Abstract
Through a multiplex promoter spanning 218 kb, the phase II UDP-glucuronosyltransferase 1A (UGT1) gene encodes at least eight differently regulated mRNAs whose protein products function as the principal means to eliminate a vast array of steroids, heme metabolites, environmental toxins, and drugs. The orphan nuclear receptors pregnane X receptor (PXR) and constitutive androstane receptor (CAR) were originally identified as sensors able to respond to numerous environmentally derived foreign compounds (xenobiotics) to promote detoxification by phase I cytochrome P450 genes. In this report, we show that both receptors can induce specific UGT1A isoforms including those involved in estrogen, thyroxin, bilirubin, and carcinogen metabolism. Transgenic mice expressing a constitutively active form of human PXR show markedly increased UGT activity toward steroid, heme, and carcinogens, enhanced bilirubin clearance, as well as massively increased steroid clearance. The ability of PXR and constitutive androstane receptor and their ligands to transduce both the phase I and phase II adaptive hepatic response defines a unique transcriptional interface that bridges the ingestion and metabolism of environmental compounds to body physiology.
The metabolism of steroid hormones, other endogenous compounds, and xenobiotics occurs in the hepatogastrointestinal tract. This catabolic process is mediated by phase I enzymes, such as the monooxygenase CYP enzymes (1), as well as the phase II conjugating enzymes, such as UDP-glucuronosyltransferase (UGTs), sulfotransferases, and GSTs (2). The UGT 1A (UGT1A) locus is controlled by 13 promoters (A1–A12), spanning >200 kb of upstream sequence encoding overlapping but distinct mRNAs (3). Using UDP-glucuronic acid as a cosubstrate, UGT enzymes convert a diverse set of lipophilic substances to water-soluble glucuronides and function as the principal means to eliminate steroids, heme metabolites, environmental toxins, and drugs from the body (2). Crigler–Najjar syndrome type I results in a lethal accumulation of bilirubin due to a defect in the 1A1 promoter, whereas Gilbert's disease (7% of the population) is a more mild 1A1 promoter defect typified by increased sensitivity to certain drugs like Tylenol (2).
Nuclear receptor (NR) pregnane X receptor (PXR, also known as the steroid and xenobiotic receptor or SXR) and the constitutive androstane receptor (CAR) were originally shown to act through NR response elements localized in the promoters of the target CYP3A and CYP2B genes (4–10). Pharmaceutical compounds such as phenobarbital and rifampicin (RIF) have been reported as UGT inducers, although the molecular basis remains to be defined (11, 12). Both phenobarbital and RIF have been empirically used to treat hyperbilirubinemia, a clinical accumulation of serum bilirubin due to insufficient glucuronidation (11, 13). These compounds are activators of PXR and CAR, suggesting a plausible signaling pathway for UGT transcription.
In this report, we show that both receptors can induce specific UGT1A isoforms including those specialized for bilirubin, carcinogens, estrogen, and thyroxin metabolism. Moreover, activation of PXR in transgenic mice is sufficient to increase levels of UGT expression and activity, as well as bilirubin and steroid clearance.
Materials and Methods
Animals.
The generation of VP-hPXR, hPXR (previously known as VPSXR and SXR) transgenic mice and PXR null mice has been described (8).
UGT1A1 Promoter Cloning and Site-Directed Mutagenesis.
The human UGT1A1 promoter was amplified by using a BAC clone containing the entire UGT1 locus template (2). All primers were designed based on the sequence encoding the human UGT1A locus published in the National Center for Biotechnology Information GenBank (accession no. AF297093). Site-directed mutagenesis was performed by PCR and confirmed by DNA sequencing.
DNA-Binding Analysis.
Electrophoretic mobility-shift assays (EMSA) were performed by using in vitro-transcribed and -translated protein (TNT, Promega) as described (14). Oligonucleotides were: UGT1A1, 5′-CTAACGGTTCATAAAGGGTATTAGGT-3′; UGT1A6, 5′-CGAGTAGGTCATAAAGGTCACA-3′; and UGT1A6m, 5′-CGAGTAGAACATAAAGAACACA-3′.
Plasmid Construction and Transfection.
The tk-UGT1A6/DR3-Luc and its mutant variants were generated by insertion of corresponding annealed oligonucleotides into the tk-Luc vector. The expression vectors for hPXR, VP-hPXR, and VP-CAR have been described (14). CV-1 cell transfection using N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (Roche Biochemicals) and HepG2 cell transfections using Lipofectamine Plus (Invitrogen) were carried out as before (14, 15).
Northern and Western Blot Analysis.
Total RNA was prepared from liver tissues by using TRIzol reagent (Invitrogen). Northern hybridization was carried out as described (8). Liver microsomes were prepared and analyzed for UGT1A expression. Western blot profiles were conducted by using an anti-UGT1A antibody, which recognizes only the UGT1A family of proteins, and an anti-UGT1A1 antibody that is specific for UGT1A1.
UGT Analysis.
The UGT analysis was similar to that described before (16). In brief, mouse liver microsomes were prepared as described (16). The [14C]UDP-GlcUA was used as the sugar donor, and TLC was used to separate glucuronidation products. The products were visualized with a Molecular Dynamics Storm 820 PhosphorImager. Alternatively, silica gel in zones corresponding to the glucuronide bands were visualized by autoradiography, or corresponding areas from control lanes were scraped into scintillation vials, and radioactivity was measured by liquid scintillation counting.
Bilirubin Clearance.
Adult males were given a single does of bilirubin (10 mg/kg body weight). Blood samples were collected in untreated tubes 1 h after injection. Serum was prepared by centrifugation at 4,000 rpm for 10 min. Total and conjugated bilirubin levels were measured by Antech Diagnostic (Lake Success, NY). The statistical analysis was performed with INSTAT 2.03 software.
Blood and Urine Collection and Hormone Analysis.
Mouse blood samples were collected in EDTA-coated tubes (Becton Dickinson) at 4 p.m. by retroorbital eye bleeding within 1 min of initial disturbance. The 24-h urine was collected by using mouse metabolic cages (Nalge). Corticosterone levels in plasma or urine were measured with a Corticosterone [125I]RIA kit from ICN. The statistical analysis was performed with INSTAT 2.03 software.
Results
Activation of PXR Induces UGT Expression and Glucuronidation in Transgenic Mice.
The regulatory locus of the UGT1A gene is highly complex. It harbors 12 promoters that control the independent expression of a specific UGT isoform with distinctive substrate specificity (2). The isoforms vary at the amino terminus and share a common body coded by exons 2–5. For example, the 1A1 isoform metabolizes estrogens but not androgens or other steroids. It also metabolizes bilirubin, carcinogens, and Tylenol. To explore whether individual isoforms of UGTs are transcriptional targets for PXR, we examined profiles of hepatic proteins and mRNAs isolated from transgenic mice harboring an activated hPXR in the liver (8). Liver microsomal proteins were prepared and subjected to Western blot analysis using a pan-UGT1A antibody or a UGT1A1 isoform-specific antibody.
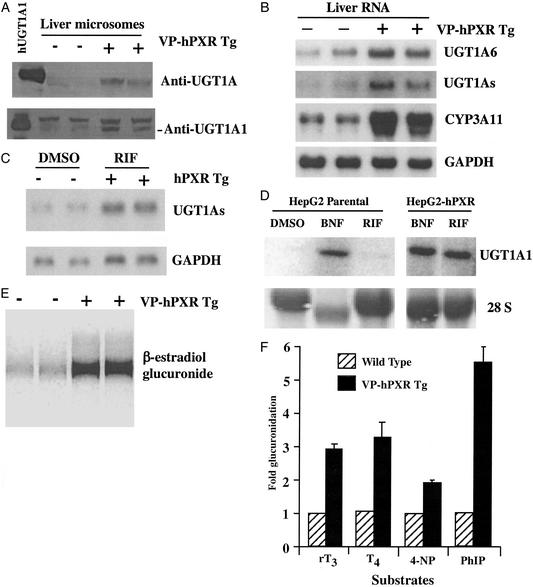
As shown in Fig. 1A, the VP-hPXR mice have increased levels of both general UGT1A protein and the UGT1A1 isoform. In addition to UGT1A1, Northern blot analysis revealed that UGT1A6 mRNA is up-regulated (Fig. 1B). As expected, up-regulation of general UGT1A mRNAs was observed when a probe containing the common 2–5 exons was used. This probe encompasses exons that are shared by all UGT1A family members and thus measures combined regulation of the entire locus (17). Whereas the induction of UGT in VP-hPXR mice is clear, it is not as dramatic as that of CYP3A11, probably because not all UGT isoforms are PXR targets (Fig. 1B). The up-regulation is liver-specific, as no change in UGT expression was seen in tissues that do not express the transgene, such as the small intestine (data not shown). A ligand-dependent induction of UGT1A was also observed in the humanized mice that express a full-length hPXR transgene (8). As expected, treatment of these mice with the prescription antibiotic RIF, a potent hPXR-specific activator, elevated UGT1As mRNA within 24 h of a single oral dose (Fig. 1C). This establishes that human PXR and its hormone-specific ligands can regulate the UGT1A locus in vivo.
Figure 1.
Induction of UGT1A expression and glucuronidation by PXR activation. (A) Liver microsomes were prepared from WT and VP-hPXR transgenic mice and subject to Western blot profiling by using a pan anti-UGT1A antibody and a specific anti-UGT1A1 antibody. (B) Mouse liver total RNAs were subjected to Northern blot analysis. The membranes were probed for UGT1A6, UGT1As, CYP3A11, and GAPDH as a loading control. (C) Induction of UGT1A mRNA by RIF in hPXR transgenic mice. WT or transgenic males were gavaged with a single dose of solvent or RIF (50 mg/kg) 24 h before death. (D) The parental HepG2 cells or the HepG2-hPXR stable cells were treated with DMSO or 25 μM RIF for 24 h and subsequently harvested for Northern blot analysis to detect UGT1A1 mRNA. (E) Glucuronidation activity toward β-estradiol. The result is shown as the autoradiograph of a TLC plate. (F) Glucuronidation toward thyroid hormones (rT3 and T4) and xenobiotics (4-nitrophenol and 4-OH-PhIP). Results are presented as fold increase in glucuronidation activity over WTs and represent the mean and standard error.
The sufficiency of PXR to mediate transactivation of the 1A1 “Gilbert's disease” isoform was examined in cultured cells. For these studies, HepG2 cells stably expressing hPXR were generated, and the expression of the transduced hPXR was confirmed by Northern blot analysis (data not shown). In contrast to the parental HepG2 line, the HepG2-hPXR stable cells are readily responsive to RIF, inducing UGT1A1 to a level that is comparable to the PXR-independent inducer β-naphthoflavone (Fig. 1D). β-Naphthoflavone is a prototypical aromatic hydrocarbon standard that induces UGT1A1 in PXR-deficient HepG2 cells. A sensitive cotransfection screen confirms that β-naphthoflavone does not activate PXR even at concentrations exceeding its maximal activation of UGT1A1 (data not shown). Together, these results suggest that at least two UGT1A isoforms (A1 and A6) are potential transcriptional targets of PXR. This is consistent with a recent quantitative PCR analysis that UGT1A mRNAs are regulated in vivo by PXR-specific ligands (18). Presumably, increased UGT expression would be associated with increased enzymatic activity. Compared with WT littermates, liver microsomes prepared from VP-hPXR mice exhibit profoundly higher glucuronidation activity toward β-estradiol, a known 1A1-selective substrate (Fig. 1E), thyroid hormones (rT3 and T4) classified as 1A1-preferred substrates (19), as well as the xenobiotic carcinogens 4-nitrophenol and 4-OH-PhIP, which are substrates for multiple 1A isoforms (20) (Fig. 1F). Microsomes prepared from RIF-treated HepG2-hPXR cells also exhibited higher glucuronidation activity (data not shown).
Regulation of UGT1A Gene Promoters by PXR and CAR.
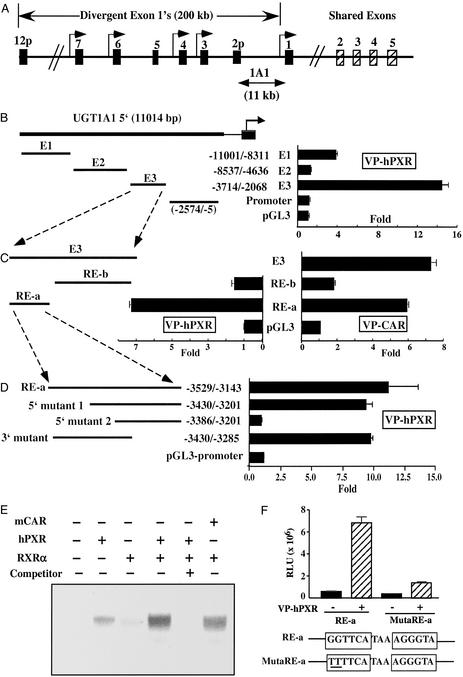
To delineate the molecular basis of UGT1A regulation, we have cloned the human UGT1 gene locus and analyzed its potential regulation by PXR and CAR. As mentioned above, the 200-kb human UGT1 gene locus has a highly specialized genomic organization, in that each of the UGT1A family members has a unique first exon but shares common exons 2–5 (Fig. 2A and ref. 17). Each first exon is proximal to its own unique promoter, which, in turn, is expressed in a characteristic tissue pattern and each UGT isoform has its substrate preferences. For example, expressed in liver, gut, and kidney, UGT1A1 metabolizes estrogen, bilirubin, thyroid hormone, and many other substrates. An 11-kb sequence of the 5′ regulatory region of the human UGT1A1 gene was cloned by PCR. Four fragments of regulatory sequences, a promoter and three enhancer elements (E1–3), that encompass the 11-kb sequence were subcloned into the pGL3 vector to generate a series of luciferase reporter constructs. The reporter genes were cotransfected with expression vectors for constitutively active forms of the receptors, VP-hPXR and VP-CAR, to test their ability to mediate transactivation by these receptors. As shown in Fig. 2 B and C, the E3 sequence that flanks −3,714 to −2,068 relative to the transcription start site is readily and profoundly activated in the presence of VP-hPXR and VP-CAR. A further dissection of the E3 fragment revealed that the PXR-responsive region is localized to the 386-bp RE-a (nucleotides −3,529 to −3,143) region (Fig. 2C). The 5′ deletion mutant 1 of RE-a does not affect the inducibility, whereas deletion mutant 2 completely abolishes the activation (Fig. 2D). Therefore, the 47-bp sequence between deletion mutants 1 and 2 is required for PXR transactivation. Inspection of this 47-bp enhancer element revealed a DR-3-like NR response element (GGTTCATAAAGGGTA). EMSA revealed that the PXR/RXRα heterodimers could bind to this element (Fig. 2E). Interestingly, a low level of binding was also seen when only PXR was present, and the migration was more consistent with a homodimer than a monomer (Fig. 2E). The binding is specific as efficient competition can be achieved by excessive unlabeled oligonucleotides. The CAR/RXRα heterodimers, but not CAR by itself, also exhibit measurable binding to the same element (Fig. 2E and data not shown), consistent with the transactivation of RE-a by CAR and our previous observation that CAR can share the DR-3 element with PXR (14). Mutation of the DR-3 in the context of RE-a abolishes VP-hPXR-dependent induction (Fig. 2F), indicating that this element is necessary for PXR transactivation. In addition to the 47-bp enhancer element, RE-a contains three additional previously reported putative NR binding sites, one of which binds to CAR (21). Our EMSA revealed that two of the DR-4-type elements can also bind to PXR (data not shown). Therefore, these additional sites may also contribute to 1A1 transactivation by PXR and CAR.
Figure 2.
Cloning of the UGT1A1 gene promoter and its activation by PXR and CAR. (A) Schematic representation of the UGT1 gene locus. The 5′ regulatory sequences for UGT1A1 isoform are indicated. (B) Analysis of the UGT1A1 5′ regulatory sequences. The Enhancer 1 (−11,001 to −8,311), Enhancer 2 (−8,537 to −4,636), Enhancer 3 (−3,714 to −2,068), and Promoter (−2,574 to −5), relative to the transcription start site, were amplified by PCR and cloned into pGL3-Luc reporter vector. The reporter genes were transfected into HepG2 cells in the presence of an expression vector for VP-hPXR. The fold induction was calculated from the luciferase activity of VP-hPXR cotransfected cells over empty vector transfected cells. (C) Enhancer 3 was dissected into RE-a and RE-b and tested for transactivation in the presence of VP-hPXR or VP-CAR. (D) Deletion mutants used in transfection experiments are shown diagrammatically. (E) EMSA of the putative DR-3 element using in vitro-translated proteins of hPXR, CAR, and RXRα. The competition was performed by using a 50-fold excess of unlabeled probe. (F) Mutation of the DR-3 in the context of RE-a blocks response to VP-hPXR.
Consistent with the induction of UGT1A6 mRNA in VP-hPXR mice, we have previously identified a putative DR-3-binding site in the UGT1A6 gene (Fig. 3A and ref. 4). As expected, the WT hPXR (Fig. 3A, lane 5), CAR (data not shown), and their respective activated variants VP-hPXR (Fig. 3A, lane 7) and VP-CAR (Fig. 3A, lane 9) bound to this element in an RXR-dependent manner (Fig. 3B). The specific binding was abrogated when this DR-3 was disrupted (Fig. 3A, lanes 11 and 12). To examine whether this DR-3 element can mediate transactivation by PXR and CAR, a luciferase reporter gene containing three copies of this element was placed upstream of a minimal thymidine kinase promoter and transfected into monkey kidney CV-1 cells together with expression vectors for hPXR, mPXR, or CAR. Significant activation of this UGT1A6 reporter by hPXR was seen when RIF, clotrimazole, and an extract of St. John's wort, but not androstenol or 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), were added to the culture medium (Fig. 3C). mPXR-mediated UGT activation was seen in the presence of St. John's wort, clotrimazole, as well as PCN, an mPXR-specific ligand. As predicted, CAR activates this UGT reporter in a ligand-independent manner. The activation by CAR is inhibited or potentiated by androstenol or TCPOBOP, respectively. The inhibitory effect of androstenol was compromised when TCPOBOP was coadded.
Figure 3.
Characterization of PXR and CAR binding in UGT1A6 promoter. (A) The sequence of the DR-3 NR response element found in the UGT1A6 gene. A mutant variant (mutated nucleotides underlined) and a previously identified DR-3 type of PXR response element found in the rat CYP3A23 gene are also shown. (B) PXR/RXR and CAR/RXR heterodimers bind to the UGT1A6/DR-3. EMSA was performed by using in vitro-translated hPXR, VP-hPXR, CAR, and RXRα proteins and radiolabeled oligonucleotides of UGT1A6 (lanes 1–10) and its mutant UGT1A6m (lanes 11 and 12). The binding of PXR/RXR heterodimers to CYP3A23 was also included as positive controls (lanes 13 and 14). (C) The tk-UGT1A6/DR-3-Luc reporter gene was transfected into CV-1 cells in the presence of the empty vector or the expression vectors for hPXR, mPXR, or CAR. The transfected cells were subsequently mock-treated or treated with indicated compounds for 24 h before luciferase assays. Results are shown as fold induction over solvent controls and represent the average and standard error from triplicate assays. The concentrations are 10 μM for PCN, RIF, and clotrimazole; 300 μg/ml for St. John's wort; 5 μM for androstenol; and 250 nM for TCPOBOP.
Activation of PXR Enhances Bilirubin Clearance.
UGT1A1 is the principal UGT isoform that facilitates bilirubin glucuronidation and subsequent clearance. The identification of UGT1A1 as direct target of PXR prompted us to examine the effect of PXR activation on bilirubin conjugation and clearance. The VP-hPXR mice were subjected to a single dose of bilirubin, and the serum bilirubin levels were monitored. As shown in Fig. 4, 1 h after injection, the remaining serum levels of both total and conjugated bilirubin in transgenic mice were less than half of their WT littermates. Therefore, activation of PXR in transgenic mice is sufficient to promote bilirubin conjugation and clearance, consistent with the up-regulation of UGT1A1 activity in these animals.
Figure 4.
Increased bilirubin clearance in VP-hPXR mice. Adult males of WT and VP-hPXR mice were treated with a single dose of bilirubin (10 mg/kg), and serum levels of total (A) and conjugated (B) bilirubin were measured 1 h after injection. The P value for total bilirubin is 0.001 between WT and VP-hPXR.
Increased Corticosterone Clearance in VP-hPXR Mice.
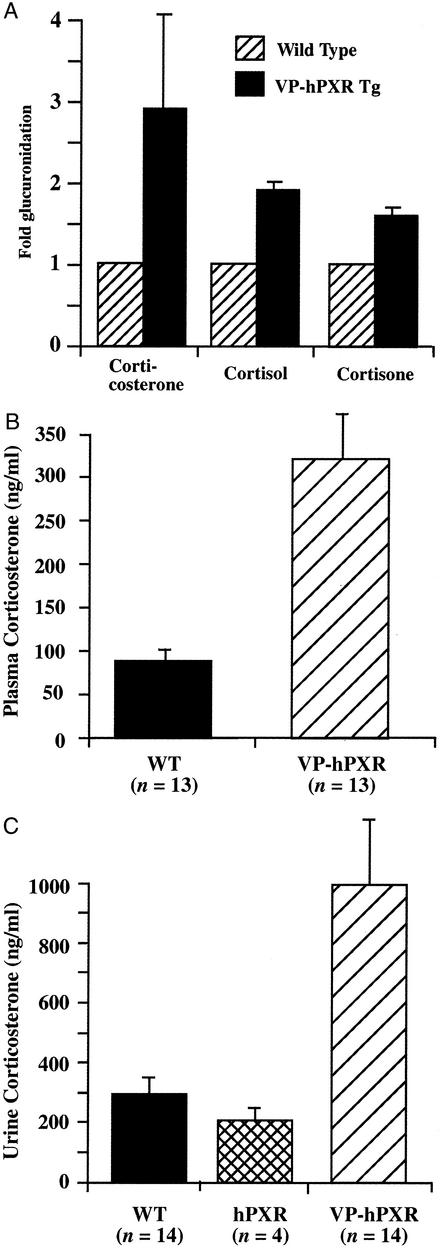
In addition to xenobiotics, UGTs are essential for the metabolism and elimination of steroid hormones. The up-regulation of UGT enzyme levels prompted us to assess a potential role of PXR in promoting steroid elimination and up-regulating the adrenal axis. As shown in Fig. 5A, liver microsomes of the VP-hPXR mice exhibited increased glucuronidation of several glucocorticoids, such as corticosterone, cortisol, and cortisone. To assess whether increased glucuronidation is associated with increased output of glucocorticoid, mouse plasma and urine samples were collected and measured for corticosterone, the principal glucocorticoid in rodents. The average plasma corticosterone concentration in WT males is 88.78 ng/ml, similar to published results (22), whereas the levels were elevated to 322.4 ng/ml in VP-hPXR mice (Fig. 5B), indicating an activation of the pituitary adrenal axis. A corresponding increase of urine corticosterone levels was also seen in VP-hPXR mice (Fig. 5C), whereas no significant changes in urinary (Fig. 5C) or blood (data not shown) corticosterone levels were observed in the untreated hPXR transgenic mice. Corticosterone is produced in the zona fasciculate of the adrenal gland cortex and subjected to the regulation of pituitary–adrenal axis. Histological analysis of the adrenal glands revealed no significant differences between WT and transgenic mice at 2.5 months (data not shown). Therefore, whereas both elimination and synthesis of corticosterone were increased, this had not yet led to an obvious morphologic change, suggesting that the increased demand was in the normal capacity of the adrenal gland. The measurement of ACTH in plasma and immunostaining detection of ACTH producing pituitary corticotropes also revealed no significant changes between WT and VP-hPXR transgenic mice (data not shown).
Figure 5.
Increased glucuronidation and output of corticosterone in VP-hPXR mice. (A) Increased glucuronidation of three corticosteroids, corticosterone, cortisone, and cortisol by liver microsomes of VP-hPXR mice. (B) Blood samples from 6-week-old males were collected via retroorbital eye bleeding, and plasma were prepared and subjected to corticosterone RIA. Nonparametric Mann–Whitney test P < 0.0001. (C) Twenty-four-hour urine samples were collected from 8- to 10-week-old males and subjected to corticosterone measurement. The P value is 0.5739 between WT and hPXR (not significant), 0.0004 between WT and VP-hPXR, and 0.0026 between hPXR and VP-hPXR.
In summary, activation of PXR results in increased levels of steroid in both plasma and urine, consistent with the premise that PXR activation can promote steroid clearance, presumably via its induction of cytochrome P450 (CYP) and UGT enzyme synthesis.
Discussion
The identification of the UGT locus as a direct target for hPXR and CAR has implications in both xenobiotic metabolism and human diseases. UGTs have been implicated in the etiology of human genetic diseases and carcinogenesis (2). The mutations in the UGT1A1 promoter are linked to inheritable hyperbilirubinemia as a result of decreased glucuronidation and, therefore, clearance of serum bilirubin (23). Our observation is also consistent with a recent study showing the regulation of bilirubin metabolism by CAR. In a related paper, Huang et al. (28) showed that activation of CAR increases hepatic expression of genes known to be involved in bilirubin metabolism including UGT1A1, and this induction is absent in CAR null mice. The carcinogenic potential of this gene was suggested in the Gunn rat, where a mutation in the UGT1 allele leads to a decrease glucuronidation of the carcinogenic benzo(a)pyrene, leading to elevated levels of DNA adducts (24). Moreover, down-regulation of UGT1 mRNA is observed in the early stages of cancer but not in benign tumorogenesis (25). Environmental mutagens, such as PhIP and benzo(a)pyrenes, have been identified as substrates for several UGT1 proteins (15, 20, 26). Indeed, PhIP is glucuronidated at a higher rate in VP-hPXR mice (Fig. 1F). Thus, the creation of these transgenic mice not only demonstrates a role for the receptor in its regulation, it provides a potential in vivo model to assess the molecular dynamics of carcinogenesis and the contribution of glucuronidation to this process. Finally, the increased levels of corticosterone in VP-hPXR mice provide evidence that PXR activation may directly contribute to the increased metabolism and/or elimination of steroids. Of note, increased glucuronidation was also seen for many other steroid hormones, including additional glucocorticoids, thyroid hormones, and estradiol, suggesting a broader role for xenobiotic nuclear receptors in hormonal homeostasis.
Although activation of PXR in transgenic mice is sufficient to induce the UGT locus, loss of PXR in knockout mice does not suppress basal expression levels (data not shown). The maintenance of basal expression in PXR null mice was also previously seen for CYP3A (8). It is possible that the sustained basal expression is mediated, in part, by CAR, as it is capable of binding to and activating through the DR-3 response element. Therefore, this study provides another example of the proposed molecular fail-safe model in xenobiotic regulation (14).
In conclusion, the xenobiotic receptors PXR and CAR function as master sensors to control the phase I and II adaptive hepatic responses by nature of their ability to coordinate the expression of a defined network of target genes. Moreover, such coordinated regulatory mechanisms may also include control of drug-effluxing transporters (27). Therefore, by residing at the biochemical interface between mammals and their chemical environment, these xenosensors represent a key protective mechanism to ensure elimination of steroid hormones along with a plethora of endotoxins and xenotoxins.
Acknowledgments
We thank Susan Nowell for Phip glucuronidation assay; Joanna Little, Ruth Yu, and Yanhong Shi for comments on the manuscript; Zheng Ma and Lingyun Zhao for technical advice; Henry Juguilon and Jing Xu for technical assistance; Joe Ritter for mouse anti-UGT1A1 antibody; and Elaine Stevens, Lita Ong, and Li Xu for administrative assistance. This work was conducted in part by U.S. Public Health Service Grant GM49139 (to R.H.T.). W.X. is supported by the Competitive Medical Research Fund of the University of Pittsburgh Medical Center Health System and the Susan G. Komen Breast Cancer Foundation. R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute for Biological Studies and March of Dimes Chair in Molecular and Developmental Biology.
Abbreviations
- CAR
constitutive androstane receptor
- PXR
pregnane X receptor
- RIF
rifampicin
- TCPOBOP
1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene
- UGT
UDP-glucuronosyltransferase
- NR
nuclear receptor
- EMSA
electrophoretic mobility-shift assay
References
- 1.Guengerich F P. Annu Rev Pharmacol Toxicol. 1989;29:241–264. doi: 10.1146/annurev.pa.29.040189.001325. [DOI] [PubMed] [Google Scholar]
- 2.Tukey R H, Strassburg C P. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 3.Gong Q H, Cho J W, Huang T, Potter C, Gholami N, Basu N K, Kubota S, Carvalho S, Pennington M W, Owens I S, Popescu N C. Pharmacogenetics. 2001;11:357–368. doi: 10.1097/00008571-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg B, Sabbagh W, Juguilon H, Bolado J, Jr, Ong E S, Evans R M. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kliewer S A, Moore J T, Wade L, Staudinger J L, Jones M A, McKee D D, Oliver B M, Willson T M, Zetterstrom R H, Perlmann T, Lehmann J. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 6.Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Proc Natl Acad Sci USA. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie W, Barwick J L, Downes M, Blumberg B, Simon C M, Nelson M C, Neuschwander-Tetri B A, Brunt E M, Guzelian P S, Evans R M. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 9.Wei P, Zhang J, Egan-Hafley M, Liang S, Moore D D. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- 10.Xie W, Evans R M. J Biol Chem. 2001;276:37739–37742. doi: 10.1074/jbc.R100033200. [DOI] [PubMed] [Google Scholar]
- 11.Yaffe S J, Levy G, Matsuzawa T, Balish T. N Engl J Med. 1996;275:1461–1466. doi: 10.1056/NEJM196612292752602. [DOI] [PubMed] [Google Scholar]
- 12.Jemnitz K, Lengyel G, Vereczkey L. Biochem Biophys Res Commun. 2002;291:29–33. doi: 10.1006/bbrc.2002.6400. [DOI] [PubMed] [Google Scholar]
- 13.Cancado E L, Leitao R M, Carrilho F J, Laudanna A A. Am J Gastroenterol. 1998;93:1510–1517. doi: 10.1111/j.1572-0241.1998.00472.x. [DOI] [PubMed] [Google Scholar]
- 14.Xie W, Barwick J L, Simon C M, Pierce A M, Safe S, Blumberg B, Guzelian P S, Evans R M. Genes Dev. 2000;14:3014–3023. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yueh M F, Nguyen N, Famourzadeh M, Strassburg C P, Oda Y, Guengerich F P, Tukey R H. Carcinogenesis. 2001;22:943–950. doi: 10.1093/carcin/22.6.943. [DOI] [PubMed] [Google Scholar]
- 16.Radominska-Pyrek A, Zimniak P, Irshaid Y M, Lester R, Tephly T R, Pyrek J S. J Clin Invest. 1987;80:234–241. doi: 10.1172/JCI113053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritter J K, Chen F, Sheen Y Y, Tran H M, Kimura S, Yeatman M T, Owens I S. J Biol Chem. 1992;267:3257–3261. [PubMed] [Google Scholar]
- 18.Maglich J M, Stoltz C M, Goodwin B, Hawkins-Brown D, Moore J T, Kliewer S A. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 19.Findlay K A, Kaptein E, Visser T J, Burchell B. J Clin Endocrinol Metab. 2000;85:2879–2883. doi: 10.1210/jcem.85.8.6715. [DOI] [PubMed] [Google Scholar]
- 20.Nowell S, Massengill J, Williams S, Radominska-Pandya A, Tephly T R, Cheng Z, Strassburg C P, Tukey R H, MacLeod S L, Lang N P, Kadlubar F F. Carcinogenesis. 1999;20:101–108. doi: 10.1093/carcin/20.6.1107. [DOI] [PubMed] [Google Scholar]
- 21.Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong Q H, Owens I S, Negishi M, Sueyoshi T. Hepatology. 2001;33:1232–1238. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- 22.Smith G W, Aubry J M, Dellu F, Contarino A, Bilezikjian L M, Gold L H, Chen R, Marchuk Y, Hauser C, Bentley C A, et al. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 23.Bosma P, Chowdhury J R, Jansen P H. Lancet. 1995;346:314–315. doi: 10.1016/s0140-6736(95)92203-2. [DOI] [PubMed] [Google Scholar]
- 24.Hu Z, Wells P G. J Pharmacol Exp Ther. 1992;263:334–342. [PubMed] [Google Scholar]
- 25.Strassburg C P, Manns M P, Tukey R H. Cancer Res. 1997;57:2979–2985. [PubMed] [Google Scholar]
- 26.Malfatti M A, Felton J S. Carcinogenesis. 2001;22:1087–1093. doi: 10.1093/carcin/22.7.1087. [DOI] [PubMed] [Google Scholar]
- 27.Bock K W, Eckle T, Ouzzine M, Fournel-Gigleux S. Biochem Pharmacol. 2000;59:467–470. doi: 10.1016/s0006-2952(99)00366-4. [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Zhang J, Chua S S, Qatanani M, Han Y, Granata R, Moore D D. Proc Natl Acad Sci USA. 2003;100:4156–4161. doi: 10.1073/pnas.0630614100. [DOI] [PMC free article] [PubMed] [Google Scholar]