Abstract
The extent and cause of male-biased mutation rates, the higher number of mutations in sperm than in eggs, is currently an active and controversial subject. Recent evidence indicates that this male (sperm) bias not only occurs in animals but also in plants. The higher mutation rate in plant sperm was inferred from rates of evolution of neutral DNA regions, and the results were confined to the mitochondria and chloroplasts of gymnosperms. However, the relative transmission rates of deleterious mutations, which have substantial evolutionary consequences, have rarely been studied. Here, an investigation is described by using the hermaphroditic self-compatible flowering plant Arabidopsis thaliana, in which we artificially increased the rate of mutation in pollen (i.e., sperm donor) and maternal (i.e., egg donor) parents, by using two kinds of UV irradiation in parallel and separate experiments, and assessed the deleterious effects on fitness of the F2 generation. The results show that more deleterious induced mutations are transmitted to the progeny by a sperm than by an egg. These findings provide the first experimental evidence that more deleterious mutations are inherited from sperm than from an egg in any organism. Possible causes underlying this male bias are discussed.
One approach to estimating sex-specific mutation rates is to compare the evolutionary substitution rate of selectively neutral DNA, which equals the mutation rate (1), in male- and female-inherited DNA. By using this approach, male-biased mutation rates have been suggested to exist in humans and higher primates (2–10), birds (11, 12), rodents (13), and sheep (14), and more recently, in gymnosperms (15). Few studies, outside of human diseases (16), however, have examined whether more deleterious mutations (nonneutral) are inherited from a sperm than from an egg. This is remarkable because deleterious mutations play a fundamental role in many evolutionary theories and have been the focus of much research (e.g., refs. 17 and 18). The absence of scientific information about this topic may be due to the difficulty in developing experimental methods that can distinguish between male and female mutations and the poor suitability of DNA sequence data for such analysis because of lack of knowledge about the direction (i.e., positive, negative or neutral) and magnitude of selective effects. Other, more direct approaches, therefore need to be developed. We describe here an experimental approach to testing the hypothesis that the sperm transmit more induced deleterious mutations to the progeny than the eggs in Arabidopsis thaliana.
External mutagenic agents such as UV-irradiation are an effective tool to compare the level of deleterious mutations transmitted to the progeny by a sperm and an egg. UV-B and UV-C lead to DNA damage that includes the formation of pyrimidine dimers and 6,4 photoproducts (19), which change the binding properties between DNA strands and can lead to mutational clusters or “hotspots” on DNA replication (20–24). In addition, UV-B and UV-C can increase the production of free radicals, leading to DNA strand breaks and the formation of mutations by the misincorporation of nucleotides on strand-break repair (19, 20). These mutations can be transmitted by a sperm or an egg and have a deleterious effect on the fitness of progeny (25). It is therefore possible to evaluate the relative number of deleterious mutations that are transmitted by a sperm and an egg by assessing the fitness of offspring produced by UV-treated pollen parents (i.e., sperm donors) and UV-treated maternal parents (i.e., egg donors).
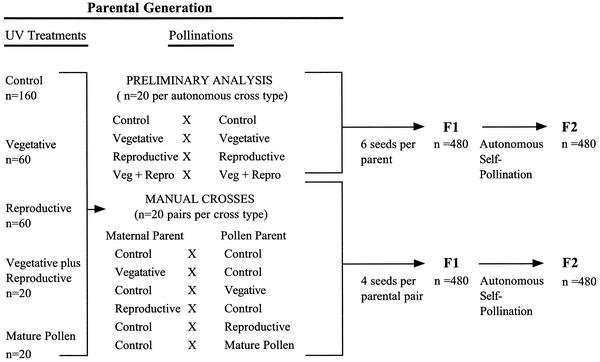
In the present investigation, we compare the fitness of two generations of progeny produced from UV-B- and UV-C-treated pollen and maternal parents in A. thaliana. Parent plants were exposed during either the vegetative stage, reproductive stage, or both, and mature pollen was also exposed. The analysis consisted of two main components (Fig. 1). First, we conducted a preliminary analysis to confirm that the doses of UV applied induced a sufficient level of mutations to have a detectable effect on the fitness of progeny. Subsequently, we performed manual crosses and compared the fitness of the F2 generation produced from UV-treated pollen and maternal parents and from UV-treated mature pollen. The F2 generation was used for all comparisons for two main reasons. Firstly, the homozygous mutant genotype was more frequent in the F2 than the F1 generation, thereby making fitness effects more evident. Secondly, and more importantly, fitness differences in the F2 generation can almost entirely be attributed to deleterious mutations, unlike in the F1 generation, which may be affected by physiological effects of UV on the quality of seed produced by the parental generation.
Figure 1.
UV-B and UV-C treatments of the parental generation and the types of crosses conducted to form the F1 and F2 generations for the preliminary analysis and for the manual crosses. The design was used separately for the UV-B and the UV-C experiments.
Materials and Methods
Genetically identical A. thaliana seeds produced from the self-pollination of a single plant were used as the parental generation in both the UV-C and UV-B experiments (catalog no. cs907, Arabidopsis Biological Resource Center, Columbus, OH). All plants were maintained in 2-cm-deep pots placed in trays measuring 25 cm × 40 cm in Schultz Cactus Mix. Fertilization was provided in deionized water by using Schultz General Nutrient Solution (seven drops per liter) approximately every three to four days. Plants were always watered from below by covering the bottom of trays and were well spaced to prevent shading.
UV Application.
For the UV-B experiment, the plants were grown under pairs of Plant and Aquarium bulbs (GE Lighting, Cleveland, OH) spaced 42 cm apart and suspended 38 cm above the surface of the trays for 18-hour days at 25°C. Individual fluorescent sunlamps (UV-B 313, Q-Panel, Cleveland, OH) were evenly spaced at 21-cm intervals with one lamp located between each pair of Plant and Aquarium bulbs. At the time of UV-B application, the plants were placed under wooden frames covered with either mylar (0.13 mm thick), to absorb UV-B radiation (no treatment), or with cellulose acetate film (0.075 mm thick), which transmits UV-B (for the various treatments). Both the mylar and the acetate were pretreated with UV-B for 8 h to increase their effectiveness in transmitting UV-B radiation (26). UV-B was measured with an Optronic OL 754 spectroradiometer with an integrating sphere that was calibrated by using an Optronic OL 752-irradiance standard (Optronic, Orlando, FL). Because the biological action spectrum that is most appropriate for a particular biological process remains largely uncertain, it is important to weight irradiance by each function that could be relevant. We therefore calculated the biologically active radiation by weighting the spectral irradiance by both a plant action spectrum (27, 28) and a DNA damage spectrum (29). The UV-B treatments consisted of a dose of 10.1 KJ/m2/day according to the plant action spectrum (28) applied over 18 h, followed by a 6-h period of darkness, and a subsequent treatment of 4.5 KJ/m2 over 8 h. This was the maximum dose that could be applied without affecting plant survival. These values were 3.7 and 1.7 KJ/m2/day, respectively, according to the DNA damage spectrum (29). Photosynthetically active radiation was measured with a quantum sensor (Apogee Instruments, Model QMSW-SS) and was 54 μmol/m2/s during the light cycle. The spectroradiometer and the quantum sensor were placed at 10.5-cm intervals along the bench, and mean values for the room were determined.
All plants other than the parental UV-B generation were grown for 18 h of light per day at 25°C under high-pressure sodium Superbulbs (P.L. Lighting Systems, Ontario, Canada) suspended ≈1.8 m above the trays at 1.2-m intervals. For the UV-C treatment, plants were irradiated with 1,000 J/m2 at 254 nm for 30 s by using a Minerallight-Lamp R-52 (UV-Products, San Gabriel, CA) held 3 cm above the rosette for all treatments except the pollen treatment, where it was held 3 cm from the pollen-producing flower. Average photosynthetically active radiation during the light cycle was 120 μmol/m2/s.
Experimental Design.
The same design was used in the separate UV-B and UV-C experiments (Fig. 1). This consisted of two main components. First, a preliminary analysis was conducted to assess the fitness of progeny produced following autonomous self-pollination of individuals treated with UV during the vegetative and/or reproductive stage. Second, a series of manual crosses was conducted to evaluate the fitness of progeny produced by pollen parents (sperm donor) and maternal parents (egg donor) that were UV-treated during the vegetative or reproductive stage. Fitness of progeny produced by UV-treated mature pollen was also assessed. The vegetative treatment was applied 25 days after germination at the rosette stage, and the reproductive treatment was applied on day 32, near the initiation of bolting. A total of 320 individuals was used in the parental generation. Of these, 160 were treated with UV. Specifically, 60 randomly chosen plants were treated with UV during the vegetative stage, 60 were treated during the reproductive stage, 20 were treated during both the vegetative and reproductive stages and 20 were treated during pollen release (i.e., the pollen was treated following the opening of the anther). The 160 plants that were not treated with UV were used as controls. Treated and control individuals were randomly assigned to undergo autonomous self-pollination (n = 80), for the preliminary analysis, or manual crossing (n = 240, Fig. 1). Manual crosses were conducted between the controls and pollen and maternal parents from the vegetative, reproductive and mature pollen treatments to produce the F1 generation. Manual crosses were also conducted among the controls to account for any effect of emasculation on progeny fitness. For both the preliminary analysis and manual crosses, the F1 generation subsequently underwent autonomous self-pollination to form the F2 generation. Each individual in the parental generation was used only once, and thus all crosses were independent.
Offspring Measurements and Analysis.
Seeds comprising the F1 generation for both the UV-C and UV-B treatments were sown in the growth room that was used for UV-C treatments. The seeds were placed in trays, each containing 24 pots. For the preliminary analysis, each tray contained six offspring from a single randomly chosen parent from the vegetative treatment, the reproductive treatment, the vegetative plus reproductive treatment and from the controls (four types of treatments per tray). Six seeds of each parent per treatment were randomly placed among the pots and trays were randomly positioned on the bench. For the manually crossed plants, each tray contained 4 seeds per parent plant per cross type (six types of crosses per tray, see Fig. 1). Following self-pollination the seeds produced by the F1 individuals were collected and these comprised the F2 generation. One seed was randomly chosen per individual and was sown as described for the F1 generation, with one parent (i.e., from the parental generation) per tray. For the F1 and F2 generation there were 480 individuals for the preliminary analysis and for the manual crosses, for each UV-type. On maturity, measurements were taken of the seed number per flower (averaged over three flowers) and the number of flowers per individual for both F1 and F2. Total fitness for each individual was determined as the number of flowers × the average number of seeds per flower. The mean total fitness was determined for each treatment per parent or grandparent for the F1 and for F2 generations, respectively.
A separate analysis was conducted for each combination of UV-type (UV-B and UV-C) and each generation category (the F1 and F2 generations). For the preliminary analysis, we conducted a one-way ANOVA with four treatment categories (control, vegetative stage, reproductive stage, and both vegetative and reproductive stages) by using the mean fitness for each parent within a tray (maximum n = 20 parents per treatment type per tray). For the manual crosses, a one-way ANOVA was conducted by using the six treatment categories that were based on the timing of UV-application and the sex of the UV-exposed parent (i.e., control, maternal parent-vegetative stage, maternal parent-reproductive stage, pollen parent-vegetative stage, pollen parent-reproductive stage, and mature pollen) by using the mean fitness for each treatment type within a tray. Pairwise comparisons of treatments were conducted by using Tukey post hoc tests (30).
Calculation of the Sperm-to-Egg Mutation Transmission Ratio.
The sperm-to-egg mutation transmission ratio was estimated by using fitness data from the F2 generation. When proportional reductions in fitness combine in a multiplicative fashion across loci, as appears reasonable (31, 32), the relative fitness of an individual with i heterozygous and j homozygous mutations is w̃ = (1 − hs)i (1 − s)j where h is the dominance coefficient of mutant alleles, and s is the selection coefficient against a homozygous mutant locus (e.g., ref. 33). Although the F2 was produced by self-fertilization of the F1, in which any new mutations were presumably heterozygous, the number of heterozygous (i) and homozygous (j) mutations in the F2 was unknown. We therefore treated relative fitnesses in the F2 as w̃ = (1 − hs)i (1 − s)j = (1 − x)n where n = i + j is the combined number of heterozygous and homozygous mutant loci. 1 − x equals (1 − hs)i(i+j) (1 − s)j(i+j) and is therefore a measure of the geometric mean relative fitness based on the two types of loci. It is thus straightforward to calculate the sperm-to-egg mutation transmission ratio as
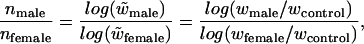 |
where wk is the mean absolute fitness of treatment k. This ratio was calculated for both the vegetative and reproductive treatments within each UV type for cases where mean control > mean maternal treatment > mean paternal treatment. Confidence intervals (95%) were estimated for the mean and median by using a bootstrap program written in Mathematica (34). For each treatment (control, treated maternal, treated paternal) F2 individuals were randomly resampled with replacement within trays, and the mean (or median) was obtained. The mean and median over trays were then used in the male-bias ratio, and the procedure was iterated 10,000 times.
Results
The preliminary analysis was conducted to assess whether there was a detectable effect of deleterious mutations on the progeny in A. thaliana. Fitness of the F1 and the F2 generation produced from UV-B and UV-C exposed parents differed among treatment categories (one-way ANOVAs, Table 1). Specifically, the fitness of the vegetative, the reproductive and the vegetative plus reproductive treatments were each statistically significantly lower than the controls (Table 2). No statistically significant differences were detected between the vegetative, reproductive, and the vegetative plus reproductive treatments for either the F1 or F2 generation.
Table 1.
ANOVAs for the effect of UV on the fitness of the F1 and F2 generations for the preliminary analysis and for the manual crosses
| df | Mean square | F ratio | P < | R2 | |
|---|---|---|---|---|---|
| Preliminary analysis | |||||
| UV-C, F1 | 3 | 4 803 757 | 6.8 | 0.003 | 0.22 |
| UV-B, F1 | 3 | 8 172 055 | 14.7 | 0.0000003 | 0.38 |
| UV-C, F2 | 3 | 3 285 750 | 14.1 | 0.0000003 | 0.38 |
| UV-B, F2 | 3 | 2 021 530 | 8.3 | 0.0001 | 0.29 |
| Manual crosses | |||||
| UV-C, F1 | 5 | 412 136 | 2.0 | 0.091 | 0.13 |
| UV-B, F1 | 5 | 3 546 860 | 2.9 | 0.021 | 0.23 |
| UV-C, F2 | 5 | 4 186 215 | 7.5 | 0.000013 | 0.37 |
| UV-B, F2 | 5 | 5 784 270 | 14.8 | 0.00000001 | 0.60 |
Model: fitness = treatment category + constant + error.
Table 2.
Means, SE, and P values of Tukey pairwise comparisons for the preliminary analysis
| UV-C
|
UV-B
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean total fitness (SE) |
P value
|
Mean total fitness (SE) |
P value
|
|||||
| Vegetative stage | Reproductive stage | Vegetative + reproductive | Vegetative stage | Reproductive stage | Vegetative + reproductive | |||
| F1 generation | ||||||||
| Controls | 2491 (211) | 0.0007 | 0.0015 | 0.0143 | 2664 (170) | 0.0002 | 0.0002 | 0.0032 |
| Vegetative stage | 1274 (206) | 0.9925 | 0.7007 | 1283 (170) | 0.9998 | 0.9442 | ||
| Reproductive stage | 1355 (206) | 0.8533 | 1301 (166) | 0.9576 | ||||
| Veg. + Repro. | 1586 (200) | 1466 (281) | ||||||
| F2 generation | ||||||||
| Controls | 1853 (116) | 0.0002 | 0.0002 | 0.0002 | 1590 (113) | 0.0062 | 0.0022 | 0.0029 |
| Vegetative stage | 898 (113) | 0.8890 | 0.6820 | 917 (113) | 0.959 | 0.935 | ||
| Reproductive stage | 1014 (110) | 0.9801 | 996 (110) | 0.774 | ||||
| Veg. + Repro. | 1074 (110) | 788 (186) | ||||||
Bold indicates statistically significant (∝ = 0.05) P value after Tukey correction for multiple comparisons. Veg., vegetative; Repro., reproductive.
For the manual crosses, total fitness of the F1 generation differed among the six treatment categories for the UV-B but not for the UV-C exposures (one-way ANOVAs, Table 1). Only two of the pairwise comparisons were near statistical significance, however, namely the vegetative UV-B treatment of pollen parents and of mature pollen were lower than the controls (Table 3). In contrast, for the F2 generation, mean fitness differed among treatment categories for both UV-B and UV-C (Table 1), and many pairwise differences were evident (Table 3). Specifically, the mean fitness was statistically significantly lower for the progeny produced by UV-treated pollen parents than by treated maternal parents for both the vegetative and the reproductive treatments with UV-C and with UV-B (Table 3). Mean fitness of the UV-B and UV-C vegetative and reproductive treatments was statistically significantly lower for pollen parents than for the controls. No statistically significant differences, in contrast, were detected between the controls and the UV-treated maternal parents. Several differences were detected involving the treatment of mature pollen. The fitness of progeny produced from UV-B mature pollen was statistically significantly lower than the controls (Table 3). In addition, UV-B exposed mature pollen produced less fit F2 progeny than the maternal parents treated during the reproductive stage. Overall, the pattern of significant treatment effects is similar in the UV-B and UV-C experiments. The sperm-to-egg mutation transmission ratio (and the 95% confidence interval; CI), calculated by using mean fitnesses of the F2 generation was 7.5 (CI, −67 to 70) for the UV-B vegetative treatment and 25 (CI, −251 to 270) for the reproductive treatment. When median fitnesses were used, the ratios were 5.0 (CI, 1.7 to 9.0) for the UV-B vegetative treatments and 11.1 (CI, −162 to 190) for the reproductive treatment. Ratios were not calculable for the UV-C treatments.
Table 3.
Means, SE, and P values of Tukey pairwise comparisons for the manual crosses
| UV-C
|
UV-B
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean total fitness (SE) |
P value
|
Mean total fitness (SE) |
P value
|
|||||||||
| Maternal parent veg. | Maternal parent repro. | Pollen parent veg. | Pollen parent repro. | Mature pollen parent | Maternal parent veg. | Maternal parent repro. | Pollen parent veg. | Pollen parent repro. | Mature pollen parent | |||
| F1 generation | ||||||||||||
| Controls | 1403 (126) | 0.1386 | 0.5034 | 0.2056 | 0.1677 | 0.1913 | 1846 (122) | 0.1312 | 0.3513 | 0.3320 | 0.0576 | 0.0715 |
| Maternal parent veg. | 936 (137) | 0.9228 | 1 | 0.9998 | 0.9999 | 1207 (219) | 0.9291 | 0.9769 | 0.9999 | 0.9999 | ||
| Maternal parent repro. | 1109 (110) | 0.9419 | 0.9694 | 0.9556 | 1460 (147) | 0.9999 | 0.9330 | 0.8768 | ||||
| Pollen parent veg. | 931 (161) | 0.9998 | 0.9999 | 1412 (173) | 0.9845 | 0.9550 | ||||||
| Pollen parent repro. | 980 (121) | 0.9999 | 1253 (163) | 0.9989 | ||||||||
| Mature pollen parent | 954 (143) | 1185 (200) | ||||||||||
| F2 generation | ||||||||||||
| Controls | 1898 (207) | 0.6739 | 0.9683 | 0.0316 | 0.0377 | 0.6389 | 2516 (156) | 0.9032 | 0.9999 | 0.0001 | 0.0001 | 0.0036 |
| Maternal parent veg. | 2353 (225) | 0.9660 | 0.0008 | 0.0006 | 0.0633 | 2185 (278) | 0.9492 | 0.0071 | 0.0086 | 0.2471 | ||
| Maternal parent repro. | 2118 (186) | 0.0029 | 0.0022 | 0.2041 | 2482 (188) | 0.0002 | 0.0002 | 0.0096 | ||||
| Pollen parent veg. | 852 (264) | 0.9956 | 0.6624 | 873 (219) | 0.9999 | 0.7222 | ||||||
| Pollen parent repro. | 1021 (199) | 0.8442 | 924 (207) | 0.7905 | ||||||||
| Mature pollen parent | 1398 (249) | 1347 (254) | ||||||||||
Bold indicates statistically significant (∝ = 0.05) P value after Tukey correction for multiple comparisons. Veg., vegetative; repro., reproductive.
Discussion
Preliminary Analysis.
For the preliminary analysis, we exposed parent plants to UV-B or UV-C during different stages of development, specifically the vegetative stage, the reproductive stage, or both (Fig. 1). Individuals thus became +/− at certain loci for each individual, where “+/−” denotes the wild type and “−” represents the mutant allele. Subsequently, all parental individuals underwent autonomous self-pollination to form the F1 generation, which, on maturity, underwent self-pollination to form the F2 generation. Accordingly, the predicted genotypic frequencies for any locus experiencing mutation were ≈1/4 +/+, 1/4 −/−, and 1/2 +/− for the F1 generation and ≈3/8 +/+, 3/8 −/−, and 1/4 −/m for the F2 generation. That we found statistically significantly lower fitness, measured as total seed production, for the F2 generation produced from plants treated during each developmental stage relative to the controls (Table 2) indicates that a detectable level of deleterious mutations induced in the parental generation were transmitted to the progeny, such that the main analysis using manual crosses could be conducted.
Fitness of Progeny of Pollen Versus Maternal Parents.
We manually crossed UV-treated parents with untreated plants to form the F1 generation, which underwent autonomous self-pollination to form the F2 generation. The estimated genotypes for any mutant locus in the F1 generation were thus entirely +/− and were ≈1/4 +/+, 1/4 −/− and 1/2 +/− in the F2 generation. The data from the F2 generation, containing some homozygous mutants and minimal/no residual physiological effects from the UV exposures, demonstrate that more deleterious mutations were transmitted to the progeny by sperm than by eggs. For the UV-C and the UV-B treatments, the mean total fitness was statistically significantly lower for F2 progeny of exposed pollen parents than the maternal parents, for both the vegetative and reproductive treatments (Table 3). In fact, the fitness of progeny of UV-treated pollen parents was statistically significantly lower than maternal parents in all pairwise comparisons, even when the timing of UV application differed. The finding of statistically significantly lower fitness of the progeny of UV-treated pollen parents than UV-treated maternal parents for the vegetative treatment indicates, remarkably, that more somatic mutations, which arose long before sexual differentiation, and even before formation of the reproductive apex, are transmitted to offspring by the sperm than the eggs. Further supporting evidence for sperm-biased transmission of mutations includes the statistically significantly lower total fitness of the F2 generation produced from UV-B- and UV-C-treated pollen parents than the controls (Table 3). In addition, the fitness of progeny produced by UV-B-treated mature pollen was statistically significantly lower than the maternal parents that were UV-treated during the reproductive stage as well as the controls. That the results were consistent for the UV-B and UV-C exposures strengthen the results beyond those which would have been possible by using only a single UV type.
Although the fitness differences show evidence that male-bias exists in these plants, the magnitude of this effect is less certain. This is largely because the sperm-to-egg mutation transmission ratios were estimated by using the ratio of the logarithm of two ratios (and thus have large errors). We used mean fitnesses to estimate the sperm-to-egg mutation transmission ratio for the F2 generation as between 7.5 (95% CI, −35 to 79) and 25 (95% CI, −17 to 375) for the UV-B treatments. Use of median fitness, however, gave less variable results: the transmission bias ratio was 5.0 (CI, 1.7 to 9.0), which excludes unity, for the UV-B vegetative treatment and was 11.1 (CI, −162 to 190) for the reproductive treatment. The ratios were not calculable for UV-C because the mean fitness of the controls in the F2 generation was smaller than the female treatments, largely resulting from unusually low control values in two trays. It is probable that larger sample sizes within each treatment would have allowed calculation of the ratios for the UV-C treatments and narrowed each of the confidence intervals for UV-B treatments to exclude unity (we maintained ≈1,900 plants per generation, the maximum permitted in our growth facilities). Nevertheless, it is notable that the estimates of the sperm-to-egg mutation ratio, of between 5 and 25, are consistent with the sex-specific mutation ratios predicted by using male- and female- inherited mitochondrial and chloroplastidial DNA sequences in gymnosperms (15), which were between 2 and 10. One would not necessarily expect these ratios to be similar, however, because, in the gymnosperm study, the sperm-to-egg mutation ratio represents naturally occurring and selectively neutral mutations, whereas in the present study it represents the transmission of induced deleterious mutations to progeny. Further study will be needed to narrow the confidence intervals of the sperm-to-egg mutation transmission ratio of deleterious mutations in plants.
Our results indicate that the differential transfer of mutations largely involves nuclear genes, for the following reason. Because mitochondrial and chloroplastidial DNA is maternally inherited in A. thaliana, UV-induced mutations in these organelles would reduce fitness in the F2 progeny of UV-treated maternal parents. We observed, however, that the fitness of the F2 generation produced by UV-treated maternal parents was higher than for UV-treated pollen parents, despite the effects of any deleterious mutations in the organellar DNA. This suggests that more mutations in nuclear genes are transmitted by the sperm than the eggs. This result for nonneutral, nuclear mutations thus extends the previous findings that sperm transmit more selectively neutral chloroplastidial and mitochondrial mutations to progeny in plants (15). Further study is needed to determine whether different levels of deleterious mutations in the chloroplastidial and mitochondrial DNA are transmitted by the sperm than the eggs.
What Causes Male-Biased Mutation Transmission?
There are at least four plausible explanations for the increased transmission of deleterious mutations through sperm than egg. First, fewer UV-induced somatic mutations may be removed by cell lineage selection (selection against mutant diploid cells during development, ref. 35) for a sperm than for an egg. Second, the egg (or megagametophyte) may experience stronger selection than the sperm (or pollen). Third, there may be no difference in mutation number, but rather the deleterious mutations have greater effect on progeny produced by mutant sperm than mutant eggs. Although this could partially explain a sex-specific bias in the F1 generation, it is an unlikely explanation for our results because a mutation in the F2 generation, which we examined, would have the same effect regardless of whether it was inherited from a sperm or from an egg. Fourth, it is possible that UV radiation has different physiological effects on sperm and egg production, causing a different number of mutations to arise during gametogenesis. This also seems unlikely, because the results were consistent for two kinds of UV radiation that were applied well before gametogenesis, at different stages of development. It therefore appears most likely that the sperm transmission bias is caused either by greater cell lineage selection or stronger selection at the gametophyte stage for an egg than for a sperm.
Acknowledgments
We thank Dr. J. Cullen for use of the spectroradiometer. We are grateful to the anonymous reviewers for this journal as well as another helpful reviewer. This work was supported by Natural Sciences and Engineering Research Council (Canada) research and equipment grants (to M.O.J.) and by a Natural Sciences and Engineering Research Council graduate scholarship, a Killam Memorial graduate scholarship, and a Natural Resources Canada Natural Sciences and Engineering Research Council supplement (to C.-A.W.).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kimura M. The Neutral Theory of Molecular Evolution. New York: Cambridge Univ. Press; 1983. [Google Scholar]
- 2.Agulnik A I, Bishop C E, Lerner J L, Agulnik S I, Solovyev V V. Mamm Genome. 1997;8:134–138. doi: 10.1007/s003359900372. [DOI] [PubMed] [Google Scholar]
- 3.Miyata T, Hayashida H, Kuma K, Misuyasa K, Yasunaga T. Cold Spring Harbor Symp Quant Biol. 1987;52:863–867. doi: 10.1101/sqb.1987.052.01.094. [DOI] [PubMed] [Google Scholar]
- 4.Bohossian H B, Skaletsky H, Page D C. Nature. 2000;406:622–625. doi: 10.1038/35020557. [DOI] [PubMed] [Google Scholar]
- 5.Huttley G A, Jacobsen I B, Wilson S R, Easteal S. Mol Biol Evol. 2000;17:929–937. doi: 10.1093/oxfordjournals.molbev.a026373. [DOI] [PubMed] [Google Scholar]
- 6.Makova K D, Li W-H. Nature. 2002;416:624–626. doi: 10.1038/416624a. [DOI] [PubMed] [Google Scholar]
- 7.Nachman M W, Crowell S L. Genetics. 2000;156:297–304. doi: 10.1093/genetics/156.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellegren H. Nat Genet. 2000;24:400–402. doi: 10.1038/74249. [DOI] [PubMed] [Google Scholar]
- 9.Huang W, Chang B H-J, Gu X, Hewitt-Emmett D, Li W H. J Mol Evol. 1997;44:463–465. doi: 10.1007/pl00006166. [DOI] [PubMed] [Google Scholar]
- 10.Shimmin L C, Chang B H J, Li W H. Nature. 1993;362:745–747. doi: 10.1038/362745a0. [DOI] [PubMed] [Google Scholar]
- 11.Kahn N W, Quinn T W. J Mol Evol. 1999;49:750–759. doi: 10.1007/pl00006597. [DOI] [PubMed] [Google Scholar]
- 12.Ellegren H, Fridolfsson A K. Nat Genet. 1997;17:182–184. doi: 10.1038/ng1097-182. [DOI] [PubMed] [Google Scholar]
- 13.Chang B H, Shimmin L C, Shyue S K, Hewett-Emmett D, Li W H. Proc Natl Acad Sci USA. 1994;91:827–831. doi: 10.1073/pnas.91.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson L J, Hewitt G M. J Mol Evol. 2002;54:54–61. doi: 10.1007/s00239-001-0017-x. [DOI] [PubMed] [Google Scholar]
- 15.Whittle C A, Johnston M O. Mol Biol Evol. 2002;19:938–949. doi: 10.1093/oxfordjournals.molbev.a004151. [DOI] [PubMed] [Google Scholar]
- 16.Crow J F. Proc Natl Acad Sci USA. 1997;94:8380–8386. doi: 10.1073/pnas.94.16.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake J W, Charlesworth B, Charlesworth D, Crow J F. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch M, Blanchard J, Houle D, Kibota T, Schultz S, Vassilieva L, Willis J. Evolution (Lawrence, Kans) 1999;53:645–663. doi: 10.1111/j.1558-5646.1999.tb05361.x. [DOI] [PubMed] [Google Scholar]
- 19.Friedberg E C, Walker G C, Wolfram S. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. Microbiol.; 1995. [Google Scholar]
- 20.Hutchingson G. Phytochem Photobiol. 1987;45:897–903. [Google Scholar]
- 21.Jiang N, Taylor J-S. Biochemistry. 1993;32:472–481. doi: 10.1021/bi00053a011. [DOI] [PubMed] [Google Scholar]
- 22.You Y H, Lee D H, Yoon J H, Nakajima S, Yasui A, Pfeifer G P. J Biol Chem. 2001;276:44688–44694. doi: 10.1074/jbc.M107696200. [DOI] [PubMed] [Google Scholar]
- 23.Lee J H, Hwang G S, Choi B S. Proc Natl Acad Sci USA. 1999;96:6632–6636. doi: 10.1073/pnas.96.12.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovalchuk I, Kovalchuk O, Hohn B. EMBO J. 2000;19:4431–4438. doi: 10.1093/emboj/19.17.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strid A, Chow W S, Anderson J M. Photosynth Res. 1994;39:475–489. doi: 10.1007/BF00014600. [DOI] [PubMed] [Google Scholar]
- 26.Middleton E M, Teramura A H. Photochem Photobiol. 1993;57:744–751. [Google Scholar]
- 27.Caldwell M M. In: Photophysiology. Giese A C, editor. Vol. 6. New York: Academic; 1971. pp. 131–177. [Google Scholar]
- 28.Green A E S, Sawada T, Shettle E P. Photochem Photobiol. 1974;19:251–259. [Google Scholar]
- 29.Setlow R B. Proc Natl Acad Sci USA. 1974;71:3363–3366. doi: 10.1073/pnas.71.9.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson L, Hill M, Vang E. systat: Statistics. Evanston, IL: Syatat; 1992. , Version 5.2. [Google Scholar]
- 31.Willis J H. Evolution (Lawrence, Kans) 1993;47:864–876. doi: 10.1111/j.1558-5646.1993.tb01240.x. [DOI] [PubMed] [Google Scholar]
- 32.Elena S F, Lenski R E. Nature. 1997;390:395–398. doi: 10.1038/37108. [DOI] [PubMed] [Google Scholar]
- 33.Charlesworth D, Charlesworth B. Evolution (Lawrence, Kans) 1992;46:703–720. doi: 10.1111/j.1558-5646.1992.tb02077.x. [DOI] [PubMed] [Google Scholar]
- 34.Wolfram S. mathematica: A System For Doing Mathematics by Computer. New York: Wolfram Media/Cambridge Univ. Press; 1999. [Google Scholar]
- 35.Otto S P, Hastings I M. Genetica. 1998;102/103:507–524. [PubMed] [Google Scholar]