Abstract
To test the hypothesis that β-chemokine levels may be relevant to the control of HIV in vivo, we compared RANTES, MIP-1α, and MIP-1β production from purified CD8+ T cells from 81 HIV-infected subjects and from 28 uninfected donors. Asymptomatic HIV+ subjects produced significantly higher levels of MIP-1α and MIP-1β, but not RANTES, than uninfected donors or patients that progressed to AIDS. In contrast, β chemokines in plasma were either nondetectable or showed no correlation with clinical status. The high β-chemokine-mediated anti-HIV activity was against the macrophage tropic isolate HIV-1BAL, with no demonstrable effect on the replication of the T-cell tropic HIV-1IIIB. These findings suggest that constitutive β-chemokine production may play an important role in the outcome of HIV-1 infection.
Immune control of HIV-1 infection by CD8+ T cells appears to be mediated in part by HLA class I restricted cytolytic processes (1). Virus-specific cytotoxic T lymphocytes have been detected in exposed but uninfected individuals (2, 3). Anti-HIV-1 cytotoxic T-lymphocyte activity correlates with the down-regulation of viremia early in infection (4, 5) and with slower disease progression (6, 7). Further, a strong inverse correlation was recently described between the frequency of HIV-1-specific CD8+ T cells that bind peptide–MHC tetramers and viral load for individuals undergoing highly active antiretroviral therapy (8). The contribution of CD8+ T lymphocytes in controlling viremia is further supported by animal studies (9, 10). CD8+ T lymphocytes can also suppress HIV replication in vitro by a nonlytic mechanism (11), and this antiviral activity is mediated by a soluble factor(s) (12, 13).
The noncytolytic antiviral response of CD8+ T cells is present soon after infection and is associated with a decrease in plasma viremia (14). This antiviral activity has been correlated directly with the clinical stage of HIV-1 infection (15–17).
In a previous study, we characterized three β chemokines released by activated CD8+ T cells, RANTES, macrophage inflammatory protein (MIP)-1α, and MIP-1β, as the major factors responsible for soluble suppressor activity against macrophage tropic HIV-1 isolates (18). It is now established that RANTES, MIP-1α, and MIP-1β bind to a receptor, CC chemokine receptor 5 (CCR5), that is also required by macrophage tropic HIV-1 strains as a coreceptor for entry into host cells (19, 20). More recent studies showed this receptor-ligand binding renders CCR5 unavailable to the virus either by competitively blocking virus interactions or by causing down-regulation from host-cell surfaces (21, 22). T-cell tropic HIV-1 isolates are instead characterized by the usage of another chemokine receptor, CXC chemokine receptor 4 (CXCR4) (23), and the α chemokine stromal-derived factor 1 (SDF-1), a ligand for CXCR4, was shown to suppress replication of T-cell tropic HIV-1 isolates (24, 25). The profound impact of reduced coreceptor accessibility on HIV infection in vivo is strongly supported by evidence that individuals homozygous for a mutant CCR5 allele encoding a fusion-defective molecule (Δ32) are strongly resistant to infection (26, 27). Moreover, the heterozygous condition provides a limited resistance to disease progression (28, 29).
Collectively, these findings suggest that antiviral immunity might also be afforded via the release of HIV-suppressive chemokines from activated CD8+ T cells because these chemokines down-regulate CCR5. However, the issue of the effects of increased production of inhibitory β chemokines in HIV infection and pathogenesis is still unsettled. In HIV-1-exposed but -uninfected individuals, an association between overproduction of β chemokines by CD4+ T cells and resistance to in vitro infection with HIV-1 macrophage tropic isolates has been described (30). Moreover, in a cohort of uninfected hemophiliacs, despite repeated exposure to contaminated blood products, protection has been associated with the ability of peripheral blood mononuclear cells (PBMCs) to produce higher levels of RANTES, MIP-1α, and MIP-1β compared with hemophiliacs who were never treated with contaminated blood products (31). A specific immune response involving a high production of β chemokines by CD4+ T cells seems to play a role of protection in exposed uninfected individuals (32) and may contribute to the control of viral replication in long-term nonprogressors (33). Higher β-chemokine secretion by PBMCs has been described in nonprogressors compared with rapid progressors (34), and higher production of MIP-1β by PBMCs has been associated with an asymptomatic status and decreased risk of disease progression (35). Antigen-induced chemokine production is also significantly decreased in HIV+ subjects with AIDS compared with asymptomatic HIV+ subjects (36). In accordance with these findings, sustained suppression of plasma HIV RNA is associated with an increase in the production of mitogen-induced MIP-1α and MIP-1β (37). Moreover, recent results indicate that human alloimmunization elicits very significant increases in the three β chemokines RANTES, MIP-1α, and MIP-1β, and resistance of CD4+ T cells to HIV infection with macrophage-tropic HIV-1 strains (38). However, when the production of β chemokines by unfractionated PBMCs, purified CD4+, or CD8+ T cells was examined in small numbers of HIV-1 positive subjects, the analysis failed to demonstrate any association between chemokine levels and disease stage (39, 40). Furthermore, plasma levels of β chemokines did not reveal any substantial differences between progressors and nonprogressors (41–46). Thus, conclusive evidence for the clinical relevance of CD8+ T-cell β-chemokine-mediated antiviral activity in the natural history of HIV-1 infection in vivo is still lacking.
To examine this issue, we performed cross-sectional analyses of RANTES, MIP-1α, and MIP-1β production by purified CD8+ T cells from 81 HIV-1-infected subjects at various stages of disease and from 28 uninfected donors. We analyzed the correlation between β-chemokine production and antiviral activity in vitro by using the macrophage tropic HIV-1BAL. The effect of anti-β-chemokine-neutralizing antibodies (NAb) on the CD8+ T-cell-mediated antiviral activity was also studied. Moreover, we determined whether circulating levels of β chemokines reflect the ability of immunocompetent T cells to produce these molecules by comparing CD8+ T-cell-mediated production with plasma levels measured in the same blood sample. Additionally, because of recent reports suggesting the potential for these chemokines to enhance HIV replication of T-cell tropic HIV-1 strains (47, 48), we determined whether RANTES, MIP-1α, and MIP-1β produced by activated CD8+ T cells could enhance virus spread of the T-cell tropic HIV-1IIIB by using an acute infectivity assay. Finally, in this study, we addressed the question of whether the α-chemokine SDF-1 plays a role in CD8+ T-cell-mediated antiviral activity against the T-cell tropic HIV-1IIIB.
Materials and Methods
HIV-1+ Subjects and Control Cases.
Heparinized peripheral blood samples were obtained from HIV-1-infected subjects at different stages of disease progression. We included 81 HIV-1-infected subjects in our studies. The only selection criteria were to ensure that there was representation of patients with AIDS vs. those without AIDS. All infected cases were being treated with antiviral drugs at the time of the study. Twenty-eight HIV-1 seronegative blood donors were analyzed for β-chemokine production as comparisons. All samples were collected after informed consent (approved by the Institutional Review Board of the University of Maryland), and by using standard protocols. In this study, AIDS was defined either by CD4+ T-cell count <200 per μl or by clinical diagnosis (Kaposi's sarcoma, opportunistic infection) according to the Center for Disease Control Criteria (49).
Cell Isolation.
PBMCs were obtained from Ficoll-isopaque gradient separation (Amersham Pharmacia). The PBMCs were washed twice in PBS (Biofluids, Rockville, MD) and resuspended in RPMI medium 1640 (GIBCO) containing 10% FCS (Biofluids), streptomycin (BioWhittaker), and penicillin. CD8+ T cells were positively selected by using magnetic beads coated with monoclonal antibodies against CD8 (Dynabeads; Dynal, Great Neck, NY) according to the manufacturer's instructions. In brief, PBMCs were incubated with beads (10 beads per target cell) on a rotator for 45 min at 4°C. Rosseted cells were isolated by placing the tube in a magnetic device (Dynal MPC-6). The positively selected CD8+ T cells were detached from the beads by using a polyclonal goat anti-mouse Fab reagent (Detachabeads; Dynal). Purity of CD8+ T cells was routinely >95% as determined by flow cytometry.
Production of RANTES, MIP-1α, and MIP-1β by Purified CD8+ T Lymphocytes.
β-Chemokine production by CD8+ T cells was analyzed after phytohemagglutinin (PHA) (Abbott) stimulation. The cells were cultured in RPMI 1640 complete medium in the presence of 1 μg/ml of PHA and 10 ng/ml of recombinant human IL-2 (Rh IL-2) (R & D Systems) at a cell concentration of 2 × 106 cells per ml. After 3 days of PHA stimulation of the culture, supernatants were collected and stored frozen at −70°C until analyzed for β-chemokine levels by ELISA (Quantikine Kits, R & D Systems) according to the manufacturer's instructions. The β-chemokine response to PHA was highly reproducible within 10% in randomly selected samples where we repeated the analysis three times at weekly intervals. In parallel, we also measured plasma levels.
Production of SDF-1 by Purified CD8+ T Lymphocytes.
The CD8+ T cells were cultured in RPMI 1640 complete medium in the presence of 1 μg/ml of PHA and 10 ng/ml of Rh IL-2 at a cell concentration of 2 × 106 cells per ml. After 3 days of PHA stimulation, the culture supernatants were collected and stored frozen at −70°C until analyzed for SDF-1 levels by ELISA. ELISA was performed by microQuant Service Laboratory, Institute of Human Virology, as previously described (50), except ELISA plates were coated with polyclonal goat antibody directed against recombinant SDF-1α (Santa Cruz Biotechnology).
Virus Suppression Assay.
We used an acute infection assay because it allowed us to evaluate the CD8+ T-cell response among different individuals by using different HIV-1 strains. PBMCs from a normal donor previously activated with PHA (1 μg/ml) for 3 days were acutely infected with HIV-1–1BAL or HIV-1–1IIIB, by using the same titer for both viruses (multiplicity of infection = 0.01) for 1 h at 37°C. Cells were washed twice with PBS to remove unabsorbed virus and resuspended in complete RPMI medium containing Rh IL-2 (10 ng/ml). After infection, PBMCs were cultured in the presence or absence of CD8+ T-cell-free supernatants obtained from HIV-1 subjects (50% vol/vol). Duplicate cultures were established with 1 × 105 cells per well in 96-well flat-bottom microtiter plates. Every 2 days, half of the medium was replaced with fresh medium or cell-free supernatants from activated CD8+ T cells. The supernatant from the cultures was harvested at day 8 after infection and tested for HIV-1 replication by p24 ELISA (Du Pont).
Statistical Analysis.
Descriptive statistics were generated by using Statistical Package for the Social Sciences. Analysis was conducted by using parametric and nonparametric methods where appropriate after assessment for normalcy. Relationships between continuous variables were assessed by using the Pearson correlation coefficient or its nonparametric equivalent, Spearman's rank coefficient. For categorical independent variables, differences between groups were assessed by using Student's t tests or analysis of variance, or nonparametric tests, including the Mann–Whitney and the Kruskal–Wallis tests. For dependent observations (measured on the same sample), we used the t test or the nonparametric alternative, Wilcoxon's matched-pairs test.
Activated CD8+ T cells mediate HIV-1 suppression via soluble factors. Levels of this activity appear to decrease with the progression of HIV-1 infection. In view of the large body of evidence showing that RANTES, MIP-1α, and MIP-1β are major components of this activity, we evaluated the potential clinical relevance of these β chemokines in HIV-1 infection.
Results
Higher MIP-1α and MIP-1β Production by CD8+ T Cells from HIV-Infected Individuals Correlates with Better Clinical Status.
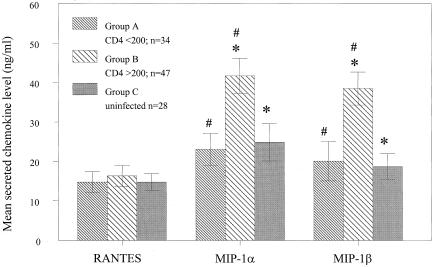
The production of RANTES, MIP-1α, and MIP-1β by CD8+ T cells obtained from HIV-1-infected individuals was assayed by ELISA 72 h after activation with PHA and IL-2. The data were then grouped and compared to determine whether chemokine production varies significantly in association with certain clinical profiles. The data were first grouped according to whether the CD4+ T-cell counts of the donors were less than (Group A, n = 34) or greater than (Group B, n = 47) 200 cells per μl. A CD4+ T-cell count of 200 per μl or less is an accepted criterion for diagnosis of AIDS (49). CD8+ T cells obtained from uninfected donors were also analyzed to provide a third data group (Group C, n = 28) for further comparisons. As shown in Fig. 1, the mean secreted levels of MIP-1α and MIP-1β in Group B were significantly higher than observed with either Group A [MIP-1α (P = 0.004); MIP-1β (P = 0.008)] or Group C [MIP-1α (P = 0.012); MIP-1β (P = 0.001)]. In contrast, there were no significant differences in the mean levels of these chemokines between groups A and C. Thus, higher production of MIP-1α and MIP-1β was associated with HIV+ donors demonstrating higher CD4+ T-cell counts. In contrast to MIP-1α and MIP-1β, mean RANTES levels were not significantly different among the three subject groups.
Figure 1.
Comparison of β-chemokine production by CD8+ T cells from HIV-1-infected cases stratified by CD4+ T-cell count and uninfected cases. CD8+ T cells were isolated from HIV+ individuals with CD4+ T-cell counts less than (Group A) or greater than (Group B) 200 cells per μl. CD8+ T cells were also obtained from uninfected donors (Group C) for further comparisons. The cells were isolated from freshly collected PMBCs by immunomagnetic beads, and 2 × 106 cells per ml were activated with PHA (1 μg/ml) and Rh IL-2 (10 ng/ml). β-Chemokine production by CD8+ T cells was analyzed in the culture supernatants after 3 days of PHA stimulation by ELISA. The indicated p-values were generated from t tests. For analyses of MIP-1α: *, P = 0.012, #, P = 0.004. For analyses of MIP-1β: *, P = 0.001, #, P = 0.008. The differences in RANTES values were not significant. Bars indicate the standard errors of the means.
The relationship between chemokine production and CD4+ T-cell count in HIV+ individuals was further studied by Pearson analysis (Table 1). In accordance with the previous analyses (Fig. 1), a significant linear correlation was evident between CD4+ T-cell count and production of MIP-1α (r = 0.266, P = 0.017) and MIP-1β (r = 0.293, P = 0.008). Notably, a correlation was also found between MIP-1α (r = 0.343, P = 0.002) and MIP-1β (r = 0.310, P = 0.006) production and peripheral blood CD8+ T-cell count. Again, there was no significant correlation between RANTES production and CD4+ and CD8+ T-cell counts.
Table 1.
Relationship between lymphocyte subsets and β-chemokine production from CD8+ T cells and matched plasma
| Plasma
|
CD8+ T cells
|
|||||
|---|---|---|---|---|---|---|
| RANTES | MIP-1α | MIP-1β | RANTES | MIP-1α | MIP-1β | |
| CD4 count | ||||||
| Pearson correlation | −.160 | .317 | .027 | .014 | .266 | .293 |
| Sig. (2-tailed) | .225 | .011 | .829 | .901 | .017 | .008 |
| N | 59 | 64 | 64 | 81 | 81 | 81 |
| CD8 count | ||||||
| Pearson correlation | −.144 | .358 | .136 | .040 | .343 | .310 |
| Sig. (2-tailed) | .293 | .005 | .301 | .734 | .002 | .006 |
| N | 55 | 60 | 60 | 76 | 76 | 76 |
Maximum number, 81 HIV-1 cases; lower numbers reflect missing data; Sig., significant; N, number of samples.
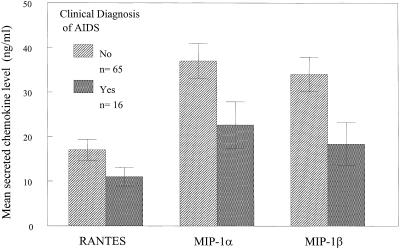
The data were also grouped according to whether the HIV-infected donors were clinically diagnosed with AIDS based on Kaposi's sarcoma or opportunistic infection. As shown in Fig. 2, mean MIP-1α and MIP-1β production was significantly higher with activated CD8+ T cells from HIV-1-infected individuals who remain clinically asymptomatic as compared with those with a clinical diagnosis of AIDS [MIP-1α (P = 0.035); MIP-1β (P = 0.016)]. As in the previous analyses, there was no significant association between mean RANTES production among the HIV+ subject groups.
Figure 2.
Lower production of MIP-1α and MIP-1β by CD8+ T cells from HIV-1+ individuals with clinical diagnosis of AIDS defined by CDC criteria compared with asymptomatic subjects. CD8+ T cells were isolated from HIV+ individuals who were clinically asymptomatic or diagnosed with AIDS based on Kaposi's sarcoma or opportunistic infection. The cells were isolated, cultured, and analyzed as described in Fig. 1 and in the text. The indicated p-values were generated from t tests. For analyses of MIP-1α: P = 0.035 or MIP-1β: P = 0.016. The difference in RANTES values was not significant. Bars indicate the standard errors of the means.
β-Chemokine Levels in CD8+ T Cell Supernatants Suppress Macrophage Tropic Viruses but Do Not Enhance Replication of T Tropic Viruses.
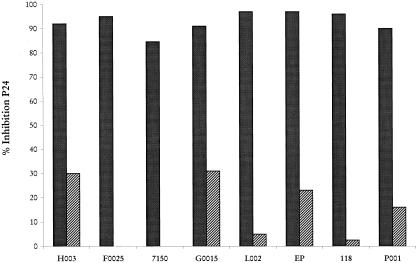
We evaluated the relationship between the β-chemokine levels and the ability of cell-free supernatants from 48 subjects to inhibit the macrophage tropic HIV-1BAL or the T-cell tropic HIV-1IIIB. However, because of lack of supernatants, only 21 subjects were evaluated by using T-cell tropic HIV-1IIIB. The effect of cell-free supernatants obtained from PHA-stimulated purified CD8+ T cells was tested for the antiviral activity in in vitro infected mitogen-activated primary PBMCs system. The PBMCs were cultured in the presence or absence of CD8+ T-cell-free supernatant (50% vol/vol). The antiviral effect was determined by the extent of inhibition of viral replication relative to results with PBMCs cultured alone. Our analysis demonstrated a correlation between β-chemokine production and inhibition of HIV-1BAL (r = 0.340, P = 0.018). As illustrated in Fig. 3, pretreatment with polyclonal goat IgG NAb against RANTES, MIP-1α, and MIP-1β completely reversed the suppressive activity secreted by primary CD8+ T cells in four of the eight HIV+ subjects tested, whereas in the other four patients, the antiviral activity was neutralized between 70 and 80%. On the contrary, and in agreement with our previous results, there was no correlation between the levels of these molecules and the antiviral activity against HIV-1IIIB (18). Nevertheless, β-chemokine levels in CD8+ T-cell supernatants had no effect on replication of HIV-1IIIB as measured by p24 antigen by ELISA (data not shown).
Figure 3.
The effect of anti-β chemokine NAb on the CD8+ T-cell-mediated antiviral activity against the macrophage tropic HIV-1BAL. The CD8+ T-cell culture supernatants obtained from 8 HIV+ subjects were assayed for antiviral activity in the presence of either a nonspecific control antibody (300 μg/ml) (■) or a mixture of NAb specific to RANTES (100 μg/ml), MIP-1α (100 μg/ml), and MIP-1β (100 μg/ml) (▨). The neutralization test was performed by incubating the antibody (R & D Systems) for 30 min at room temperature. Antibodies were added at the initiation of the culture and were replaced after 48 h on medium exchange. The effect on HIV replication was determined at day 8 by p24 ELISA.
Role of the Antiviral Chemokine SDF-1 in the CD8+ T-Cell-Mediated Antiviral Activity.
The production of the α-chemokine SDF-1 by CD8+ T cells obtained from 21 HIV-1-infected individuals was tested by ELISA 72 h after PHA and IL-2 stimulation. Our analyses revealed very low levels of this chemokine (<0.5 ng/ml, data not shown). Consistent with this finding, when we examined the association between SDF-1 levels and the ability of cell-free supernatants from CD8+ T cells to inhibit the T-cell tropic HIV-1IIIB, our analyses demonstrated a lack of correlation (data not shown).
Lack of Relationship Between Plasma Levels of RANTES, MIP-1α, MIP-1β, and Clinical Status.
We examined the association between plasma levels of RANTES, MIP-1α, and MIP-1β and clinical status in the same cohort of HIV+ individuals. We also examined whether β-chemokine production by PHA-stimulated CD8+ T cells correlates with plasma levels measured in the same samples. Our results did not reveal any association between plasma levels of RANTES, MIP-1α, and MIP-1β and clinical status (data not shown). Of interest, when the relationship between plasma levels of RANTES, MIP-1α, and MIP-1β and cell production of this chemokine was examined, the analysis did not reveal a correlation between plasma levels and the ability of CD8+ T cells to release these molecules after in vitro activation (data not shown). However, as illustrated in Table 1, plasma levels of MIP-1α correlated with both CD4+ T-cell count (r = 0.317, P = 0.011) and CD8+ T-cell count (r = 0.358, P = 0.005). On the contrary, RANTES and MIP-1β production did not correlate with either CD4+ or CD8+ T-cell count.
Discussion
Activated CD8+ T cells have been characterized as major sources of a soluble noncytolytic HIV-suppressive activity (12, 13). Consequently, numerous studies have been carried out to examine how the production of this activity in response to nonspecific activation by mitogen or anti-CD3 antibody changes during disease progression in HIV-infected persons (15–17). Despite the establishment of RANTES, MIP-1α, and MIP-1β as major mediators of this activity, and that in terms of T lymphocytes CD8+ T cells are a more potent source of β chemokine (51), there is still no conclusive evidence for the role of CD8+ T-cell β-chemokine-mediated anti-HIV responses in HIV-1 pathogenesis. To address this issue, we performed a cross-sectional study of freshly isolated CD8+ T cells from 81 seropositive patients at different stages of infection along with 28 healthy controls to evaluate differences in chemokine release after nonspecific activation in vitro. Analysis of the data has demonstrated that MIP-1α and MIP-1β production by CD8+ T cells from asymptomatic HIV-1+ subjects was substantially greater than production observed in uninfected donors. In contrast, no significant difference in RANTES production was observed. The activation of CD8+ T cell subsets after exposure to HIV-1 antigens might be responsible for the increased β-chemokine production observed in asymptomatic HIV-1+ individuals. This may represent a specific anti-HIV immune response, which plays a role in viral control. This hypothesis is supported by recent studies where HIV-1-specific CD8+ T cells have been shown to synthesize the β-chemokine RANTES, MIP-1α, and MIP-1β along with cytolytic activity (52).
In this study, we demonstrated that the mean levels of MIP-1α and MIP-1β released by CD8+ T cells are significantly lower among HIV+ persons with AIDS as defined by CD4+ T-cell counts below 200 per μl or by the clinical diagnosis compared with asymptomatic HIV+ subjects. Moreover, MIP-1α and MIP-1β production by CD8+ T lymphocytes correlated with CD4+ and CD8+ T-cell counts.
These findings suggest that activated CD8+ T cells may represent an important source of MIP-1α and MIP-1β in vivo. The neutralization of CD8+ T-cell-mediated antiviral activity in assay with macrophage-tropic HIV-1 isolates after pretreatment with NAb to RANTES, MIP-1α, and MIP-1β indicated that the levels of chemokines released in vitro are sufficient to suppress HIV and represent the major component of the anti-HIV activity, thus suggesting the potential role of these molecules in controlling HIV replication in vivo. The increase in CD8+ T-cell β-chemokine-mediated anti-HIV-1 effects may result in greater antiviral activity and consequentially contribute to slower progression. The association between high levels of MIP-1β production by activated CD8+ T cells and the asymptomatic status reported here is consistent with its specificity for the CCR5 coreceptor (53). Because MIP-1β is less promiscuous than MIP-1α or RANTES and may bind solely to CCR5, high levels of this chemokine may suppress HIV-1 more efficiently.
The decreased ability of CD8+ T lymphocytes to produce MIP-1α and MIP-1β in HIV-1+ subjects who developed AIDS may contribute to less sustained suppression of HIV-1 replication that contributes to ultimate disease development. HIV-1 disease progression is not necessarily associated with virus insensitivity to the β-chemokine effect (54, 55), because 50% of the HIV-1+ subjects who developed AIDS harbor nonsyncytia inducing viruses (56). The loss of viral control in these patients, independent of the sensitivity of their primary isolates to the antiviral effect of the recombinant β chemokine in vitro (54, 55), might instead reflect a decreased ability of the CD8+ T cells to produce the antiviral β-chemokine MIP-1α and MIP-1β.
Because the release of other HIV-suppressive chemokines (24, 25, 57) and perhaps other soluble antiviral factors yet to be identified may also contribute to the inhibition of HIV infection (58), we investigated the potential role of the antiviral chemokine SDF-1 in the CD8+ T-cell-mediated antiviral activity against T-cell tropic HIV-1 strains. Our results indicated that SDF-1 is unlikely involved in the CD8+ T-cell-mediated antiviral activity against T-cell tropic HIV-1, in agreement with a previous report showing extremely low levels of SDF-1 mRNA transcript in CD8+ T cells and a lack of correlation between these levels and the CD8+ T-cell-mediated suppressive activity (59).
In contrast to the results with cells, we found no difference in plasma levels of RANTES, MIP-1α, and MIP-1β between HIV+ subjects differentiated by CD4+ T-cell counts. Moreover, our analysis demonstrated a lack of correlation between CD4+ and CD8+ T-cell counts and plasma levels of MIP-1β or RANTES, although a statistically significant correlation was observed with MIP-1α. Given that chemokines aggregate, adhere to extracellular carbohydrates, and arise from platelets during sample collection (60, 61), these results should not be surprising. Indeed, our analysis showed no correlation between plasma levels of β chemokines and the ability of CD8+ T cells to release these molecules. These data underscore the inherent inaccuracies in plasma chemokine measurements, suggesting that circulating levels are unlikely to reflect what is present at the immune effector sites, and probably explain why such studies failed to demonstrate any association between chemokine levels and disease stage (41–46).
Our data demonstrated that in a cross-sectional analysis, higher production of MIP-1α and MIP-β by CD8+ T lymphocytes is associated with better clinical status. A statistically significant correlation was detected between suppression of the macrophage tropic HIV-1BAL and β-chemokine levels produced by activated CD8+ T cells; moreover, NAb to RANTES, MIP-1α, and MIP-1β were able to reverse the suppressive activity. These chemokine levels, although sufficient for suppression of the macrophage tropic HIV-1BAL, played no role in enhancing the replication of the T-cell tropic HIV-1IIIB. This effect apparently involves receptor activation and stimulation of colocalization of CD4 and CXCR4 that facilitate more efficient viral entry (62). Our data suggest that it is unlikely that the physiologic levels of these molecules produced in vivo could play a role in enhancing the replication of T-cell tropic HIV-1.
Future longitudinal studies to elucidate the mechanism of these effects should identify CD8+ T-cell subsets that can be activated by mitogen to secrete MIP-1α and MIP-1β to characterize which changes in CD8+ T cells ultimately cause the reduction in chemokine release. There may be selective loss of a subset that gives rise to chemokine-secreting effector cells or progressive impairment in chemokine production on a per cell basis, which occur with AIDS development.
Acknowledgments
We thank Anne Sill for her skillful help with the statistical analysis, Kristin Wilson and Lewis Davis for their excellent technical help, and Yvette Gordon and Anna Mazzuca for their editorial assistance. This work was funded by National Institutes of Health Grant R21AI44735.
Abbreviations
- MIP
macrophage inflammatory protein
- NAb
neutralizing antibodies
- PBMCs
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- Rh IL-2
recombinant human IL-2
- CCR5
CC chemokine receptor 5
- SDF-1
stromal-derived factor 1
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240469997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240469997
References
- 1.Tsubota H, Lord C I, Watkins D I, Morimoto C, Letvin N L. J Exp Med. 1989;169:1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langlade-Demoyen P, Ngo-Giang H, Ferchal F, Oksenhendler E. J Clin Invest. 1993;93:1293–1297. doi: 10.1172/JCI117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, et al. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 4.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaldo C, Huang X L, Fan Z F, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, et al. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, et al. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, et al. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lifton M A, Lord C I, Forman M A, Letvin N L. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 11.Walker C M, Moody D J, Stites D P, Levy J A. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 12.Walker C M, Levy J A. Immunology. 1989;66:628–630. [PMC free article] [PubMed] [Google Scholar]
- 13.Brinchmann J E, Gaudernack G, Vartdal F. J Immunol. 1990;144:2961–2966. [PubMed] [Google Scholar]
- 14.Mackewicz C E, Yang L C, Lifson J D, Levy J A. Lancet. 1994;344:1671–1673. doi: 10.1016/s0140-6736(94)90459-6. [DOI] [PubMed] [Google Scholar]
- 15.Gomez A M, Smaill F M, Rosenthal K L. Clin Exp Immunol. 1994;97:68–75. doi: 10.1111/j.1365-2249.1994.tb06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackewicz C E, Ortega H W, Levy J A. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackbourn D J, Mackewicz C E, Barker E, Hunt T K, Herndier B, Haase A T, Levy J A. Proc Natl Acad Sci USA. 1996;93:13125–13130. doi: 10.1073/pnas.93.23.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 19.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 20.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 21.Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkhatib G, Locati M, Kennedy P E, Murphy P M, Berger E A. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. [Google Scholar]
- 24.Bleul C C, Farzan M, Choe H, Prolin C, Clark-Lewis I, Sodroski J, Springer T A. Nature (London) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 25.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, et al. Nature (London) 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 26.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, et al. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. , and erratum (1996) 274, 1069. [DOI] [PubMed] [Google Scholar]
- 27.Michael N L, Chang G, Louie L G, Mascola J R, Dondero D, Birx D L, Sheppard H W. Nat Med. 1997;3:338–340. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 28.Eugen-Olsen J, Iversen A K N, Garred P, Koppelhus U, Pedersen C, Benfield T L, Sorensen A M, Katzenstein T, Dickmeiss E, Gerstoft J, et al. AIDS. 1997;11:305–310. doi: 10.1097/00002030-199703110-00007. [DOI] [PubMed] [Google Scholar]
- 29.De Roda Husman A M, Koot M, Cornelissen M, Keet I P, Brouwer M, Broersen S M, Bakker M, Ross M T, Prins M, deWolf F, et al. Ann Intern Med. 1997;127:882–890. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Paxton W A, Martin S R, Tse D, O'Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 31.Zagury D, Lachgar A, Chams V, Fall L S, Bernard J, Zagury J F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, et al. Proc Natl Acad Sci USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furci L, Scarlatti G, Burastero S, Tambussi G, Colognesi C, Quillent C, Longhi R, Loverro P, Borgonovo B, Gaffi D, et al. J Exp Med. 1997;186:455–460. doi: 10.1084/jem.186.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 34.Zagury D, Lachgar A, Chams V, Fall L S, Bernard J, Zagury J F, Bizzini B, Gringeri A, Santagostino E, Rappaport J, et al. Proc Natl Acad Sci USA. 1998;95:3851–3856. doi: 10.1073/pnas.95.7.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullum H, Cozzi A, Victor J, Aladdin A, Phillips A N, Gerstoft J, Skinhej P, Pederson B K. J Infect Dis. 1998;177:331–336. doi: 10.1086/514192. [DOI] [PubMed] [Google Scholar]
- 36.Garzino-Demo A, Moss R B, Margolick J B, Cleghorn F, Sill A, Blattner W A, Cocchi F, Carlo D J, DeVico A L, Gallo R C. Proc Natl Acad Sci USA. 1999;96:11986–11991. doi: 10.1073/pnas.96.21.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar D, Parato K, Kumar A, Sun E, Cameron D W, Angel J B. AIDS Res Hum Retroviruses. 1999;15:1073–1077. doi: 10.1089/088922299310368. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Tao L, Mitchell E, Bravery C, Berlingieri P, Armstrong P, Vaughan R, Underwood J, Lehner T. Nat Med. 1999;5:1004–1009. doi: 10.1038/12440. [DOI] [PubMed] [Google Scholar]
- 39.Blazevic V, Heino M, Ranki A, Jussila T, Krohn K J. AIDS. 1996;10:1435–1436. doi: 10.1097/00002030-199610000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Clerici M, Balotta C, Trabattoni D, Papagno L, Ruzzante S, Rusconi S, Fusi M L, Colombo M C, Galli M. AIDS. 1996;10:1432–1433. doi: 10.1097/00002030-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Zanussi S, D'Andrea M, Simonelli C, Tirelli U, De Paoli P. AIDS. 1996;10:1431–1432. doi: 10.1097/00002030-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Weiss L, Si-Mohamed A, Giral P, Castiel P, Ledur A, Blondin C, Kazatchkine M D, Haeffner-Cavaillon N. J Infect Dis. 1997;176:1621–1624. doi: 10.1086/517341. [DOI] [PubMed] [Google Scholar]
- 43.McKenzie S W, Dallalio G, North M, Frame P, Means R T. AIDS. 1996;10:F29–33. doi: 10.1097/00002030-199610090-00001. [DOI] [PubMed] [Google Scholar]
- 44.Krowka J F, Gesner M L, Ascher M S, Sheppard H W. Clin Immunol Immunopathol. 1997;85:21–27. doi: 10.1006/clin.1997.4411. [DOI] [PubMed] [Google Scholar]
- 45.Kakkanaiah V N, Ojo-Amaize E A, Peter J B. Clin Diag Lab Immunol. 1998;5:499–502. doi: 10.1128/cdli.5.4.499-502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polo S, Veglia F, Malnati M S, Gobbi C, Farci P, Raiteri R, Sinicco A, Lusso P. AIDS. 1999;13:447–454. doi: 10.1097/00002030-199903110-00002. [DOI] [PubMed] [Google Scholar]
- 47.Dolei A, Biolchini A, Serra C, Curreli S, Gomes E, Dianzani F. AIDS. 1998;12:183–190. doi: 10.1097/00002030-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Gordon C J, Muesing M A, Proudfoot A E, Power C A, Moore J P, Trkola A. J Virol. 1999;73:684–694. doi: 10.1128/jvi.73.1.684-694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castro K G, Ward J W, Slutsker L, Buehler J W, Jaffe H W, Berkelman R L, Curran J W. MMWR Morb Mortal Wkly Rep. 1992;41:1. [Google Scholar]
- 50.Derdeyn C A, Costello C, Kilby J M, Sfakianos G, Saag M S, Kaslow R, Bucy R P. AIDS Res Hum Retroviruses. 1999;15:1063–1071. doi: 10.1089/088922299310359. [DOI] [PubMed] [Google Scholar]
- 51.Conlon K, Llyod A, Chattopadhyay U, Lukacs N, Kunkel S, Schall T, Taub D, Morimoto C, Osborne J, Oppenheim J, et al. Eur J Immunol. 1995;25:751–756. doi: 10.1002/eji.1830250319. [DOI] [PubMed] [Google Scholar]
- 52.Wagner L, Yang O O, Garcia-Zepeda E A, Ge Y, Kalams S A, Walker B D, Pasternack M S, Luster A D. Nature (London) 1998;391:908–911. doi: 10.1038/36129. [DOI] [PubMed] [Google Scholar]
- 53.Premack B, Schall T. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 54.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, et al. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 55.Jansson M, Popovic M, Karlsson A, Cocchi F, Rossi P, Albert J, Wigzell H. Proc Natl Acad Sci USA. 1996;93:15382–15387. doi: 10.1073/pnas.93.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tersmette M, Gruters R A, deWolf F, DeGoede R E Y, Lange J M A, Schellekens P T A, Goudsmit J, Huisman J G, Miedema F. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 58.Levy J A, Mackewicz C E, Barker E. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 59.Lacey S F, McDanal C B, Horuk R, Greenberg M L. Proc Natl Acad Sci USA. 1997;94:9842–9847. doi: 10.1073/pnas.94.18.9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klinger M H, Wilhelm D, Bubel S, Sticherling M, Schroder J M, Kuhnel W. Int Arch Allergy Immunol. 1995;107:541–546. doi: 10.1159/000237097. [DOI] [PubMed] [Google Scholar]
- 61.Kameyoshi Y, Dorschner A, Mallet A I, Christophers E, Schroder J M. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinter A, Catanzaro A, Monaco J, Ruiz M, Justement J, Moir S, Arthos J, Oliva A, Ehler L, Mizell S, et al. Proc Natl Acad Sci USA. 1998;95:11880–11885. doi: 10.1073/pnas.95.20.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]