Abstract
Metalloneurochemistry is the study of metal ion function in the brain and nervous system at the molecular level. Research in this area is exemplified through discussion of several forefront areas where significant progress has been made in recent years. The structure and function of ion channels have been elucidated through high-resolution x-ray structural work on the bacterial K+ ion channel. Selection of potassium over sodium ions is achieved by taking advantage of key principles of coordination chemistry. The role of calcium ions in neuronal signal transduction is effected by several Ca2+-binding protein such as calmodulin, calcineurin, and synaptotagmin. Structural changes in response to calcium ion concentrations allow these proteins to function in memory formation and other neurochemical roles. Metallochaperones help to achieve metal ion homeostasis and thus prevent neurological diseases because of metal ion imbalance. Much detailed chemical information about these systems has become available recently. Zinc is another important metal ion in neuroscience. Its concentration in brain is in part controlled by metallothionein, and zinc is released in the hippocampus at glutamatergic synapses. New fluorescent sensors have become available to help track such zinc release.
The discovery that metal ions perform structural and catalytic functions in proteins and enzymes spawned new research at the interface of chemistry and biology. Bioinorganic chemistry is the amalgamation of the concepts that provide the foundation for the disciplines of biochemistry and inorganic chemistry. Before the inception of this field, biochemistry focused primarily on organic biomolecules like proteins and DNA, and many metal ions were dismissed as inconsequential trace elements. Purely inorganic chemists may tend to concentrate exclusively on metal coordination chemistry and overlook the importance of macromolecular structures. The bioinorganic chemist has the unique challenge of blending knowledge about the chemistry of metal ions with appreciation for biomolecules to understand how complex living systems function.
The study of the brain and central nervous system (CNS) traditionally has been the domain of neurochemists and neurophysiologists because of the specialization required for investigating their intricacies. Although metal ions and other inorganic species have numerous vital roles and unidentified functions in the CNS, the interest of bioinorganic chemists in this area is often minimal because many of the problems in the field involve studying spectroscopically silent metal ions like Ca2+, Mg2+, K+, and Na+. Investigating problems in neurochemistry may be less appealing to bioinorganic chemists because of the inability to apply conventional instrumental techniques, such as EPR, Mössbauer, UV-visible, and extended x-ray absorption fine structure (EXAFS) spectroscopy, to analyze such metal ions. Although neurochemists and neurophysiologists have made great strides in elucidating the functions of metal ions in the CNS, these researchers are often unable to apply the principles of inorganic chemistry. As the study of the CNS continues to progress, there is a tremendous opportunity for bioinorganic chemists to contribute to the understanding of the roles that metal ions have in synaptic transmission, in memory formation, and in the causes and treatments of neurological diseases.
To recognize the unique nature of this subdiscipline of bioinorganic chemistry, we introduce the term metalloneurochemistry to describe the study of metal ions in the brain and nervous system at the molecular level.† Although much work in this area is in its early stages, there are many examples where researchers in the field have made significant progress. In this brief perspective, we highlight from the recent literature a few topics in metalloneurochemistry and speculate about the future direction of research. In addition, we emphasize specific areas where bioinorganic chemists potentially can make important contributions to the field of metalloneurochemistry.
The Structure and Function of Ion Channels
The earliest and most enduring examples of research in metalloneurochemistry are the longstanding investigations into the functions of the alkali metal ions sodium and potassium in neurotransmission. The opening and closing of ion channels creates electrochemical gradients across membranes that transmit information and regulate cellular function. The elucidation of the x-ray crystal structure of the bacterial KcsA K+ channel, initiated nearly 5 years ago (1), is a landmark advance in the study of ion channel metalloneurochemistry (Fig. 1). This structural characterization had been elusive because ion channels are multicomponent, membrane-spanning proteins. The rapid propagation of neurochemical signals requires the K+ channel to discriminate against Na+ ions and to pass K+ ions at a rate of 107 to 108 sec−1. A convincing description of the mechanism of how the K+ ion channel functions has emerged from analysis of the structural data and correlation with previous electrophysiological and mutagenesis studies.
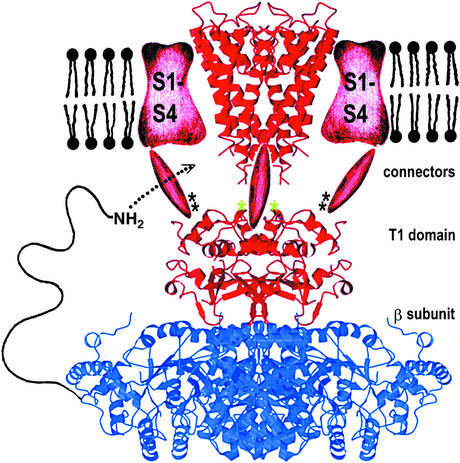
Figure 1.
Composite model of a voltage-dependent K+ channel. The α subunit containing the selectivity filter is shown in red, and the β subunit is in blue. An NH2-terminal inactivation peptide is shown entering a lateral opening to gain access to the pore. [Reproduced with permission from ref. 4 (copyright 2000, AAAS, www.sciencemag.org).]
The potassium channel is a 4-fold symmetric tetramer resembling a teepee that surrounds a central pore. Each of the four subunits of the channel has a sequence of five amino acids, Thr-Val-Gly-Tyr-Gly, on the extracellular side of the channel that comprises the selectivity filter. The carbonyl oxygen atoms of these five amino acid residues are oriented in a configuration capable of coordinating completely dehydrated K+ but not the smaller Na+ ions (2). Ions within the hydrophobic interior of the channel are stabilized by the presence of an aqueous cavity and helices of the protein with negatively charged side chains oriented toward the pore region. In addition to stabilizing interactions within the channel, electrostatic repulsion between K+ ions, which pass through the selectivity filter pore in single file, provides the force necessary to drive the ions through the channel at a high rate of speed (3).
Crystallizing this integral membrane protein was a remarkable achievement, but only the first in a series of accomplishments that are revolutionizing the understanding of ion channel function. The initial structure revealed only the membrane-spanning subunit of the K+ channel, including the selectivity filter. The x-ray structure of the cytoplasmic β subunit-T1 assembly has subsequently been determined (4). Like the membrane component of the channel, the cytoplasmic subunit exists as a tetramer, T14β4. The β subunits are oxidoreductase proteins containing catalytic residues and a NADPH cofactor. Although the structure of the β subunit has been elucidated, the substrate and the biological function of the oxidoreductase protein remain to be identified. One of the key features of the β subunit is a sequence of 20 amino acid residues at the N terminus, which serve as an inactivation gate (5). During inactivation, the protein tail binds to the cytoplasmic channel surfaces and then enters the pore region, blocking the passageway for the flow of K+ ions. Quaternary ammonium compounds also appear to inhibit channel function in a similar fashion (5).
The original structure determination of the KcsA K+ channel was carried out on a truncated version of the protein having 35 of the 160 amino acids removed from the C terminus. Although this truncation facilitated crystallization, it also produced a protein that prefers a closed conformation, limiting the utility of this form of the channel in other studies. To overcome this deficiency, a semisynthesis of the potassium channel was devised whereby the 73 amino acid residues of a recombinant N-terminal peptide were ligated with a chemically synthesized 52-aa C-terminal peptide (6). This synthetic channel folds into a tetrameric state on incorporation into lipid vesicles, but also remains in the closed conformation because it is not the full-length protein. The methodology developed in this synthesis, however, paves the way for future construction of the full-length protein as well as other ion channel proteins. In addition, the synthetic techniques will allow greater flexibility in studying structure–function relationships by facilitating site-specific modification of the ion channel proteins.
There are many remaining questions about the function of K+ channels, including the mechanism of gating (7). The two main classes of ion channel proteins are ligand-gated and voltage-gated. Voltage-gated channels, like the KcsA K+ channel, open and close in response to changes in membrane potential. Ligand-gated channels, like the MthK K+ channel, require the binding of an external substrate to open the channel. The MthK K+ channel opens and closes in a Ca2+-dependent fashion, and crystallographic analysis reveals two Ca2+-binding sites on the intracellular side of the protein (8). The Ca2+-binding sites reside on a protein domain that contains three carboxylate ligands from either glutamate or aspartate residues, and two serine residues in close proximity. Free energy supplied by Ca2+-binding induces structural changes that open and close the pore. Despite the difference in mechanism, the two types of channel appear to share a common structural basis for gating (7).
New insight gained through x-ray crystallographic analysis has sparked the reevaluation of previous work involving ion channels. Most of the structural reports thus far have addressed K+ channels; however, the structure of the ClC chloride channel has recently been reported (9), generating even more enthusiasm for new research on ion channels. Like the K+ channel, the Cl− channel must select for the passage of one ion over other similar ones. The initial structural work on this channel provided some insight into the structural basis for such discrimination, but more details are required to understand completely the mechanism of anion channel selectivity. In addition to the importance of ion channels as a fundamental component of signal transduction and neurochemical events in the cell, many neurological diseases are correlated with mutation of ion channel genes (10). Understanding the relationships between ion channel structure and function may provide insight into the causes and potential treatments for these genetic disorders. This area is certain to be of interest to medicinal bioinorganic chemists.
Ca2+ Proteins and Synaptic Transmission
Perhaps the most important metal ion in signal transduction is Ca2+. Changes in intracellular Ca2+ concentrations initiate a cascade of signaling events. On Ca2+ binding, calmodulin, an intracellular protein capable of coordinating four Ca2+ ions, binds to a wide variety of enzyme targets, modulating their states of activation. Years of research dedicated to Ca2+ signaling have provided enough information that many of the functions of calmodulin are now understood (11). The study of calmodulin is one of the original areas of focus in metalloneurochemistry, and research continues to provide intriguing insight into neurological function. Evidence now exists that Ca2+-dependent translocation of calmodulin in neuronal nuclei is critical both for rapid signaling and for memory formation (12).
Calcineurin, which was first isolated from mammalian brain (13), is one target of calmodulin involved in Ca2+- dependent cellular signaling. Calcineurin is a heterodimeric phosphatase containing a catalytic subunit with a calmodulin binding domain and a Ca2+-binding subunit (14). It has been implicated in many biological functions including apoptosis (15), transcription (13), T cell expression (16), and heart development (17). Calcineurin is also involved in synaptic plasticity (18). One possibility for contribution to synaptic function is involvement in an estradiol-dependent pathway in the hippocampus (19), the center of learning and memory in the brain. In the putative signaling mechanism, binding of estradiol to a receptor decreases Ca2+ influx to neurons. At lower Ca2+ concentrations the Ca2+/ calmodulin-dependent phosphatase activity of calcineurin is diminished. Although the exact significance of these results is not certain, they may explain the facilitation of long-term potentiation associated with rising estrogen levels, and they may have implications in hormone replacement therapy for improving memory.
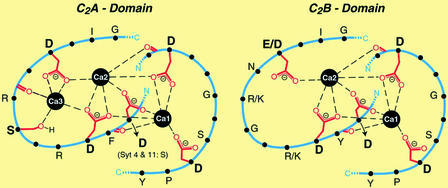
As progress in the study of the metallochemistry of calmodulin and calcineurin unfolded, another family of Ca2+-containing proteins involved in the release of neurotransmitters was identified (20). Twelve members of this family of proteins, known as synaptotagmins, have been characterized (21). They are typically localized on the membranes of synaptic vesicles and have two Ca2+-binding domains, C2A and C2B (Fig. 2). The C2A domain contains a trinuclear Ca2+-binding site with four bridging aspartate residues, and the C2B domain consists of a dinuclear site with three bridging aspartate residues. The functions and properties of many members of the synaptotagmin family remain unknown (21); however, extensive studies have been carried out on synaptotagmin I.
Figure 2.
Structure of the Ca2+-binding sites of the C2A and C2B domains of synaptotagmins. The C2A domain contains three Ca2+ ions via five aspartate and one serine residue, whereas the C2B domain lacks the binding site for Ca3 and coordinates two Ca2+ ions. [Reproduced with permission from ref. 21 (copyright 2002, American Society for Biochemistry and Molecular Biology).]
The affinity of synaptotagmin I for Ca2+ is decreased by a factor of two when an arginine residue in close proximity to the C2A domain is substituted by a glutamine. This point mutation does not modify the structure of the protein (22). The consequence of the diminished binding affinity is a 2- to 3-fold decrease in the sensitivity of Ca2+-dependent neurotransmitter release from synaptic vesicles of hippocampal neurons. The implication is that synaptotagmin I acts a Ca2+ sensor, or is at least part of a cascade of events in synaptic transmission. Conflicting reports exist, however. In one study, an aspartate residue of the C2A binding domain was mutated to an asparagine, but the Ca2+-dependent binding of synaptotagmin to its targets and the release of neurotransmitters were unaffected in vivo (23). In addition, synaptotagmin IV, which does not bind Ca2+ in its C2A domain, elicited similar responses. This work raises the possibility that Ca2+-dependent neurotransmitter release involves the dinuclear C2B site.
Evidence for the importance of the C2B domain for synaptic transmission comes from site directed mutagenesis studies of the Ca2+-binding residues in synaptotagmin I. Mutating two of the key aspartate residues in the C2B binding site decreases the release of neurotransmitter by >95% (24). Because the two Ca2+-binding sites are in close proximity, Ca2+-binding to the C2B domain may act cooperatively with the C2A domain by facilitating binding of synaptotagmin I to its target membranes. Investigation of the cooperative interactions between the domains with fluorescent probes suggests that both are involved in binding interactions with vesicular membranes (25).
Although the exact mechanism of Ca2+-mediated synaptic transmission by synaptotagmin remains to be elucidated, evidence suggests that the neurotoxicity of heavy metals such as lead may arise from competitive binding to the Ca2+ locale in the C2 domains of the protein (26). Competitive metal binding studies indicate that the affinity of synaptotagmin for Pb2+ is significantly higher than for Ca2+, and that both metals coordinate to the protein in a similar manner. Pb2+-binding alters the ability of synaptotagmin to bind to target membranes involved in neurotransmitter release. Pb2+-synaptotagmin binds phosphoslipid targets at concentrations 1,000 times lower than that required by the Ca2+-loaded protein, but impairs its ability to bind to its protein partner syntaxin (27). Further experiments are required to determine whether and how this binding influences neurotransmitter release.
Although not a substitute for biochemical investigations, modeling studies on metalloenzymes have provided considerable insight into protein function. The field of synaptotagmin seems primed for contributions by bioinorganic chemists in the modeling field. In particular, understanding the basis for Pb2+ toxicity would well be facilitated by investigation of model complexes. Although synaptotagmin appears to bind Ca2+ and Pb2+ in a similar manner, the detailed molecular structure has yet to be reported. In addition, modeling studies may provide a means to address potential treatments, such as the design of drugs for Pb2+ removal by chelation therapy.
Metallochaperones and Metal Ion Homeostasis
Many neurological disorders have been associated with the accumulation of toxic amounts of metal ions in the nervous system (28). The maintenance of metal ion homeostasis is an important topic in metalloneurochemistry. The seminal work on the iron-transport protein transferrin and the iron-storage protein ferritin epitomizes the importance of tight regulation of metal ion concentrations (11). More recently, research has been carried out on copper chaperone proteins (29–32). The molecular handshake that occurs between the copper-zinc superoxide dismutase (SOD1) and the copper chaperone for superoxide dismutase (CCS) (33) ensures delivery of copper to the enzyme and prevents indiscriminate binding to inappropriate targets. It may also preserve the stability of the Cu+, which would otherwise disproportionate to Cu2+ and Cu(s) under physiological conditions. A breakdown in copper trafficking can have detrimental consequences. Accumulation of toxic amounts of copper in the brain results in neuronal degradation, and may be directly linked to copper chaperone proteins that deliver copper to ATPases involved in translocation of heavy metals across cell membranes (34). Metallochaperones of Ni2+ have also been identified (35), although a connection between nickel and neuronal damage has not yet been implicated. Given the importance and complexity of the known metal delivery systems, it is likely that additional proteins with trafficking functions will be discovered.
ZnTs and Synaptic Zn2+
Like the complex cellular mechanism for copper distribution, several proteins involved in Zn2+ transport have been characterized. These zinc transporters (ZnTs) are widely expressed, transport Zn2+ across membranes, and compartmentalize the metal ion into vesicles. This family of integral membrane proteins includes ZnT-1, which is found in intestinal cells and is responsible for uptake of dietary Zn2+ (36), and ZnT-2, which occurs in the kidney (37). ZnT-4 in breast cells may be involved in delivering zinc into milk (38), ZnT-5, which is found abundantly in the pancreatic cells loads Zn2+ into secretory granules (39), and ZnT-6 may transport Zn2+ from the cytoplasm into the Golgi apparatus (40). Of particular interest to the discussion of Zn2+ metalloneurochemistry, however, is ZnT-3, which is found exclusively in brain and testis (41). The occurrence of ZnT-3 on the membranes of presynaptic vesicles of Zn2+-enriched neurons in the hippocampus suggests this transporter is the primary protein responsible for Zn2+- loading in the brain (42).
The function of synaptic Zn2+ has been the subject of much research. For example, high concentrations of Zn2+ in the olfactory bulb of mice and the occurrence of two kinds of zinc-enriched (ZEN) terminals suggest a role in olfaction (43). Although none of the functions of synaptic Zn2+ has been definitively established, there are several potential targets for released metal ion (Fig. 3) (44). Zinc may bind to receptors on the postsynaptic neuron and modulate the flow of ions through channels, or it may act as an intracellular signal following entry of postsynaptic neuron through Zn2+-permeable channels. Alternatively the Zn2+ may simply diffuse away freely, or be taken back up into the presynaptic neuron. Zn2+ released from presynaptic neurons during high-frequency electrical stimulation induces long-term potentiation (LTP) between the mossy fiber and CA3 synapses of the hippocampus (45). Moreover, the presence of [CaEDTA]2− blocks the signal, and glutamate that is coreleased from synaptic vesicles is required for induction of LTP. Studies indicate that, once released by electrical stimulation, Zn2+ traverses the synaptic cleft and can permeate ion channels on postsynaptic neurons gated by glutamate. This work suggests that Zn2+ may act as a second messenger, similar to Ca2+ (46).
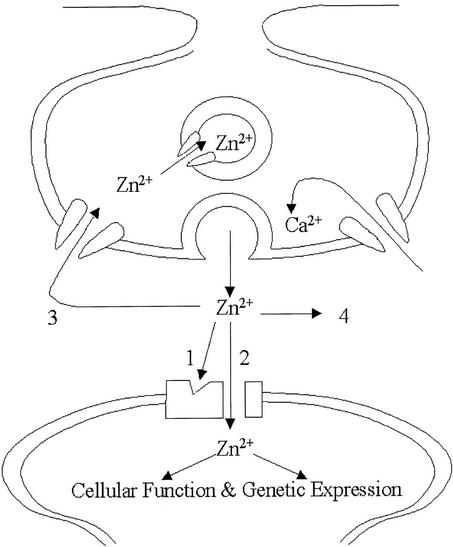
Figure 3.
Schematic illustration of putative Zn2+-signaling pathways. After its release from presynaptic neurons, Zn2+ can (1) bind to receptors of ion channels of postsynaptic neurons modulating their activity; (2) enter postsynaptic neurons via Zn2+-permeable ion channels (i.e., voltage-gated Ca2+ channels); (3) be taken back up into the presynaptic neuron and vesicles; or (4) diffuse away into the extracellular fluid. [Reproduced with permission from ref. 45 (copyright 2001, American Physiological Society).]
The requirement of ZnT-3 for sequestering Zn2+ into synaptic vesicles was examined by generating ZnT-3 knockout mice (47). Plasma emission spectroscopy indicates a 20% decrease in hippocampal Zn2+ in these mice, and histochemical staining reveals no evidence for synaptic Zn2+. The mice were confronted with a battery of tests designed to evaluate the sensitivity of their auditory and olfactory senses and to test their learning and memory (48). These measurements were chosen for their relevance to roles and functions postulated for synaptic Zn2+. The ZnT-3 knockout mice displayed no appreciable differences in behavior compared with control (normal) mice. This result raises the possibility either that the neuromodulatory Zn2+ is not relevant for brain function under normal conditions, or simply that these tests were not sensitive enough to detect subtle changes in behavior. More likely, however, because these mice developed from birth without the ZnT-3 gene, they compensated for the loss of synaptic Zn2+ by reprogramming the affected functions. Not only do the studies raise interesting questions about the function of synaptic Zn2+ in vivo, but they also highlight the difficulty in studying metal ions in biological systems as complex as the CNS.
One salient feature of the ZnT-3 deficient mice appeared when they were crossed with transgenic mice expressing a mutant human amyloid precursor protein that induces cerebral amyloid plaque pathology (49). The aggregation of β-amyloid plaques is well established in the neuropathology of Alzheimer's disease (50, 51). Abnormal amounts of Zn2+ have been detected in amyloid plaques taken from Alzheimer's patients, and Zn2+ has been implicated in the aggregation of the peptides that form the senile plaques (52). In ZnT-3-null mice that also exhibit amyloid plaque pathology, a drastically decreased plaque formation was observed in the brain tissue compared with that found in mice having the ZnT-3 gene (49). This study strongly suggests that synaptic Zn2+ may contribute to the deposition of Alzheimer's plaques in vivo.
In addition to neurodegenerative disorders (53), acute disruption of Zn2+ homeostasis during seizures, ischemia, and head trauma may play a role in neuronal damage (54, 55). The basis for such Zn2+-induced toxicity is corroborated by the ability of Zn2+ chelators to prevent neuronal death. Administration of [CaEDTA]2−, which binds Zn2+ but does not significantly buffer Ca2+ concentrations, before or immediately after traumatic injury prevents Zn2+ accumulation in postsynaptic neurons and thus neuronal death (56, 57). The cause of cell death is not fully understood. It appears, however, that Zn2+ enters the neurons through voltage-gated Ca2+ channels (58) and may trigger cell death by a number of mechanisms. Included would be cytotoxic up-regulation of NMDA receptor activity (59) or by downstream generation of reactive oxygen species (60, 61). Despite some evidence that Zn2+ is lost from synaptic vesicles during trauma (57, 62), the fact that the ZnT-3 knockout mice also suffer from Zn2+-induced neuronal toxicity argues against vesicular origin (63). Interestingly, there is also evidence that synaptic Zn2+ may induce the production of a neuroprotective protein after traumatic injury (64).
MTs and Zn2+
Another class of proteins involved in metalloneurochemistry are the metallothioneins (65). These cysteine-rich proteins have a number of functions in the central nervous system, including heavy-metal detoxification (66). MT-III is a brain-specific member of this family of proteins that is particularly abundant in zinc-containing neurons (67). In contrast to other MT isoforms, MT-III binds Zn2+ in a noncooperative manner and can house a greater number of metal ions. MT-III typically contains seven Zn2+ ions but has a capacity for at least nine (68). In addition, proteins containing fewer than seven ions are stable. The binding properties of MT-III suggest that it may have a chaperone-like function, delivering Zn2+ to vesicular membranes where the ion is compartmentalized by ZnT-3. One observation about MT-III is that the levels of this protein are lower in the brains of Alzheimer's patients (69, 70), although Alzheimer's disease neuronal changes may not be a consequence of such down-regulation (71). Because free Zn2+ promotes the formation of β-amyloid aggregation (72), the depletion of MT-III in Alzheimer's tissue suggests that a breakdown in the expression of proteins capable of sequestering Zn2+ may contribute to the onset of symptoms or deterioration of function.
There also seems to be a synergistic relationship between Zn2+ and the neurotransmitter nitric oxide (NO). Upon reaction with cysteine residues that bind Zn2+, NO is capable of releasing Zn2+ from MTs with concomitant formation of a disulfide bond (73, 74). S-nitrosation of Zn2+-bound cysteine residues in MT-III can occur either by direct reaction with NO or by transnitrosation with S-nitrosothiols (75). This reactivity suggests that MT-III may be responsible for converting NO signals into Zn2+ signals. In addition, release of Zn2+ due to oxidative stress may serve as a source of cytotoxic metal ion (53). Understanding the exact function of synaptic Zn2+ in vivo, and the consequences of loss of Zn2+ homeostasis, remain interesting topics in metalloneurochemisty.
Fluorescent Sensors for Zn2+ and NO
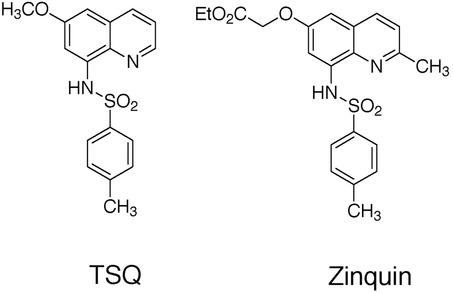
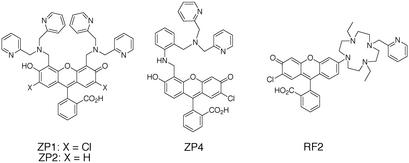
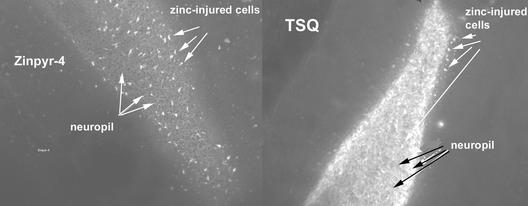
Our interest in metalloneurochemistry, in particular synaptic transmission, required the development of new tools for studies at the interface of bioinorganic chemistry and neurochemistry. Studying spectroscopically silent metal ions like Zn2+, and small inorganic radicals like NO, require methodologies to visualize these otherwise undetectable species in neuronal cells. Small molecule fluorescent sensors have been instrumental in the study of Ca2+, an important intracellular second messenger (76). When we initiated our work, the primary fluorescent probes available for Zn2+ were quinoline sulfonamides such as TSQ (77) and Zinquin (78) (Fig. 4); however, their fluorescence properties are not ideal for application to metalloneurochemistry (79). In particular, these sensors require excitation with near-UV wavelength light, whereas longer wavelength absorption is desired to prevent damage of living tissues. In addition, these probes have a limited range of dissociation constants (sub-nM), whereas effective study of the numerous functions of Zn2+ requires multiple sensors with a wide array of binding affinities. With these objectives in mind, we have designed, prepared, and characterized a series of sensors based on fluorescein fluorophores that include Zinpyr 1 (ZP1) (80), ZP2 (81), ZP4 (82), and Rhodafluor 2 (RF2) (83) (Fig. 5). These sensors are especially amenable to biological investigation. They employ a fluorescein fluorophore and therefore can be excited with and emit visible light. Although all of the ZP sensors prepared thus far have sub-nM dissociation constants, the RF2 sensor has a Kd of ≈15 μM. The synthetic strategy developed during the preparation of these sensors also provides the groundwork to access additional new probes with better fluorescence properties and a greater range of dissociation constants. Apart from our sensors, several others using fluorescein as the reporting group have been prepared (84–87). This combined collection of probes provides a new set of powerful tools for studying the metalloneurochemistry of Zn2+. Our current efforts are focused on making sensors with improved fluorescence properties as well as application of the probes to problems in neurochemistry. In pursuit of the latter goal, we discovered ZP4 to be superior to all other sensors screened for imaging neurons damaged by Zn2+ during seizure and blunt head trauma (Christopher J. Frederickson, S.C.B., Cathy J. Frederickson, S. L. Sensi, J. H. Weiss, R. Balaji, E. Bedell, D. S. Prough, and S.J.L., unpublished data). Unlike TSQ, which permeates vesicular membranes and images synaptic Zn2+ in addition to Zn2+ in damaged cells, ZP4 is membrane impermeant and selectively images the injured neurons (Fig. 6). In addition to Zn2+ sensors, we have ongoing efforts to develop NO sensors, and have had some initial success (88, 89). Access to both Zn2+ and NO sensors would be useful for studying such problems as the transformation of NO signals to Zn2+ ones by MTs as well as other questions in metalloneurochemistry.
Figure 4.
Chemical structure of the quinoline sulfonamide Zn2+ sensors TSQ and Zinquin.
Figure 5.
Chemical structures of the fluorescein-based sensors for Zn2+ ZP1, ZP2, ZP4, and RF2. The ZP refers to the Zinpyr family and RF stands for Rhodafluor. The name Zinpyr comes both from the utilization of pyridine ligands and the ability to “peer” at the Zn2+ concentrations in cells. The name Rhodafluor reflects the hybrid nature of the fluorophore, which has chemical features of both rhodamine and fluorescein.
Figure 6.
Comparison of the imaging of Zn2+ damaged neurons between TSQ and ZP4 stained slices from a mouse following drug-induce traumatic seizure. Because TSQ is membrane permeable, it images the Zn2+-containing synaptic vesicles in intact neurons (neuropil). [Reproduced with permission from ref. 82 (copyright 2003, American Chemical Society).]
Future Prospects
In this perspective we have highlighted progress in several areas in the field of metalloneurochemistry. This sampling is not meant to be comprehensive, for there are many other areas of interest, including release of intracellular Ca2+ triggered by extracellular Zn2+ (90), Zn2+-containg polypeptides such as nerve growth factor, a neurotrophin that is essential for the development and maintenance of neuronal populations (91, 92), and S100 proteins that bind Ca2+, Zn2+, and Cu2+ and have been linked to neurodegenerative disorders (93). Neurochemists and neurophysiologists have carried out much of the research in metalloneurochemistry, so there is tremendous potential for bioinorganic chemists to contribute to this field because of their expertise in coordination chemistry. In order for progress in unraveling the fundamental concepts of synaptic transmission and neurodegenerative conditions to continue, it is essential that a connection be made between the fields of bioinorganic chemistry and neurobiology. Such union would provide a powerful coalition to improve our knowledge of metalloneurochemistry.
Footnotes
Burdette, S. C., Woodroofe, C. C. & Lippard, S. J. (2002) 224th ACS National Meeting, August 18–22, 2002, Boston, MA (Am. Chem. Soc., Washington, DC), INOR-582 (abstr.).
References
- 1.Doyle D A, Cabral J M, Pfuetzner R A, Kuo A, Gulbris J M, Cohen S L, Chait B T, MacKinnon R. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Morais-Cabral J H, Kaufman A, MacKinnon R. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 3.Morais-Cabral J H, Zhou Y, MacKinnon R. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 4.Gulbis J M, Zhou M, Mann S, MacKinnon R. Science. 2000;289:123–127. doi: 10.1126/science.289.5476.123. [DOI] [PubMed] [Google Scholar]
- 5.Zhou M, Morais-Cabral J H, Mann S, MacKinnon R. Nature. 2001;411:657–661. doi: 10.1038/35079500. [DOI] [PubMed] [Google Scholar]
- 6.Valiyaveetil F I, MacKinnon R, Muir T W. J Am Chem Soc. 2002;124:9113–9120. doi: 10.1021/ja0266722. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Lee A, Chen J, Cadene M, Chait B T, MacKinnon R. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, Lee A, Chen J, Cadene M, Chait B T, MacKinnon R. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 9.Dutzler R, Campbell E B, Cadene M, Chait B T, MacKinnon R. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 10.Weinreich F, Jentsch T J. Curr Opin Neurobiol. 2000;10:409–415. doi: 10.1016/s0959-4388(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 11.Lippard S J, Berg J M. Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books; 1994. pp. 145–148. and 184–192. [Google Scholar]
- 12.Mermelstein P G, Deisseroth K, Dasgupta N, Isaksen A L, Tsien R Y. Proc Natl Acad Sci USA. 2001;98:15342–15347. doi: 10.1073/pnas.211563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crabtree G R. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- 14.Olson E N, Williams R S. BioEssays. 2000;22:510–519. [Google Scholar]
- 15.Kim M-J, Jo D-G, Hong G-S, Kim B J, Lai M, Cho D-H, Kim K-W, Bandyopadhyay A, Hong Y-M, Kim D H, et al. Proc Natl Acad Sci USA. 2002;99:9870–9875. doi: 10.1073/pnas.152336999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bueno O F, Brandt E B, Rothenberg M E, Molkentin J D. Proc Natl Acad Sci USA. 2002;99:9398–9403. doi: 10.1073/pnas.152665399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bueno O F, Wilkins B J, Tymitz K M, Glascock B J, Kimball T F, Lorenz J N, Molkentin J D. Proc Natl Acad Sci USA. 2002;99:4586–4591. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aramburu J, Rao A, Klee C B. Curr Top Cell Regul. 2001;36:237–295. doi: 10.1016/s0070-2137(01)80011-x. [DOI] [PubMed] [Google Scholar]
- 19.Sharrow K M, Kumar A, Foster T C. Neuroscience. 2002;113:89–97. doi: 10.1016/s0306-4522(02)00151-3. [DOI] [PubMed] [Google Scholar]
- 20.Augustine G J. Curr Opin Neurobiol. 2001;11:320–326. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 21.Sudhoff T C. J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Chacon R, Konigstorfer A, Gerber S H, Garcia J, Matos M F, Stevens C F, Brose N, Rizo J, Rosenmund C, Sudhof T C. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 23.Robinson I M, Ranjan R, Schwarz T L. Nature. 2002;418:336–340. doi: 10.1038/nature00915. [DOI] [PubMed] [Google Scholar]
- 24.Mackler J M, Drummond J A, Loewen C A, Robinson I M, Reist N E. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- 25.Bai J, Wang P, Chapman E R. Proc Natl Acad Sci USA. 2002;99:1665–1670. doi: 10.1073/pnas.032541099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godwin H A. Curr Opin Chem Biol. 2001;5:223–227. doi: 10.1016/s1367-5931(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 27.Bouton C M L S, Frelin L P, Forde C E, Godwin H A, Pevsner J. J Neurochem. 2001;76:1724–1735. doi: 10.1046/j.1471-4159.2001.00168.x. [DOI] [PubMed] [Google Scholar]
- 28.Gabay S, Harris J, Ho B T, editors. Metal Ions in Neurology and Psychiatry. New York: Alan R. Liss; 1985. [Google Scholar]
- 29.Rosenzweig A C, O'Halloran T V. Curr Opin Chem Biol. 2000;4:140–147. doi: 10.1016/s1367-5931(99)00066-6. [DOI] [PubMed] [Google Scholar]
- 30.Rosenzweig A C. Acc Chem Res. 2001;34:119–128. doi: 10.1021/ar000012p. [DOI] [PubMed] [Google Scholar]
- 31.Huffman D L, O'Halloran T V. Annu Rev Biochem. 2001;70:677–701. doi: 10.1146/annurev.biochem.70.1.677. [DOI] [PubMed] [Google Scholar]
- 32.Rosenzweig A C. Chem Biol. 2002;9:673–677. doi: 10.1016/s1074-5521(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 33.Lamb A L, Torres A S, O'Halloran T V, Rosenzweig A C. Nat Struct Biol. 2001;8:751–755. doi: 10.1038/nsb0901-751. [DOI] [PubMed] [Google Scholar]
- 34.Wernimont A K, Huffman D L, Lamb A L, O'Halloran T V, Rosenzweig A C. Nat Struct Biol. 2000;7:766–771. doi: 10.1038/78999. [DOI] [PubMed] [Google Scholar]
- 35.Song H K, Mulrooney S B, Huber R, Hausinger R P. J Biol Chem. 2001;276:49359–49364. doi: 10.1074/jbc.M108619200. [DOI] [PubMed] [Google Scholar]
- 36.McMahon R J, Cousins R J. Proc Natl Acad Sci USA. 1998;95:4841–4846. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmiter R D, Cole T B, Findley S D. EMBO J. 1996;15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 38.Michalczyk A A, Allen J, Blomeley R C, Ackland M L. Biochem J. 2002;364:105–113. doi: 10.1042/bj3640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kambe T, Narita H, Yamaguchi-Iwait Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M. J Biol Chem. 2002;277:19049–19055. doi: 10.1074/jbc.M200910200. [DOI] [PubMed] [Google Scholar]
- 40.Huang L, Kirschke C P, Gitschier J. J Biol Chem. 2002;277:26389–26395. doi: 10.1074/jbc.M200462200. [DOI] [PubMed] [Google Scholar]
- 41.Palmiter R D, Cole T B, Quaife C J, Findley S D. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel H J, Cole T B, Born D E, Schwartzkroin P A, Palmiter R D. Proc Natl Acad Sci USA. 1997;94:12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jo S M, Won M H, Cole T B, Jensen M S, Palmiter R D, Danscher G. Brain Res. 2000;865:227–236. doi: 10.1016/s0006-8993(00)02227-7. [DOI] [PubMed] [Google Scholar]
- 44.Frederickson C J, Bush A I. Biometals. 2001;14:353–366. doi: 10.1023/a:1012934207456. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Hough C J, Frederickson C J, Sarvey J M. J Neurosci. 2001;21:8015–8025. doi: 10.1523/JNEUROSCI.21-20-08015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Hough C J, Suh S W, Sarvey J M, Frederickson C J. J Neurophysiol. 2001;86:2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- 47.Cole T B, Wenzel H J, Kafer K E, Schwartzkroin P A, Palmiter R D. Proc Natl Acad Sci USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole T B, Martyanova A, Palmiter R D. Brain Res. 2001;891:253–265. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- 49.Lee J-Y, Cole T B, Palmiter R D, Suh S W, Koh J-Y. Proc Natl Acad Sci USA. 2002;99:7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush A I. Curr Opin Chem Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 51.Bush A I, Tanzi R E. Proc Natl Acad Sci USA. 2002;99:7317–7319. doi: 10.1073/pnas.122249699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suh S W, Jensen K B, Jensen M S, Silva D S, Kesslak P J, Danscher G, Frederickson C J. Brain Res. 200;852:274–278. doi: 10.1016/s0006-8993(99)02096-x. [DOI] [PubMed] [Google Scholar]
- 53.Cuajungco M P, Lees G J. Neurobiol Dis. 1997;4:137–169. doi: 10.1006/nbdi.1997.0163. [DOI] [PubMed] [Google Scholar]
- 54.Choi D W, Koh J Y. Ann Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- 55.Weiss J H, Sensi S L, Koh J Y. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 56.Koh J-Y, Suh S W, Gwag B J, He Y Y, Hsu C Y, Choi D W. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 57.Suh S W, Chen J W, Motamedi M, Bell B, Listiak K, Pons N F, Danscher G, Frederickson C J. Brain Res. 2000;852:268–273. doi: 10.1016/s0006-8993(99)02095-8. [DOI] [PubMed] [Google Scholar]
- 58.Canzoniero L M T, Sensi S L, Choi D W. Neurobiol Dis. 1997;4:275–279. doi: 10.1006/nbdi.1997.0160. [DOI] [PubMed] [Google Scholar]
- 59.Manzerra P, Behrens M M, Canzoniero L M T, Wang X Q, Heidinger V, Ichinose T, Yu S P, Choi D W. Proc Natl Acad Sci USA. 2001;98:11055–11061. doi: 10.1073/pnas.191353598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim Y-H, Kim E Y, Gwag B J, Sohn S, Koh J-Y. Neuroscience. 1999;89:175–182. doi: 10.1016/s0306-4522(98)00313-3. [DOI] [PubMed] [Google Scholar]
- 61.Noh K-M, Koh J-Y. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suh S W, Listiack K, Bell B, Chen J, Motamedi M, Silva D, Danscher G, Whetsell W, Thompson R, Frederickson C J. J Histochem Cytochem. 1999;47:969–972. doi: 10.1177/002215549904700715. [DOI] [PubMed] [Google Scholar]
- 63.Lee J-Y, Cole T B, Palmiter R D, Koh J-Y. J Neurosci. 2000;20:RC79. doi: 10.1523/JNEUROSCI.20-11-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee J-Y, Park J, Kim Y-H, Kim D H, Kim C G, Koh J-Y. Exp Neurol. 2000;161:433–441. doi: 10.1006/exnr.1999.7297. [DOI] [PubMed] [Google Scholar]
- 65.Vasak M, Hasler D W. Curr Opin Chem Biol. 2000;4:177–183. doi: 10.1016/s1367-5931(00)00082-x. [DOI] [PubMed] [Google Scholar]
- 66.Hidalgo J, Aschner M, Zatta P, Vasak M. Brain Res Bull. 2001;55:133–145. doi: 10.1016/s0361-9230(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 67.Masters B A, Quaife C J, Erickson J C, Kelly E J, Froelick G J, Zambrowicz B P, Brinster R L, Palmiter R D. J Neurosci. 1994;14:5844–5857. doi: 10.1523/JNEUROSCI.14-10-05844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palumaa P, Eriste E, Njunkova O, Pokras L, Jornvall H, Sillard R. Biochemistry. 2002;41:6158–6163. doi: 10.1021/bi025664v. [DOI] [PubMed] [Google Scholar]
- 69.Uchida Y, Takio K, Koiti T, Yasue I, Tomonaga M. Neuron. 1991;7:337–347. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- 70.Yu W H, Lukiw W J, Bergeron C, Niznik H B, Fraser P E. Brain Res. 2001;894:37–45. doi: 10.1016/s0006-8993(00)03196-6. [DOI] [PubMed] [Google Scholar]
- 71.Thomas S Z, Froelick G J, Palmiter R D. J Neurosci. 1997;17:1271–1281. doi: 10.1523/JNEUROSCI.17-04-01271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown A M, Tummolo D M, Rhodes K J, Hofmann J R, Jacobsen J S, Sonnenberg-Reines J. J Neurochem. 1997;69:1204–1212. doi: 10.1046/j.1471-4159.1997.69031204.x. [DOI] [PubMed] [Google Scholar]
- 73.Kronke K, Fehsel K, Schmidt T, Zenke F T, Dasting I, Wesener J R, Bettermann H, Breunig K D, Kolb-Bachofen V. Biochem Biophys Res Commun. 1994;200:1105–1110. doi: 10.1006/bbrc.1994.1564. [DOI] [PubMed] [Google Scholar]
- 74.Berendji D, Kolb-Bachofen V, Meyer K L, Grapenthin O, Weber H, Wahn V, Krönke K-D. FEBS Lett. 1997;405:37–41. doi: 10.1016/s0014-5793(97)00150-6. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, Irie Y, Keung W M, Maret W. Biochemistry. 2002;41:8360–8367. doi: 10.1021/bi020030+. [DOI] [PubMed] [Google Scholar]
- 76.Tsien R Y. Ann Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- 77.Frederickson C J, Kasarskis E J, Ringo D, Frederickson R E. J Neurosci Methods. 1987;20:91–103. doi: 10.1016/0165-0270(87)90042-2. [DOI] [PubMed] [Google Scholar]
- 78.Mahadevan I B, Kimber M C, Lincoln S F, Tiekink E R T, Ward A D, Betts W H, Forbes I J, Zalewski P D. Aust J Chem. 1996;49:561–568. [Google Scholar]
- 79.Fahrni C J, O'Halloran T V. J Am Chem Soc. 1999;121:11448–11458. [Google Scholar]
- 80.Walkup G K, Burdette S C, Lippard S J, Tsien R Y. J Am Chem Soc. 2000;122:5644–5645. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 81.Burdette S C, Walkup G K, Tsien R Y, Lippard S J. J Am Chem Soc. 2001;123:7831–7841. doi: 10.1021/ja010059l. [DOI] [PubMed] [Google Scholar]
- 82.Burdette S C, Frederickson C J, Bu W, Lippard S J. J Am Chem Soc. 2003;125:1778–1787. doi: 10.1021/ja0287377. [DOI] [PubMed] [Google Scholar]
- 83.Burdette S C, Lippard S J. Inorg Chem. 2002;41:6816–6823. doi: 10.1021/ic026048q. [DOI] [PubMed] [Google Scholar]
- 84.Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T. Angew Chem Int Ed. 2000;39:1052–1058. doi: 10.1002/(sici)1521-3773(20000317)39:6<1052::aid-anie1052>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 85.Hirano T, Kikuchi K, Urano Y, Higuchi T, Nagano T. J Am Chem Soc. 2000;122:12399–12400. [Google Scholar]
- 86.Hirano T, Kikuchi K, Urano Y, Nagano T. J Am Chem Soc. 2002;124:6555–6562. doi: 10.1021/ja025567p. [DOI] [PubMed] [Google Scholar]
- 87.Gee K R, Zhou Z-L, Qian W-J, Kennedy R. J Am Chem Soc. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 88.Franz K J, Singh N, Lippard S J. Angew Chem Int Ed. 2000;39:10504–10512. doi: 10.1002/1521-3773(20000616)39:12<2120::aid-anie2120>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 89.Franz K J, Singh N, Spingler B, Lippard S J. Inorg Chem. 2000;39:4081–4092. doi: 10.1021/ic000344q. [DOI] [PubMed] [Google Scholar]
- 90.Hershfinkel M, Moran A, Sekler I. Proc Natl Acad Sci USA. 2001;98:11749–11754. doi: 10.1073/pnas.201193398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shamovsky I L, Ross G M, Riopelle R J, Weaver D F. J Am Chem Soc. 1999;121:9797–9806. [Google Scholar]
- 92.Shooter E M. Annu Rev Neurosci. 2001;24:601–629. doi: 10.1146/annurev.neuro.24.1.601. [DOI] [PubMed] [Google Scholar]
- 93.Heizmann C W, Cox J A. Biometals. 1998;11:383–397. doi: 10.1023/a:1009212521172. [DOI] [PubMed] [Google Scholar]