytic domain. J. Biol. Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- 52.Lin LL, et al. Cytoplasmic PLA2 is activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 53.Borsh-Haubold AG, et al. Identification of the phosphorylation sites on cytosolic phospholipase A2 in agonist-stimulated human platelets and HeLa cells. J. Biol. Chem. 1998;273:4449–4458. doi: 10.1074/jbc.273.8.4449. [DOI] [PubMed] [Google Scholar]
- 54.de Carvalho MGS, et al. Identification of the phosphorylation sites of the 85-kDa cytosolic phospholipsae A2 expressed in insect cells and present in human monocytes. J. Biol. Chem. 1996;271:6987–6997. doi: 10.1074/jbc.271.12.6987. [DOI] [PubMed] [Google Scholar]
- 55.Gijón MA, et al. Role of phosphorylation sites and the C2 domain in regulation of cytosolic phospholipase A2. J. Cell Biol. 1999;145:1219–1232. doi: 10.1083/jcb.145.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonventre JV, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 57.Evans JH, et al. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J. Biol. Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 58.Lepley RA, Muskardin D, Fitzpatrick FA. Tyrosine kinase activity modulates catalysis and translocation of cellular 5-lipoxygenase. J. Biol. Chem. 1996;271:6179–6184. doi: 10.1074/jbc.271.11.6179. [DOI] [PubMed] [Google Scholar]
- 59.Lepley RA, Fitzpatrick FA. Inhibition of MAP kinase kinase blocks activation and redistribution of 5-lipoxygenase in HL-60 cells. Arch. Biochem. Biophys. 1996;331:141–144. doi: 10.1006/abbi.1996.0292. [DOI] [PubMed] [Google Scholar]
- 60.Lepley RA, Fitzpatrick FA. 5-Lipoxygenase contains a functional Src homology 3-binding motif that interacts with the Src homology 3 domain of Grb2 and cytoskeletal proteins. J. Biol. Chem. 1994;269:24163–24168. [PubMed] [Google Scholar]
- 61.Werz O, et al. 5-Lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lepley RA, Fitzpatrick FA. 5-Lipoxygenase compartmentalization in granulocytes is modulated by an internal nuclear localization signal and NF-κB complex formation. Arch. Biochem. Biophys. 1998;356:71–76. doi: 10.1006/abbi.1998.0744. [DOI] [PubMed] [Google Scholar]
- 63.Bonizzi B, et al. Reactive oxygen intermediate-dependent NF-κB activation by interleukin-1 requires 5-lipoxygenase or NADPH oxidase activity. Mol. Cell. Biol. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulkani S, et al. Molecular basis of the specific subcellular localization of the C2-like domain of 5-lipoxygenase. J. Biol. Chem. 2002;277:3568–3575. doi: 10.1074/jbc.M112393200. [DOI] [PubMed] [Google Scholar]
- 65.Brock TG, Maydanski E, McNish RW, Peters-Golden M. Co-localization of leukotriene A4 hydrolase with 5-lipoxygenase in nuclei of alveolar macrophages and rat basophilic leukemia cells but not neutrophils. J. Biol. Chem. 2001;276:35071–35077. doi: 10.1074/jbc.M105676200. [DOI] [PubMed] [Google Scholar]
- 66.Devchand PR, et al. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 67.Woods JW, et al. 5-Lipoxygenase is located in the euchromatin of the nucleus in resting human alveolar macrophages and translocates to the nuclear envelope upon cell activation. J. Clin. Invest. 1995;95:2035–2046. doi: 10.1172/JCI117889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen X-S, et al. cDNA cloning, expression, mutagenesis, intracellular localization, and gene chromosomal assignment of mouse 5-lipoxygenase. J. Biol. Chem. 1995;270:17993–17999. doi: 10.1074/jbc.270.30.17993. [DOI] [PubMed] [Google Scholar]
- 69.Chen X-S, Zhang Y-Y, Funk CD. Determinants of 5-lipoxygenase nuclear localization using green fluorescent protein/5-lipoxygenase fusion proteins. J. Biol. Chem. 1998;273:31237–31244. doi: 10.1074/jbc.273.47.31237. [DOI] [PubMed] [Google Scholar]
- 70.Christmas P, Fox JW, Ursino SR, Soberman RJ. Differential localization of 5- and 15-lipoxygenases to the nuclear envelope in RAW macrophages. J. Biol. Chem. 1999;274:25594–25598. doi: 10.1074/jbc.274.36.25594. [DOI] [PubMed] [Google Scholar]
- 71.Lam BK, et al. Site-directed mutagenesis of human leukotriene C4 synthase. J. Biol. Chem. 1997;272:13923–13928. doi: 10.1074/jbc.272.21.13923. [DOI] [PubMed] [Google Scholar]
- 72.Jakobsson P-J, Mancini JA, Ford-Hutchinson AW. Identification and characterization of a novel human microsomal glutathione S-transferase with leukotriene C4 synthase activity and significant sequence identity to 5-lipoxygenase-activating protein and leukotriene C4 synthase. J. Biol. Chem. 1997;271:22203–22210. doi: 10.1074/jbc.271.36.22203. [DOI] [PubMed] [Google Scholar]
- 73.Jakobsson P-J, et al. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7220–7225. doi: 10.1073/pnas.96.13.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1β in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent prostaglandin E2 synthase expression. J. Biol. Chem. 2002;277:16355–16364. doi: 10.1074/jbc.M111246200. [DOI] [PubMed] [Google Scholar]
- 75.Murakami M, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000;275:32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 76.Tanioka T, et al. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 2000;275:32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- 77.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 78.Rouzer CA, et al. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J. Biol. Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- 79.Gupta N, Nicholson DW, Ford-Hutchinson AW. Demonstration of cell-specific phosphorylation of LTC4 synthase. FEBS Lett. 1999;449:66–70. doi: 10.1016/s0014-5793(99)00397-x. [DOI] [PubMed] [Google Scholar]
- 80.Ali A, Nicholson DW, Ford-Hutchinson AW. Characterization of human LTC4 synthase. Adv. Prostaglandin Thromboxane Leukot. Res. 1995;23:171–173. [PubMed] [Google Scholar]
- 81.Ali A, Ford-Hutchinson AW, Nicholson DW. Activation of protein kinase C down-regulates leukotriene C4 synthase activity and attenuates cysteinyl leukotriene production in an eosinophilic substrain of HL-60 cells. J. Immunol. 1994;153:776–788. [PubMed] [Google Scholar]
- 82.Kargman S, et al. Protein kinase C-dependent regulation of sulfidopeptide leukotriene biosynthesis and leukotriene C4 synthase in neutrophilic HL-60 cells. Mol. Pharmacol. 1994;45:1043–1049. [PubMed] [Google Scholar]
- 83.Reiber DC, Murphy RC. Covalent binding of LTA(4) to nucleosides and nucleotides. Arch. Biochem. Biophys. 2000;379:119–126. doi: 10.1006/abbi.2000.1851. [DOI] [PubMed] [Google Scholar]
- 84.Fabre J-E, et al. Transcellular biosynthesis contributes to the production of leukotrienes during inflammatory responses in vivo. J. Clin. Invest. 2002;109:1373–1380. doi:10.1172/JCI200214869. doi: 10.1172/JCI14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schewe T. 15-Lipoxygenase-1: a prooxidant enzyme. Biol. Chem. 2002;383:365–374. doi: 10.1515/BC.2002.041. [DOI] [PubMed] [Google Scholar]
- 86.Cyrus T, et al. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E–deficient mice. J. Clin. Invest. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moos PJ, et al. Electrophilic prostaglandins and lipid aldehydes repress redox-sensitive transcription factors p53 and hypoxia-inducible factor by impairing the selenoprotein thioredoxin reductase. J. Biol. Chem. 2003;278:745–750. doi: 10.1074/jbc.M211134200. [DOI] [PubMed] [Google Scholar]
- 88.Mullally JE, Moos PJ, Edes K, Fitzpatrick FA. Cyclopentenone prostaglandins of the J series inhibit the ubiquitin isopeptidase activity of the proteasome pathway. J. Biol. Chem. 2001;276:30366–30373. doi: 10.1074/jbc.M102198200. [DOI] [PubMed] [Google Scholar]
- 89.Kondo T, et al. 15-Deoxy-12,14-prostaglandin J2: the endogenous electrophile that induces neuronal apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7367–7372. doi: 10.1073/pnas.112212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moos PJ, Edes K, Fitzpatrick FA. Inactivation of wild-type p53 tumor suppressor by electrophilic prostaglandins. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9215–9220. doi: 10.1073/pnas.160241897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cerunda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sula D. 15-deoxy-delta 12, 14-prostagladin J2 NF-kB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 92.Straus DS, et al. 15-Deoxy-12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down-regulation of inducible nitric-oxide synthase by 15-deoxy-12,14-prostaglandin J2. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan SH, Sorof S. Preferential binding of growth inhibitory prostaglandins by the target protein of a carcinogen. Proc. Natl. Acad. Sci. U. S. A. 1990;87:9401–9405. doi: 10.1073/pnas.87.23.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rossi A, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–118. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 96.Smith WL, Langenbach R. Why there are two cyclooxygenase isozymes. J. Clin. Invest. 2001;107:1491–1495. doi: 10.1172/JCI13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 1993;244:173–176. doi:10.1038/nature01478. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 98.Narumiya S, Fitzgerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J. Clin. Invest. 2001;108:25–30. doi:10.1172/JCI200113455. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]