Abstract
The β-chemokines RANTES (regulated on activation, normal T cell expressed and secreted), macrophage inflammatory protein-1α (MIP-1α), and MIP-1β are the natural ligands of the HIV-1 coreceptor CCR5 and compete with the virus for receptor binding. We show that secretion of the β-chemokines by activated lymphocytes starts before cellular DNA synthesis is detected and demonstrate that transient prolongation of the G1 phase of the cell cycle by treatment with cytostatic drugs results in increased levels of the three chemokines in culture supernatants. Supernatants collected from peripheral blood mononuclear cells exposed to hydroxyurea, which arrests the cell cycle in late G1, contained high levels of β-chemokines. These supernatants were able to inhibit HIV-1 replication when added to cultures of infected lymphocytes. The observed antiviral effect likely was due to the increased levels of β-chemokines RANTES, MIP-1α, and MIP-1β because (i) supernatants greatly inhibited the replication of HIV-1 BaL, whereas they affected HIV-1 IIIb replication only slightly; (ii) neutralizing antibodies against the chemokines abrogated the antiviral effect of the supernatants; and (iii) the hydroxyurea concentrations shown to up-regulate chemokine levels were not sufficient to inhibit virus replication by depletion of intracellular nucleotide pools. Although antiviral properties have been reported previously for the cytostatic agents shown here to up-regulate β-chemokine levels, our results provide an additional mechanism by which these drugs may exert antiviral activity. In summary, increased extracellular levels of anti-HIV-1 β-chemokines resulting from transient prolongation of the G1 phase of the lymphocyte cell cycle by treatment with cytostatic drugs may help to control the replication of CCR5-using strains of HIV-1.
The β-chemokines RANTES (regulated on activation, normal T cell expressed and secreted), macrophage inflammatory protein-1α (MIP-1α), and MIP-1β are the natural ligands of CCR5, the main coreceptor of non-syncytium-inducing HIV-1, and have been shown to inhibit the in vitro infection of lymphocytes (1). Several studies have demonstrated that increased production of RANTES, MIP-1α, and MIP-1β correlates with resistance to infection or a more favorable clinical prognosis, likely because of competition of the chemokines with HIV-1 for binding to CCR5 (2–6).
In the present work, we have explored the concept of enhancing production of the anti-HIV β-chemokines by manipulating the cell cycle in activated lymphocytes. Zagury et al. (3) have shown that considerable protein amounts of MIP-1α, MIP-1β, and, to a lower extent, RANTES are detected in cultures of peripheral blood mononuclear cells (PBMCs) shortly after activation, before DNA synthesis occurs. In light of these observations, we reasoned that prolongation of the G1 phase of the cell cycle might increase the overall β-chemokine production by activated lymphocytes, which, in turn, could result in inhibition of HIV-1.
Methods
Tissue Culture.
PBMCs were separated from whole blood of HIV-1 seronegative donors by density centrifugation with Ficoll Histopaque (Sigma). Cells were cultured in complete medium consisting of RPMI medium 1640 supplemented with 10% heat-inactivated FBS, 2 mM glutamine, and penicillin/streptomycin (Invitrogen). In some experiments, purified CD8+ lymphocytes obtained by negative selection using the Human CD8+ T Cell Enrichment Mixture (StemCell Technologies, Vancouver) were used. Cell purity measured by flow cytometry was >80% among different donor purifications.
Cells were activated by culture for 72 h under three different conditions: phytohemagglutinin (PHA, 2.5 μg/ml; Roche, Gipf-Oberfrick, Switzerland), anti-CD3 antibody (1 μg/ml; Coulter) plus 100 units/ml recombinant IL-2 (Roche), or staphylococcal enterotoxin B at 0.03 μg/ml (Sigma). Activated cells were cultured in complete medium supplemented with recombinant IL-2 (100 units/ml), and medium was changed every 3 or 4 days.
Cell proliferation was measured by the trypan blue staining viability test, [3H]thymidine incorporation in DNA, and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Roche).
Measurement of β-Chemokine Levels and Assessment of Cell Cycle Arrest.
The impact of cell cycle arrest in β-chemokine production was evaluated by measuring chemokine levels in supernatants of cell cultures containing compounds known to cause cell cycle arrest. Levels of the β-chemokines RANTES, MIP-1α, and MIP-1β were measured by using commercial ELISA kits (R & D Systems). Cell cycle arrest in G1 was induced by culturing of the cells in the presence of aphidicolin (APH), sodium butyrate (SB), hydroxyurea (HU), roscovitine (RC), or olomoucine (OL). Arrest in late S was induced by culture of cells in the presence of resveratrol. G2 cycle arrest was induced by the compounds nocodazole and Colcemid. All compounds were purchased from Sigma except RC and OL, which were from Calbiochem. Arrest of cell cycle progression in the presence of cytostatic agents was measured by propidium iodide staining followed by fluorescence-activated cell sorter (FACS) analysis (7).
Assessment of HIV-1-Suppressive Activity in Supernatants Collected from HU-Treated PBMCs.
The antiviral activity of the supernatants collected from cultures of PBMCs that had been exposed to 100 μM HU for 7 days [supernatants referred to as conditioned medium (CM)] was evaluated in PBMCs infected with HIV-1 BaL and HIV-1 IIIb. Briefly, PHA-activated PBMCs were infected with each virus at 100 tissue culture 50% infective dose units (TCID50)/106 PBMCs or 10 TCID50/106 PBMCs for 2 h at 37°C. Infected cells were cultured in IL-2 medium alone, IL-2 medium with 100 μM HU, IL-2 medium containing 50% supernatant from HU-treated PBMCs (CM/HU), or IL-2 medium containing 50% supernatant from control-treated PBMCs (CM/control). On day 3 after infection, culture medium was replaced with fresh medium of the same kind as on day 1. Viral growth (measured by p24 levels in the supernatant) and cell viability (assayed by MTT) were determined on day 7 after infection. To determine the role of the β-chemokines RANTES, MIP-1α, and MIP-1β in the antiviral activity found in supernatants of HU-exposed PBMCs, the antiviral activities of such supernatants were evaluated in the presence of neutralizing antibodies against all three chemokines as described (8).
Results
Kinetics of β-Chemokine Secretion on Activated PBMCs.
To determine the kinetics of β-chemokine secretion in relation to DNA synthesis on cellular activation, PBMCs were activated by PHA treatment for 72 h. At 24 h after plating the cells, the entire supernatant was collected, and fresh medium containing PHA was added to the culture. Twenty-four hours later (48 h from the time cells were plated), supernatants again were collected and PHA medium was added. Supernatants were collected for the last time after 72 h. Thus, this experiment measured the amount of chemokine released to the culture medium during each 24-h interval, not the continuous accumulation of chemokine over 48 or 72 h of exposure to PHA. Cellular DNA synthesis was measured by assaying [3H]thymidine incorporation in parallel wells at 24, 48, and 72 h after plating of the cells. Results are shown in Fig. 1a. At 24 h after the beginning of PHA treatment, chemokine protein concentration in the culture supernatants was 850, 17,200, and 13,300 pg/ml for RANTES, MIP-1α, and MIP-1β, respectively (average values from three different donors). Slightly increased values in RANTES and MIP-1α secretion were detected during the 24- to 48-h culture period, whereas the MIP-1β values remained constant. During the 48- to 72-h period, levels of RANTES and MIP-1α secretion were unchanged, whereas the secretion of MIP-1β decreased. Synthesis of cellular DNA was almost undetectable at 24 h (645 cpm) and increased considerably by 48 and 72 h (62,474 and 106,402 cpm, respectively). These data show that considerable protein amounts of MIP-1α, MIP-1β, and, to a lower extent, RANTES are present in the culture supernatant of PBMCs at 24 h after activation, a time at which cellular DNA synthesis is minimal.
Figure 1.
Kinetics of RANTES, MIP-1α, and MIP-1β secretion in activated PBMCs. (a) PBMCs (1 × 106) were cultured in 1 ml of culture medium in the presence of PHA. Cultures were maintained for 72 h. Every 24 h, the entire culture medium was collected and replaced with fresh medium containing PHA. Supernatants were assayed for chemokine content in supernatants by ELISA. DNA synthesis was measured by [3H]thymidine incorporation in PBMCs cultured in parallel under identical conditions. Results are the mean ± SD of data obtained from three different donors. (b) PBMCs from four donors were cultured in the presence of PHA for 3 days and in the presence of IL-2 afterward. Culture supernatants were assayed for β-chemokine production by ELISA on days 3, 7, and 11. Values from each donor are the mean ± SD of triplicate wells.
The profile of β-chemokine secretion next was evaluated in cultures of activated PBMCs maintained in the presence of IL-2 for several days. PBMCs were activated with PHA for 3 days and then cultured in the presence of IL-2 for 8 additional days. At days 3, 7, and 11, chemokine content in the culture fluid was measured (Fig. 1b). Although variability was observed among different donors, RANTES levels usually reached a peak on day 7 after activation. In contrast, MIP-1α and MIP-1β levels peaked on day 3 or day 7, depending on the donor. Levels of all three chemokines were low by day 11. Taken together, these data indicate that secretion of the β-chemokines by PBMCs in response to activation starts before lymphocytes enter the DNA synthesis phase of the cell cycle (S phase), reaches a peak by day 3 or 7, and then declines to low levels.
Treatment of PBMC Cultures with Compounds That Arrest the Cell Cycle in G1 Results in Increased Levels of Secreted β-Chemokines.
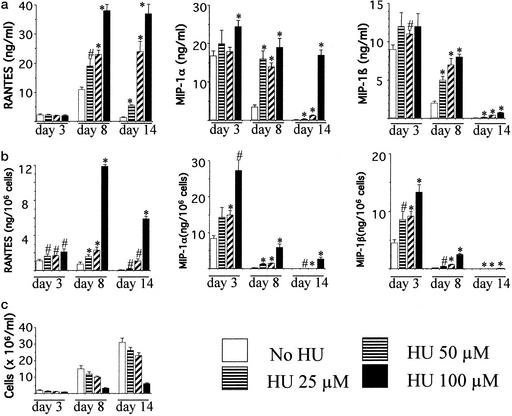
Because our previous experiments indicated that β-chemokine secretion by activated PBMCs begins before DNA synthesis occurs, we tested whether delay of entry in the S phase of the cell cycle results in an overall increase in chemokine levels. To this end, β-chemokine production by activated PBMCs cultured in the presence of HU was investigated. HU is a cytostatic drug that, by depleting intracellular nucleotide pools, arrests cell cycle progression in late G1 (9). Fig. 2 shows chemokine production by activated PBMCs cultured in the presence of different concentrations of HU for 14 days. HU treatment resulted in increased concentrations (ng/ml) of RANTES, MIP-1α, and MIP-1β in the culture supernatants in a dose-dependent manner (Fig. 2a). In the representative experiment depicted in Fig. 2, day 8 chemokine levels in cultures containing 100 μM HU were increased 3.4-fold for RANTES, 5.4-fold for MIP-1α, and 4-fold for MIP-1β compared with the untreated control. Because HU inhibits lymphocyte proliferation, chemokine values also were expressed as chemokine amount per viable cell. As expected, cell numbers were lower in the presence of the drug (Fig. 2c). Chemokine levels expressed as ng per 106 cells indicated increases of 16.2-, 25.4-, and 18.4-fold for RANTES, MIP-1α, and MIP-1β, respectively, in the presence of 100 μM HU (Fig. 2b). Similar increases were observed in PBMC cultures from the other three donors, and the increases were evident when chemokine values were expressed either as ng/ml or as ng per 106 viable cells (data not shown).
Figure 2.
HU treatment of PHA-activated PBMCs results in increased levels of secreted β-chemokines. PBMCs were cultured in the presence of PHA for 3 days and in the presence of IL-2 afterward. HU was added at the indicated concentrations at the beginning of the experiment and added fresh every time the medium was changed. Culture supernatants collected on days 3, 8, and 14 were assayed for chemokine production. Chemokine levels in the supernatants are expressed as ng/ml (a) and as ng per 106 viable cells (b); cell number was monitored by trypan blue exclusion (c). Representative values of one of four experiments, each using PBMCs from a different donor, are shown. Values are means ± SD of triplicate wells. ∗, P < 0.01; #, P < 0.05, compared with untreated control by Student's t test.
Similarly, HU treatment increased chemokine levels in PBMCs that had been activated by crosslinking of the T cell antigen receptor/CD3 complex with anti-CD3 antibodies or by occupancy of the T cell antigen receptor with the superantigen staphylococcal enterotoxin B (data not shown).
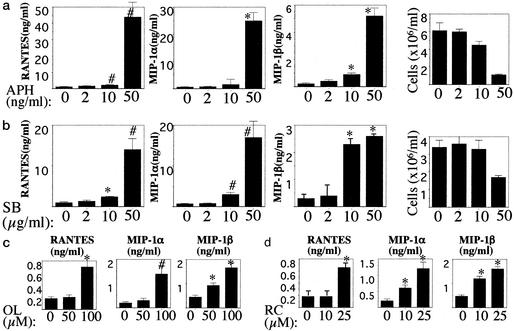
Having demonstrated that HU treatment of activated PBMCs results in increased chemokine levels, we next extended our observations to other cytostatic agents that, as does HU, arrest cell cycle progression before DNA synthesis occurs. The agents evaluated were SB, APH, RC, and OL. SB and APH arrest the cell cycle in early and late G1, respectively (10, 11). RC and OL are purine-derivative drugs that arrest cell cycle progression in late G1 through inhibition of cyclin-dependent kinases (CDKs) (12). Fig. 3a shows chemokine production by activated PBMCs cultured in the presence of APH. The number of viable cells and chemokine levels are depicted. Increased chemokine values were observed at 0.5 μM APH, a drug concentration that exhibited cytostatic effects as manifested by reduced cell proliferation. Fig. 3b shows chemokine production by activated PBMCs cultured in the presence of SB. As was the case with APH, increased chemokine levels were detected at drug concentrations of SB exerting cytostatic activity. Similarly, exposure of activated PBMCs to the CDK inhibitors RC and OL resulted in increased chemokine levels at cytostatic concentrations of the drugs (Fig. 3c and data not shown). These experiments indicate that treatment of activated PBMCs with compounds that arrest the cell cycle in the G1 phase results in increased levels of extracellular β-chemokines.
Figure 3.
Treatment of activated PBMCs with cytostatic drugs inducing G1 cell cycle arrest results in increased levels of extracellular β-chemokines. PHA-activated PBMCs were cultured in the presence of APH (a), SB (b), OL (c), or RC (d) at the indicated concentrations. Cultures were kept for 14 days, with medium changes every 3 or 4 days. Culture supernatants were tested for chemokine content by ELISA, and cell number was determined by trypan blue staining. Data show day 8 values for both APH and SB and day 3 values for RC and OL. Values are means ± SD of triplicate wells. ∗, P < 0.01; #, P < 0.05, compared with untreated control by Student's t test.
Up-Regulation of β-Chemokine Levels in Supernatants of CD8 Lymphocyte Cultures Is Specific to Cell Cycle Arrest in G1.
In the experiments described thus far, total PBMCs had been used. To demonstrate that arrest of the cell cycle in CD8 lymphocytes (the main cell type producer of the anti-HIV chemokines) results in increased chemokine levels, chemokine production by purified CD8 lymphocytes exposed to HU was evaluated next. Negatively selected CD8+ lymphocytes were activated by anti-CD3 plus IL-2 treatment for 3 days. Activated cells were cultured in the presence of IL-2 and HU (100 and 200 μM) for 24 or 48 h, time points at which cell proliferation and supernatant chemokine levels were assayed. Cell proliferation was determined by trypan blue staining, [3H]thymidine incorporation in DNA, and percentage of cells in S phase as assessed by propidium iodide staining (Fig. 4). HU cytostatic effects were evident after 48 h of exposure to the drug because both cell number and thymidine incorporation doubled between 24 and 48 h in the absence of HU, whereas they remained constant or decreased in the presence of the drug. Similarly, the percentage of cells in S phase increased in the absence of HU, whereas it decreased in its presence. CD8+ lymphocyte cycle arrest by HU resulted in increased levels of RANTES, MIP-1α, and MIP-1β after 24 and 48 h of exposure to the drug. At 48 h, levels of RANTES, MIP-1α, and MIP-1β increased by 1.9-, 3.7-, and 4.7-fold, respectively, in the presence of 100 μM HU. Slightly lower increases in chemokine production were found in CD8 cells exposed to 200 μM HU for 48 h, a drug concentration that resulted in a slightly lower number of viable cells shown by trypan blue staining in two of three donors examined (Fig. 4 and data not shown). In summary, these data demonstrate that arrest of cell cycle progression in CD8+ lymphocytes by HU treatment results in increased levels of RANTES, MIP-1α, and MIP-1β.
Figure 4.
Cell cycle arrest in G1, but not in G2, results in increased extracellular levels of β-chemokines. Purified CD8 lymphocytes were activated by anti-CD3 and IL-2 treatment for 3 days. Activated cells were cultured in the presence of IL-2 medium containing HU at the indicated concentrations. After 24 and 48 h of the addition of HU, cell number was evaluated by trypan blue staining (a), newly synthesized DNA was measured by [3H]thymidine incorporation (b), percentage of cells in S phase was determined by propidium iodide staining (c), and β-chemokine levels were determined by ELISA (d). (e) Cell cycle arrest and chemokine production in the presence of nocodazole 48 h after addition of the drug. Results are single data values, representative of three independent experiments for HU and representative of two independent experiments in the case of nocodazole.
To investigate whether increased levels of supernatant chemokines upon cell cycle arrest are cell cycle phase-specific, the effect of G2 arrest in chemokine production next was evaluated. Activated CD8 lymphocytes were arrested in G2 by treatment with 0.01 μg/ml nocodazole (13). As shown in Fig. 4e, treatment of activated CD8 cells with nocodazole for 48 h resulted in accumulation of cells in G2 compared with the untreated control. However, chemokine content in treated cultures was lower than in the untreated controls. The same results were obtained on induction of G2 cycle arrest by Colcemid (data not shown). Similarly, cell cycle arrest in late S by 10 μM resveratrol resulted in chemokine levels that were lower than the untreated control (data not shown). Together, these data suggest that increased chemokine levels are specific to cell cycle arrest in G1.
Supernatants Collected from PBMCs Arrested in G1 Inhibit HIV-1 BaL Replication.
To determine whether the augmented chemokine levels found upon G1 cell cycle arrest possess any antiviral activity, supernatants were harvested from cultures of activated PBMCs that had been exposed to 100 μM HU for 7 days. These culture supernatants were referred to as CM/HU. Culture supernatants from activated PBMCs cultured under the same conditions in the absence of HU (CM/control) also were harvested. Supernatants were harvested on day 7 because our previous experiments (Fig. 2) indicated that high levels of RANTES, MIP-1α, and MIP-1β are present at this time point.
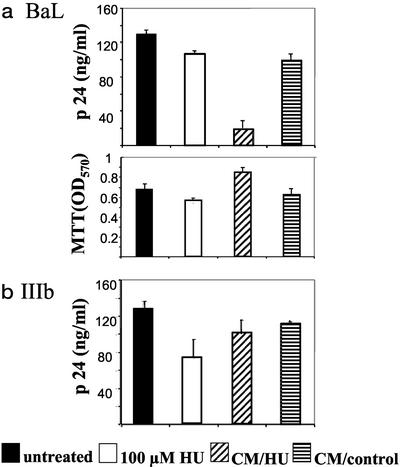
The antiviral activity of the CM was evaluated in the infection of PBMCs with HIV-1 BaL (a prototype R5-using virus) and HIV-1 IIIb (a prototype X4-using virus). Activated PBMCs from two seronegative donors were infected with each virus, and the infected cells were cultured in medium supplemented by 50% with CM/HU or CM/control. An additional culture was set up by culturing infected cells in fresh medium supplemented with 100 μM HU, the same concentration of HU as the one present in the CM/HU medium. Virus replication and cell viability were measured on day 7 after infection. Results obtained in one of the donors are shown in Fig. 5. Culture of infected PBMCs in the presence of CM/HU fluid suppressed HIV-1 BaL replication by 85% compared with the untreated control, whereas the CM/control fluid reduced virus replication by 24%. The control containing HU at the same concentration as the CM/HU inhibited HIV-1 BaL replication by 8%. Cell viability (MTT assay) was not affected by the addition of CM to the cultures. In contrast to the results obtained with HIV-1 BaL, the CM/HU and CM/control fluids suppressed HIV-1 IIIb by 22% and 10%, respectively. These data indicate that activated PBMCs grown in the presence of HU for several days release factors that strongly inhibit HIV-1 BaL replication, whereas they have a much lesser effect on the replication of HIV-1 IIIb. In addition, these results suggest that factors present in the supernatants, but not HU per se, are responsible for the inhibition of HIV-1 BaL.
Figure 5.
Supernatants collected from PBMCs exposed to HU contain suppressive factors that markedly inhibit HIV-1 BaL replication, whereas they only slightly affect the replication of HIV-1 IIIb. Activated lymphocytes from a seronegative donor were infected with HIV-1 BaL (a) or HIV-1 IIIb (b). Infected cells were cultured in IL-2 culture medium supplemented by 50% with supernatants collected from HU-treated PBMCs (CM/HU) or supernatants collected from untreated PBMCs (CM/control). In addition, a culture containing 100 μM HU in fresh medium was included. Virus replication was measured in the culture supernatant on day 7 after infection. Cell viability was assessed by the MTT assay. Data are means ± SD of triplicate wells. Representative results of one of two experiments are shown.
The Antiviral Activity Present in Supernatants of Lymphocytes Arrested in G1 Phase by HU Treatment Is Due to RANTES, MIP-1α, and MIP-1β.
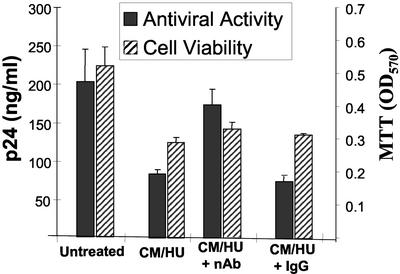
The above results demonstrated selective inhibition of the R5-using virus HIV-1 BaL by supernatants collected from HU-treated PBMCs. In addition, these results suggested that the β-chemokines RANTES, MIP-1α, and MIP-1β were the likely suppressive factors accounting for viral inhibition. To confirm that an increase in β-chemokine levels in the CM was responsible for the observed inhibition of HIV-1 BaL replication, the antiviral activity of the CM in the presence of a mixture of neutralizing antibodies to all three chemokines was assessed. As can be seen in the experiment depicted in Fig. 6, CM/HU fluid inhibited HIV-1 BaL replication by ≈60%. However, the antiviral activity of CM/HU was only ≈10% in the presence of the neutralizing antibodies mixture. An IgG antibody control did not affect the antiviral activity of the CM/HU fluid. In a different experiment, using cells from a different donor, the neutralizing antibodies mixture similarly abrogated the antiviral activity of the CM/HU supernatant (data not shown). These data demonstrate that the antiviral activity found in culture supernatants of HU-treated PBMCs primarily is due to the presence of the β-chemokines RANTES, MIP-1α, and MIP-1β.
Figure 6.
The antiviral activity of supernatants collected from HU-exposed PBMCs is reversed by neutralizing antibodies against the β-chemokines RANTES, MIP-1α, and MIP-1β. Activated lymphocytes from a seronegative donor were infected with HIV-1 BaL. Infected cells were cultured in the presence of supernatants collected from PBMCs that had been cultured for 7 days in the presence of 100 μM HU (CM/HU). CM/HU was preincubated with a mixture of neutralizing antibodies (anti-RANTES, anti-MIP1α, and anti-MIP1β; indicated as nAb) or an IgG control before addition to the culture. Fresh medium containing CM/HU and the correspondent antibodies was added again on day 3 after infection. On day 7, viral replication was measured by a p24 assay, and cell viability was assessed by the MTT assay. Representative results obtained in one of two experiments are shown. Data are mean values ± SD of duplicate wells.
Discussion
The studies described here were prompted by the observation made by Zagury et al. (3), who demonstrated that considerable levels of the β-chemokines are present in the supernatants of cultured PBMCs soon after activation, before synthesis of cellular DNA is detected. We reasoned that prolongation of the G1 phase of the cell cycle in lymphocytes might result in increased chemokine production, which, in turn, could inhibit replication of R5-using viruses.
Our experiments confirmed the presence of high levels of β-chemokines in supernatants of PBMC cultures 24 h after activation, a time at which cellular DNA synthesis could not be detected (Fig. 1a). By arresting cell cycle progression of PHA-activated PBMCs in G1 by treatment with cytostatic drugs, we have found increased levels of RANTES, MIP-1α, and MIP-1β in the culture supernatants. Increases in chemokine levels also were found when G1 cycle arrest was induced in cells activated by crosslinking of the T cell receptor (TCR)/CD3 complex with an anti-CD3 antibody or by occupancy of the TCR with staphylococcal enterotoxin B, activation conditions thought to mimic the in vivo process of antigen activation more closely than PHA (14). Increased levels of β-chemokines were specific to compounds that caused cell cycle arrest in G1, and arrest of the cell cycle in late S (by resveratrol) or in G2 (by nocodazole or Colcemid) did not result in such increases. The G1-arresting agents that in the present study demonstrated up-regulation of β-chemokine levels were SB, HU, RC, and OL. HU then was selected for additional experiments as a representative agent. Simultaneous analyses of chemokine production and induction of G1 arrest by HU in purified CD8+ lymphocytes (monitored by cell number, thymidine incorporation, and percentage of cells in S phase) confirmed the results obtained in total PBMCs.
Supernatants collected from cultures of PBMCs that had been exposed to HU for several days, referred to as CM in our experiments, were able to markedly suppress the replication of HIV-1 BaL in PBMCs. A mixture of neutralizing antibodies against all three chemokines abrogated the anti-HIV-1 BaL activity of the CM, thus demonstrating that the β-chemokines were responsible for the antiviral effect. When HU was added to fresh medium at the same concentration as in the CM, an only minor antiviral effect (<10%) was found in the replication of HIV-1 BaL. These data suggested that the antiviral effect observed was not due to inhibition of the virus reverse transcription step by HU-mediated depletion of nucleotide pools. Although it is known that HU exerts anti-HIV-1 activity by reducing the intracellular nucleotide pools (15), low concentrations of the drug (≤100 μM) are effective only when combined with nucleoside analog reverse transcription inhibitors (9). Therefore, our results showing little antiviral activity by 100 μM HU when added to fresh culture medium agree with previous reports and indicate further that the increase in chemokine levels resulting from exposure to the drug is the likely explanation for the antiviral effect observed.
Taking our results together, we have described an approach to increase the concentration of the CCR5 ligands RANTES, MIP-1α, and MIP-1β in cultures of PBMCs or CD8+ lymphocytes. This approach involves the transient arrest of activated cells in the G1 phase of the cell cycle by using low concentrations of cytostatic agents. The compounds demonstrated in this report to increase chemokine levels previously have been shown to inhibit HIV-1 (10, 15, 16). Our results provide an additional mechanism explaining the antiviral activity of these compounds, namely, increased production of β-chemokines upon arrest of the cell cycle in G1. Of note, a recent report by Achour (17) demonstrates that AY 9944, an inhibitor of sterol synthesis, increases the in vitro production of RANTES, MIP-1α, and MIP-1β by PBMCs derived from HIV-1-infected individuals. Interestingly, AY 9944 inhibits DNA synthesis in PHA-activated PBMCs (18), which agrees with our explanation for increased chemokine levels after cell cycle arrest before the S phase.
We postulate that induction of increased levels of the anti-HIV β-chemokines by administration of cytostatic drugs may be helpful in controlling replication of R5 strains in infected individuals. Augmentation of β-chemokine levels by cell cycle arrest requires conditions of cellular activation, which is one of the hallmarks of HIV-1 disease (19). In addition, cellular activation is present primarily in lymph nodes, which constitute a major anatomical site of ongoing viral replication (20). By causing transient cell cycle arrest, the agents investigated in our experiments led to a reduced number of cells over time. Despite a reduced number of cells, the cultures exposed to the G1 cytostatic drug presented greater levels of chemokines than the untreated ones. Therefore, cells exposed to the cytostatic agent were surrounded by more CCR5 ligands than the unexposed cells in these in vitro experiments. Should these in vitro observations hold true in vivo, our data suggest an approach to promote host conditions favoring virus control.
There is a reasonable concern regarding the use of cytostatic drugs in HIV-1-infected individuals. Although diminished cellular proliferation may not appear advantageous in HIV disease, it is important to underscore that conditions leading to cellular proliferation favor viral replication as well (21). Results from several clinical trials in which HU has been used in combination with anti-HIV drugs have shown an impressive control of the virus (22–24). In the majority of these studies, a significant recovery in CD4 counts has not been observed. However, in a recent study in which a HU-containing antiretroviral regimen suppressed viral replication for >2 years, CD4 lymphocytes from some of the patients were shown to be functionally competent against HIV-1 (25). Our data demonstrating increases in β-chemokine levels after HU treatment further support the use of this drug in HIV-1 patients.
Based on current experimental evidence, our in vitro observations suggest that in vivo increases in RANTES, MIP-1α, and MIP-1β levels will result in inhibition of R5 viruses, but not X4 viruses. Because R5 viruses are the dominantly transmitted strains and their presence is demonstrated throughout the course of the disease, their inhibition by β-chemokines may be of particular relevance. Although blocking of CCR5 by its natural ligands is expected to curtail virus replication, the possibility exists that effective blocking of CCR5 may lead to the premature emergence of X4 viruses, an effect that may be detrimental considering the faster progression to AIDS reported in patients harboring X4 strains (26). However, a recent in vitro study indicates that virus resistance to a CCR5 antagonist does not involve CXCR4 use (27). Moreover, the use of a cytostatic agent in combination with potent HIV-1 drugs is expected to hamper the development of viral mutations required for X4 usage. In summary, induction of anti-HIV chemokines by cytostatic drugs that, by themselves, potentiate the antiviral activities of anti-HIV drugs offers an additional strategy with which to control replication of HIV-1. This may be a particularly attractive strategy with which to inhibit the virus in African countries in which subtype C is prevalent, because these viruses use CCR5 and not CXCR4 (28).
Acknowledgments
We thank Tony DeVico and Jean Lim for helpful suggestions on the design of experiments, Peter Evans for statistical analysis of the data, and Jeff Mayer for technical assistance. We also thank the Institute of Human Virology microQUANT Facility for performance of ELISAs.
Abbreviations
- RANTES
regulated on activation, normal T cell expressed and secreted
- MIP
macrophage inflammatory protein
- PBMC
peripheral blood mononuclear cell
- PHA
phytohemagglutinin
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- APH
aphidicolin
- SB
sodium butyrate
- HU
hydroxyurea
- RC
roscovitine
- OL
olomoucine
- CM
conditioned medium
References
- 1.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 2.Paxton W A, Martin S R, Tse D, O'Brien J, Skurnick J, Van Devanter N L, Padain N, Braun J F, Kotler S M, Wolinsky S M, et al. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 3.Zagury D, Lachgar A, Chams V, Fall L S, Bernard J, Zagury J F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, et al. Proc Natl Acad Sci USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferbas J, Giorgi J V, Amini S, Grovit-Ferbas K, Wiley D J, Detels R, Plaeger S. J Infect Dis. 2000;182:1247–1250. doi: 10.1086/315849. [DOI] [PubMed] [Google Scholar]
- 5.Cocchi F, DeVico A L, Yarchoan R, Redfield R R, Cleghorn F, Blattner W A, Garzino-Demo A, Colombini-Hatch S, Margolis D D, Gallo R C. Proc Natl Acad Sci USA. 2000;97:13812–13817. doi: 10.1073/pnas.240469997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams D H, Lloyd A R. Lancet. 1997;349:490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi P D. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. New York: Wiley; 1991. pp. 5.7.1–5.7.6. [Google Scholar]
- 8.Castillo R C, Arango-Jaramillo S, John R, Weinhold K, Kanki P, Carruth L, Schwartz D H. J Infect Dis. 2000;181:897–903. doi: 10.1086/315300. [DOI] [PubMed] [Google Scholar]
- 9.Lori F, Malykh A, Cara A, Sun D, Weinstein J N, Lisziewicz J, Gallo R C. Science. 1994;266:801–805. doi: 10.1126/science.7973634. [DOI] [PubMed] [Google Scholar]
- 10.Korin Y, Zack J. J Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koostra N A, Zwart B M, Schuitemaker H. J Virol. 2000;74:1712–1717. doi: 10.1128/jvi.74.4.1712-1717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray N, Detivaud L, Doerig C, Meijer L. Curr Med Chem. 1999;6:859–875. [PubMed] [Google Scholar]
- 13.Gualberto A, Aldape K, Kozakiewicz K, Tlsty T. Proc Natl Acad Sci USA. 1998;95:5166–5171. doi: 10.1073/pnas.95.9.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss A. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott; 1999. pp. 413–415. [Google Scholar]
- 15.Gao W Y, Cara A, Gallo R C, Lori F. Proc Natl Acad Sci USA. 1993;90:8925–8928. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Fuente C, Deng L, Wang L, Zilberman I, Eadie C, Healey M, Stein D, Denny T, Harrison L, et al. J Virol. 2001;75:7266–7279. doi: 10.1128/JVI.75.16.7266-7279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achour A. AIDS. 2000;14:1454–1455. doi: 10.1097/00002030-200007070-00021. [DOI] [PubMed] [Google Scholar]
- 18.Kay G, Wilce P A. Biochem Biophys Res Commun. 1983;110:82–87. doi: 10.1016/0006-291x(83)91263-9. [DOI] [PubMed] [Google Scholar]
- 19.Cossarizza A, Ortolani C, Mussini C, Borghi V, Guaraldi G, Mongiardo N, Bellesia E, Francheschini M G, DeRienzo B, Francheschini C. J Infect Dis. 1995;172:105–112. doi: 10.1093/infdis/172.1.105. [DOI] [PubMed] [Google Scholar]
- 20.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 21.De Boer R, Boucher C, Perelson A. AIDS. 1998;12:1567–1570. doi: 10.1097/00002030-199813000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Lori F, Jessen H, Foli A, Lisziewicz J, Matteo P S. J Am Med Assoc. 1997;277:1437–1438. [PubMed] [Google Scholar]
- 23.Vila J, Biron F, Nugier F, Vallet T, Peyramond D. Lancet. 1996;348:203–204. doi: 10.1016/s0140-6736(05)66157-0. [DOI] [PubMed] [Google Scholar]
- 24.Paton N I, Aboulhab J, Karim F. Lancet. 2002;359:1667–1668. doi: 10.1016/S0140-6736(02)08557-4. [DOI] [PubMed] [Google Scholar]
- 25.Lori F, Rosenberg E, Lieberman J, Foli A, Maserati R, Seminari E, Alberici F, Walker B, Lisziewicz J. AIDS Res Hum Retroviruses. 1999;15:1333–1338. doi: 10.1089/088922299310034. [DOI] [PubMed] [Google Scholar]
- 26.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trkola A, Kuhmann S, Strizki J. Proc Natl Acad Sci USA. 2002;99:395–400. doi: 10.1073/pnas.012519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ping L H, Nelson J A, Hoffman I F, Schock J, Lamers S L, Goodman M, Vernazza P, Kazembe P, Maida M, Zimba D, et al. J Virol. 1999;73:6271–6281. doi: 10.1128/jvi.73.8.6271-6281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]