Abstract
CD40, a tumor necrosis factor receptor superfamily member, is up-regulated on intraheptatic endothelial cells (IHEC) and epithelial cells during inflammatory liver disease, and there is evidence that the functional outcome of CD40 ligation differs between cell types. Ligation of CD40 on cholangiocytes or hepatocytes results in induction of Fas-mediated apoptosis, whereas ligation of IHEC CD40 leads to enhanced chemokine secretion and adhesion molecule expression. We now report that differential activation of two transcription factors, nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), in primary human hepatocytes or IHEC, is associated with and may explain, in part, the different responses of these cell types to CD40 ligation. CD40 ligation induced a rise in NF-κB activity in hepatocytes ,which peaked at 2 h and returned to baseline by 24 h; however, IHEC CD40 ligation resulted in a sustained up-regulation of NF-κB (>24 h). In hepatocytes, CD40 ligation led to sustained up-regulation of AP-1 activity >24 h associated with increased protein levels of RelA (p65), c-Jun, and c-Fos, whereas no induction of AP-1 activity was observed in IHECs. Analysis of mitogen-activated protein kinase phosphorylation (phospho-extracellular signal-regulated kinase 1/2 and phospho-c-Jun NH2-terminal kinase 1/2) and expression of inhibitor κBα were entirely consistent, and thus confirmed the profiles of NF-κB and AP-1 signaling and the effects of the selective inhibitors assessed using electrophoretic mobility shift assay or Western immunoblotting. CD40 ligation resulted in induction of apoptosis in hepatocytes after 24 h, but on IHECs, CD40 ligation resulted in proliferation. Inhibition of (CD40-mediated) NF-κB activation prevented IHEC proliferation and led to induction of apoptosis. Selective extracellular signal-regulated kinase and c-Jun NH2-terminal kinase inhibitors reduced levels of apoptosis in (CD40-stimulated) hepatocytes by ∼50%. We conclude that differential activation of these two transcription factors in response to CD40 ligation is associated with differences in cell fate. Transient activation of NF-κB and sustained AP-1 activation is associated with apoptosis in hepatocytes, whereas prolonged NF-κB activation and a lack of AP-1 activation in IHECs result in proliferation.
INTRODUCTION
CD40, a transmembrane receptor of the tumor necrosis factor receptor (TNFR) superfamily, was first identified in B lymphocytes but has since been found on many other cell types, including those of the macrophage lineage, stromal cells, endothelium, and epithelium (Eliopoulos et al., 1996; Van Kooten and Bancherau, 1997). There is now compelling evidence that CD40 is pivotal in regulating inflammatory responses and recent studies have shown widespread expression of CD40 during inflammatory liver diseases, including allograft rejection, autoimmune disease, and viral hepatitis (Kondo et al., 1997; Afford et al., 1999). All these diseases are characterized by an inflammatory cell infiltrate associated with loss of either hepatocytes or cholangiocytes by apoptotic mechanisms (Galle et al., 1995; Harada et al., 1997; Afford et al., 1999, 2001). Apoptosis of liver cells can be induced by effector leukocytes via distinct pathways, including activation of death receptors such as Fas (CD95, Apo-1), or through the granzyme/perforin pathway (Berke, 1995; Harada et al., 1997; Afford et al., 1999, 2001). Hepatocytes undergo apoptosis directly when cell surface Fas is either cross-linked with antibody or Fas ligand (FasL)-bearing effector cells (Galle et al., 1995). We have previously shown that CD40 activation with either soluble recombinant CD40 ligand or cross-linking monoclonal antibody (mAb) can also trigger apoptosis of hepatocytes and cholangiocytes (Afford et al., 1999, 2001) in the absence of any cofactors, via Fas-dependent mechanisms.
Although it has been reported that activation of CD40 on endothelial cells results in increased chemokine secretion and adhesion molecule expression (Hollenbaugh et al., 1995), the effects of CD40 ligation on primary endothelial cell fate remain unknown. Differences in cellular responses to CD40 ligation have also been widely reported in other cell types. For example, CD40 ligation provides an antiapoptotic and proliferative signal for normal resting B cells (Gordon, 1995) and results in enhanced secretion of interleukin 6, tumor necrosis factor-α (TNF-α), and adhesion molecule expression in keratinocytes (Denfeld et al., 1996; Peguet-Navarro et al., 1997), whereas in malignant epithelial cell lines CD40 ligation results in growth inhibition and sensitization to apoptosis (Eliopoulos et al., 1996; von Leoprechting et al., 1999).
The nature and profile of the intracellular signaling pathways and transcription factors activated by CD40 have not yet been comparatively studied in primary human liver cells but will be critical in determining the diverse effects of CD40 ligation. Although the cytoplasmic C terminus of the CD40 receptor lacks intrinsic kinase activity, adapter proteins of the TNFR-associated factor (TRAF) family, particularly TRAF2 and TRAF6, mediate the activation of signaling cascades such as c-Jun NH2-terminal kinase (JNK), which lead to activation of the transcription factors AP-1 and NF-κB. Both NF-κB and AP-1 have recently been implicated in the control of epithelial proliferation or apoptosis (Eliopoulos et al., 2000) with cell fate being determined by the level of activation of pro- or antiapoptotic genes with NF-κB– and AP-1–sensitive promoters.
It is against this background that we have studied the NF-κB and AP-1/JNK signaling pathways in primary human hepatocytes and intrahepatic endothelial cells (IHECs) before and after CD40 ligation to ascertain the influence and level of involvement of the transcription factors NF-κB and AP-1 on cell survival.
MATERIALS AND METHODS
Sources of Liver Tissue and Isolation of Hepatocytes and IHECs
Liver tissue was obtained from fully informed consenting patients undergoing transplantation for a variety of end stage liver diseases or from normal donor tissue surplus to surgical requirements of the transplant program.
Hepatocytes and IHECs were isolated from freshly collected liver tissue and maintained in culture as described previously (Strain et al., 1991; Afford et al., 1999; Lalor et al., 2002). Briefly, hepatocytes were isolated from human liver tissue by using collagenase perfusion and Percoll density gradient centrifugation. Immediately after isolation, cells were resuspended in Williams E medium containing hydrocortisone (2 μg/ml) (Sigma-Aldrich, St. Louis, MO), insulin (0.124 μ/ml) (Novo Nordisk Pharmaceuticals, Brighton, United Kingdom), and glutamine (2 mM) (Sigma-Aldrich) and plated onto collagen-coated 75-ml tissue culture flasks (2 × 105 cells/ml) and allowed to adhere for 2 h. Medium was then replaced, and cells rested for 24 h before treatment.
IHECs were isolated by finely chopping human liver tissue (30 g), followed by incubation with collagenase type 1A (Sigma-Aldrich) and metrizamide (Sigma-Aldrich) density gradient centrifugation. This was followed by negative immunomagnetic selection with mAb to HEA-125 (TCS Biologicals, Buckingham, United Kingdom) to remove any contaminating cholangiocytes and then positive immunomagnetic selection with mAb to CD31 (DAKO, Ely, United Kingdom) for IHECs. After isolation, IHECs were cultured in human basal endothelial medium containing 10% human serum, 10 ng/ml hepatocyte growth factor (R & D Systems, Abingdon, Oxford, United Kingdom), and 10 ng/ml vascular endothelial growth factor (R & D Systems) and then plated in collagen-coated 25-ml tissue culture flasks. Cells were expanded in these flasks with regular medium exchanges until they became a confluent monolayer.
Assessment of NF-κB and AP-1 Functional Activity by Electrophoretic Mobility Gel Shift Assay (EMSA) and Western Immunoblotting
Hepatocytes and IHECs were cultured in the presence or absence of either a cross-linking mAb to CD40 (1:100; IgG2a isotype clone G28.5; obtained as a gift from Dr. J. Pound, Immunology, University of Birmingham, Birmingham, United Kingdom), previously shown to induce apoptosis in hepatocytes (Afford et al., 1999) or TNF-α (10 ng/ml; R & D Systems) for up to 24 h, after which cytoplasmic and nuclear extracts were prepared according to standard protocol (Abmayr and Worman, 1991). The protein content of both cytoplasmic and nuclear extracts was determined by the bicinchoninic acid protein assay (Pierce Chemical, Rockford, IL; according to manufacturer's instructions), and EMSAs were performed as described previously (Afford et al., 2001) by using 32P end-labeled NF-κB– or AP-1–binding consensus oligoduplex probes (NF-κB probe from Promega, Madison, WI; sequence 5′-AGT TGA GGG GAC TTT CCC AGG C-3′; AP-1 probe from Santa Cruz Biotechnology, Santa Cruz, CA; sequence 5′-CGC TTG ATG ACT CAG CCG GAA-3′). The specificity of the bands was confirmed by supershift assays, where hybridized nuclear extracts were incubated with specific mouse monoclonal antisera to NF-κB RelA (p65) (catalog no. SC-8008X; Santa Cruz Biotechnology), AP-1 c-jun (catalog no. SC-7481X; Santa Cruz Biotechnology), or AP-1 c-fos (catalog no. SC-8047X; Santa Cruz Biotechnology). All EMSAs were performed at least four times by using nuclear extracts from different liver preparations for each liver cell type.
For Western immunoblotting studies, 40 μg of nuclear protein (to detect RelA, c-Jun, and c-Fos) or cytoplasmic protein (to detect phospho-extracellular signal-regulated kinase [pERK1/2], phospho-c-Jun NH2-terminal kinase [pJNK1/2], and inhibitor κBα [IκBα]) was resolved on an SDS-PAGE gel and transferred to a nitrocellulose membrane (Hybond C-extra; Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom). The blotted membrane was blocked for 1 h at room temperature in Tris-buffered saline (TBS) containing 5% (wt/vol) membrane blocking reagent (nonfat dried milk). All antibody incubations were carried out at room temperature in TBS containing 1% membrane blocking reagent. The incubation steps were followed by three washing steps of 5 min with TBS containing 0.1% Tween 20. The following primary antibodies were used: 1) NF-κB RelA (p65) mouse monoclonal primary antibody used at a dilution of 1:3000 for 1 h (catalog no. SC-8008; Santa Cruz Biotechnology); 2) c-jun mouse monoclonal primary antibody used at a dilution of 1:2000 for 1 h (catalog no. SC-7481; Santa Cruz Biotechnology); 3) c-fos mouse monoclonal primary antibody used at a dilution of 1:3000 for 1 h (catalog no. SC-8047; Santa Cruz Biotechnology); 4) phospho-specific p44/p42 mitogen-activated protein kinase (pERK1/2) mouse monoclonal (1:2000 dilution; catalog no. SC-7383; Santa Cruz Biotechnology). 5) phospho-specific p46/p54 JNK (pJNK1/2) mouse monoclonal (1:2000 dilution; catalog no. SC-6254; Santa Cruz Biotechnology); and 6) IκBα mouse monoclonal (catalog no. SC-16431, 1:2000 dilution; Santa Cruz Biotechnology).
Binding of specific mAb was detected with a horseradish peroxidase-conjugated rabbit anti-mouse at a dilution of 1:5000 for 1 h. Protein bands were visualized using the enhanced chemiluminescence detection system (Amersham Biosciences UK) followed by exposure of the membranes to Hyperfilm-ECL (Amersham Biosciences UK). Quantification of the protein bands was carried out using laser densitometry. Equality of protein loading on membranes and complete transfer were checked by staining gels and membranes with Coomassie Blue. All Western immunoblots were performed at least three times by using nuclear or cytoplasmic extracts from different liver preparations for each liver cell type.
Assessment of Apoptosis and Proliferation
Apoptosis was quantitated on cytospun preparations of hepatocytes or IHECs that had been cultured in the presence or absence of either anti-CD40 mAb, TNF-α, and/or specific NF-κB/JNK/ERK inhibitors, caffeic acid phenethyl ester (CAPE), 6-dimethyl amino purine (DMAP), and PD98059 (2′amino-3′methoxyflavone), respectively, by using the in situ DNA end-labeling (ISEL) method described previously (Ansari et al., 1993; Afford et al., 1995).
Proliferation was quantified on similar cytospun preparations of cells before or after CD40 ligation by using an mAb-specific for Ki67 nuclear antigen (DAKO) according to the manufacturer's standard protocol.
For each cytospin the percentage of positive cells was calculated from five randomly selected fields or >200 cells. The mean percentage of positive cells ± SD was then calculated from a minimum of three separate experiments.
RESULTS
NF-κB Activation
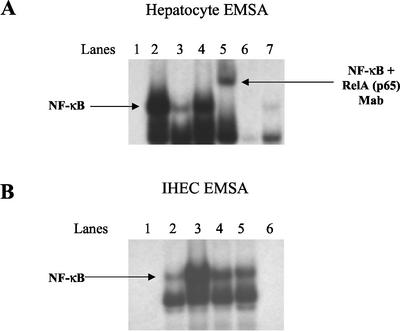
Hepatocytes and IHECs in culture expressed low levels of functional NF-κB (Figure 1A, lane 3, and B, lane 2, respectively), which was increased fourfold after 2-h incubation with TNF-α (Figure 1A, lane 2, and B, lane 3). CD40 activation resulted in a similar increase in NF-κB activity in both cell lineages at 2 h (Figure 1, A and B, lanes 4). Multiple bands were visualized after hybridization of the NF-κB–radiolabeled oligonucleotide, and the functional RelA (p65) moiety was detected using a specific mouse mAb, which resulted in a supershift of the specific RelA (p65) band (Figure 1A, lane 5). Binding of the nuclear extracts to the NF-κB consensus sequence was eliminated in the presence of a 100-fold molar excess of unlabeled oligonucleotide probe (Figure 1, A and B, lanes 6). After 24-h stimulation with the CD40 mAb, NF-κB activity had returned to baseline in hepatocytes (Figure 1A, lane 7) (in fact, NF-κB activity fell to control levels after 4 h of CD40 stimulation; our unpublished data). In contrast, increased NF-κB activity in IHECs was sustained (Figure 1B, lane 5) over the 24-h period.
Figure 1.
EMSA analysis showing changes in DNA binding activity of NF-κB in the nuclear extracts of hepatocytes (A) and IHECs (B) stimulated via CD40 or TNF-α (positive control). Aliquots of nuclear extracts (20 μg of protein) from cultured and stimulated primary human hepatocytes and IHECs were subjected to EMSA analysis with 32P-labeled oligonucleotide probe consensus sequence to NF-κB. In hepatocytes, NF-κB activation was detected at 2 h but was largely undetectable at 24 h (A), whereas NF-κB activation was sustained >24 h in the IHECs (B). (A) Hepatocyte NF-κB: lane 1, negative control (labeled probe alone); lane 2, 2-h TNF-α stimulation; lane 3, unstimulated control; lane 4, 2-h CD40 stimulation; lane 5, supershift by using anti-RelA mAb; lane 6, cold competition with 100× excess of unlabeled probe; lane 7, 24-h CD40 stimulation. (B) IHEC NF-κB: lane 1, negative control (labeled probe alone); lane 2, 2-h unstimulated control; lane 3, 2 h after TNF-α stimulation; lane 4, 2-h CD40 stimulation; lane 5, 24-h CD40 stimulation; lane 6, cold competition with 100× excess of unlabeled probe.
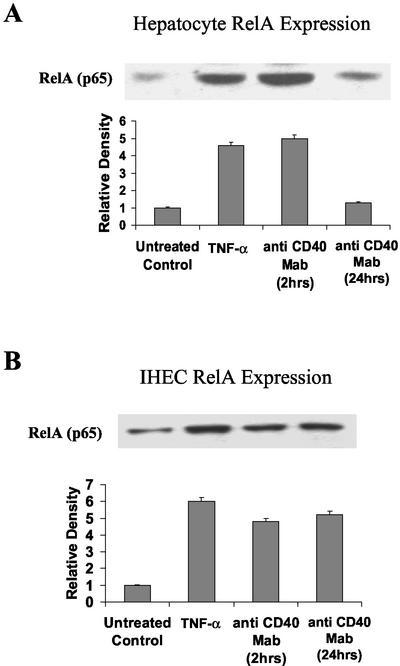
NF-κB consists of Rel A (p65), NF-κB1 (p50), NF-κB2 (p52), c-Rel, and Rel B, which form a variety of homo- and heterodimers (Baeuerle, 1991). The principle and most potent NF-κB activator of transcription is a NF-κB1/RelA heterodimer. To ascertain the level of involvement of this heterodimer after CD40 stimulation, levels of the RelA (p65) subunit were determined in hepatocytes and IHECs by Western blotting. Little RelA (p65) was detected in untreated nuclear extracts of hepatocytes or IHECs (Figure 2, A and B), but a four- to sixfold increase of protein complexes containing RelA (p65) was detected after 2-h TNF-α (Figure 2, A and B) or CD40 stimulation in both cell types (Figure 2, A and B). In parallel with the EMSA results, RelA (p65) expression fell to untreated control levels after 24-h stimulation with anti-CD40 mAb in hepatocytes (Figure 2A) but remained elevated throughout the 24-h period in IHECs (Figure 2B).
Figure 2.
Western immunoblots showing changes in RelA (p65) levels in CD40 stimulated or 2-h TNF-α (positive control) stimulated hepatocyte (A) and IHEC (B) nuclear extracts. Aliquots of nuclear extracts (40 μg of protein) from cultured and stimulated primary human hepatocytes and IHECs were subjected to Western blot analysis. Relative changes in p65 levels among the two cell types, as determined by densitometric analysis, and various experimental conditions are shown in the histograms (n = 4). Consistent with the EMSA data in Figure 1, increased levels of hepatocyte Rel A (p65) detected at 2 h were back to baseline at 24 h (A), whereas increased levels of RelA (p65) were sustained >24 h in the IHECs (B).
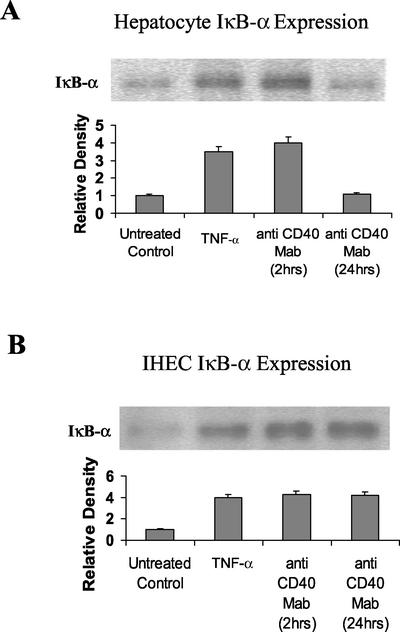
IκB proteins regulate NF-κB through cytoplasmic retention. Although five mammalian IκB proteins have been characterized, IκBα is thought to play a major role in the repression of NF-κB. In the current studies, cytoplasmic IκBα levels in hepatocytes increased after 2 h of CD40 stimulation and returned to basal levels by 24 h (Figure 3A), whereas in IHECs increased levels of IκBα were maintained throughout the 24-h stimulation period (Figure 3B). These results were entirely consistent with the EMSA results (Figure 1) and the RelA (p65) Western blot data (Figure 2).
Figure 3.
Western Immunoblots showing changes in levels of cytoplasmic IκBα in CD40-stimulated or 2-h TNF-α (positive control)–stimulated hepatocytes (A) and IHECs (B). Aliquots of cytoplasmic extracts (40 μg of protein) from cultured and stimulated primary human hepatocytes and IHECs were subjected to Western blot analysis. Relative changes in IκBα levels among the two cell types, as determined by densitometric analysis, and various experimental conditions are shown in the histograms (n = 3). Consistent with the EMSA data in Figure 1 and the Western blot data in Figure 2, increased levels of hepatocyte IκBα detected after 2 h of CD40 stimulation were back to baseline at 24 h (A), whereas increased levels of IκBα were sustained >24 h in the IHECs (B). (A) Hepatocyte IκBα: lane 1, unstimulated control; lane 2, 2-h TNF-α stimulation; lane 3, 2-h CD40 stimulation; lane 4, 24-h CD40 stimulation. (B) IHEC IκBα: lane 1, unstimulated control; lane 2, 2-h TNF-α stimulation; lane 3, 2-h CD40 stimulation; and lane 4, 24-h CD40 stimulation.
AP-1 Activation
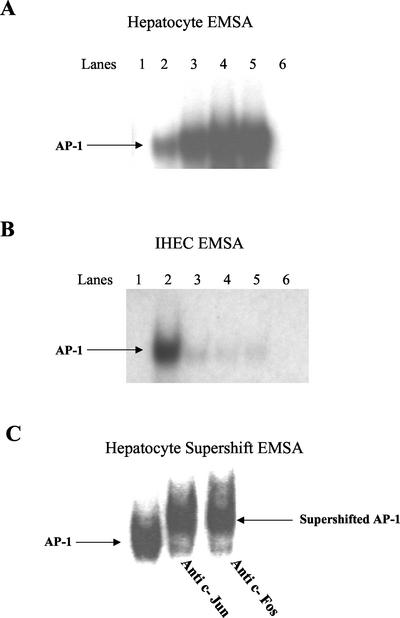
AP-1 DNA binding activity in hepatocytes stimulated with TNF-α for 2 h was increased fivefold, compared with unstimulated controls (Figure 4A, lane 3). A similar trend was seen after 2 h of CD40 stimulation with a sixfold increase in AP-1 DNA binding capacity (Figure 4A, lane 4). The increased levels of AP-1 binding were maintained for 24 h after CD40 stimulation (Figure 4A, lane 5). In IHECs, however, although there was an increase in AP-1 activity after 2 h of TNF-α stimulation (Figure 4B, lane 2), no such increase was observed after 2- or 24-h CD40 activation (Figure 4B, lanes 4 and 5). In all experiments, cold competition studies were performed using 100-fold molar excess of the unlabeled AP-1 oligonucleotide probe to confirm specificity of binding (Figure 4, A and B, lanes 6). Because AP-1 is composed of members of the Jun and Fos families (Karin, 1995), supershift EMSAs were performed with specific polyclonal antibodies against Jun and Fos to detect their presence in the DNA–protein complexes in hepatocytes after CD40 activation (Figure 4C).
Figure 4.
EMSA analysis showing changes in DNA binding activity of AP-1 in the nuclear extracts of hepatocytes (A) and IHECs (B) stimulated via CD40 or TNF-α (positive control). Aliquots of nuclear extracts (20 μg of protein) from cultured and stimulated primary human hepatocytes and IHECs were subjected to EMSA analysis with 32P-labeled oligonucleotide probe consensus sequence to AP-1. In hepatocytes, increased AP-1 binding was detected in response to CD40 at 2 and 24 h (A), whereas in IHECs little AP-1 activation was detected in response to CD40 at either 2 or 24 h (B). (A) Hepatocyte AP-1: lane 1, negative control (labeled probe alone); lane 2, unstimulated control; lane 3, 2-h TNF-α stimulation; lane 4, 2-h CD40 stimulation; lane 5, 24-h CD40 stimulation; lane 6, cold competition with 100× excess of unlabeled probe. (B) IHEC AP-1: lane 1, negative control (labeled probe alone); lane 2, 2-h TNF-α stimulation; lane 3, unstimulated control; lane 4, 2-h CD40 stimulation; lane 5, 24-h CD40 stimulation; and lane 6, cold competition with 100× excess of unlabeled probe. (C) Supershift EMSA analysis showing the presence of AP-1 components c-Jun and c-Fos in nuclear extracts of hepatocytes stimulated for 24 h with CD40. Aliquots of nuclear extracts (20 μg of protein) from cultured and stimulated primary human hepatocytes were subjected to supershift EMSA analysis containing 2 μg of antibodies against c-Jun or c-Fos components of AP-1 and 32P-labeled oligonucleotide probe consensus sequence of AP-1.
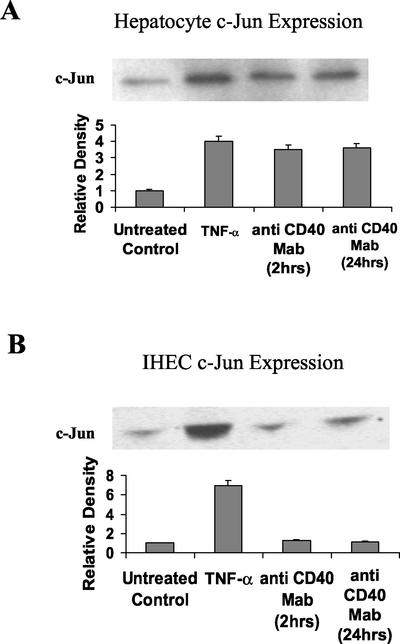
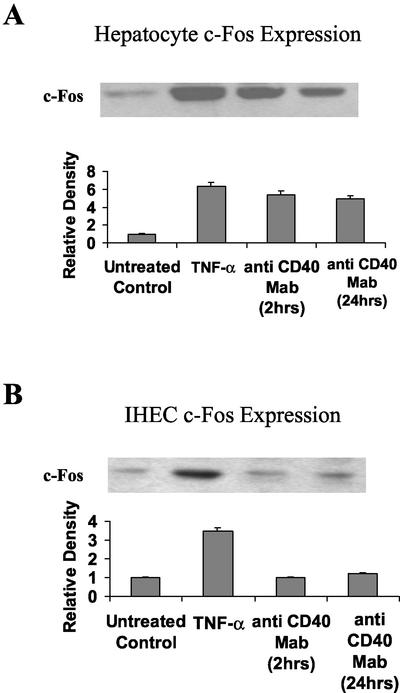
To ascertain whether the increase in AP-1 DNA binding activity was due to an increase in levels of Fos and/or Jun, the relative concentrations of these proteins was determined using Western immunoblotting. Little Jun or Fos was detected in untreated control nuclear extracts of hepatocytes and IHECs (Figures 5, A and B, and 6, A and B, respectively). TNF-α treatment for 2 h resulted in a four- to sevenfold increase in levels of Jun and Fos proteins in both cell types (Figure 5, A and B, respectively). Stimulation of hepatocytes via CD40 for 2 h produced a threefold increase in Jun protein levels (Figure 5A) and a fivefold increase in Fos protein levels (Figure 6A). These levels of protein expression were maintained after 24 h following CD40 stimulation (Figures 5A and 6A). Levels of Jun and Fos were not increased after 2- or 24-h CD40 ligation in IHECs (Figures 5B and 6B), consistent with lack of AP-1 induction.
Figure 5.
Western immunoblots showing changes in c-Jun levels in CD40-stimulated or 2-h TNF-α (positive control)–stimulated hepatocyte (A) and IHEC (B) nuclear extracts. Aliquots of nuclear extracts (40 μg of protein) from cultured and stimulated primary human hepatocytes, and IHECs were subjected to Western blot analysis. Relative changes in c-Jun levels among both cell types, as determined by densitometric analysis and various experimental conditions, are shown in the histograms (n = 3). In hepatocytes, increased c-Jun was detected at 2 and 24 h after CD40 activation (A), whereas in IHEC no activation in response to CD40 was detected at either time point (B).
Figure 6.
Western immunoblots showing changes in c-Fos levels in CD40-stimulated or 2 h TNF-α (positive control)–stimulated hepatocyte (A) and IHEC (B) nuclear extracts. Aliquots of nuclear extracts (40 μg of protein) from cultured and stimulated primary human hepatocytes and IHECs were subjected to Western blot analysis. Relative changes in c-Fos levels among both cell types, as determined by densitometric analysis, are shown in the histograms (n = 3). In hepatocytes, increased c-Fos was detected at 2 and 24 h after CD40 activation (A), whereas in IHEC no activation in response to CD40 was detected at either time point (B) consistent with the observations for c-Jun (Figure 5).
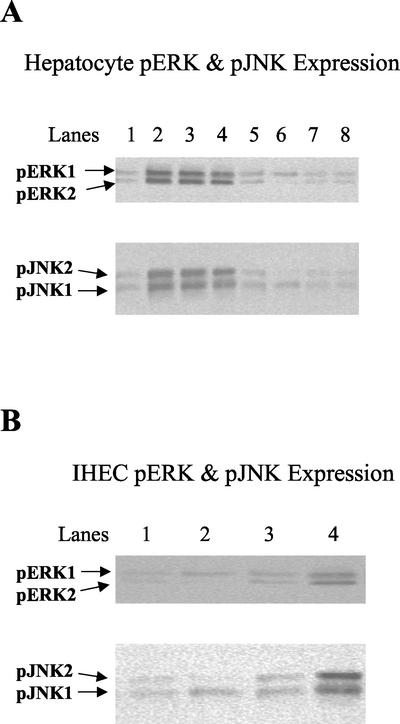
To characterize the downstream signaling systems that lead to the activation of AP-1 after CD40 stimulation, phospho-Western immunoblotting was used to determine activation of two mitogen-activated protein kinase (MAPK) pathways. MAPKs are recruited as part of three-tiered MAPK kinase kinase (MAP3K) ⇒ MAPK kinase (MKK) ⇒ MAPK core pathways. MAPKs require concomitant tyrosine and threonine phosphorylation for activity (Kyriakis and Avruch, 2001). By using antibodies specific for the phosphorylated, active forms of ERK1/2 and JNK1/2, we find that in hepatocytes both these pathways are activated after 2 h of CD40 stimulation (Figure 7A, top and bottom, lane 3) and remain elevated after 24 h (Figure 7A, top and bottom, lane 4). pERK and pJNK activation are comparable with that incurred by TNF-α, used as a positive control (Figure 7A, top and bottom, lane 2). However, in IHECs there are no increases in the levels of pERK or pJNK after CD40 ligation (Figure 7B, top and bottom, lanes 3 and 4).
Figure 7.
Western immunoblots showing changes in levels of cytoplasmic phospho-ERK1/2 and phospho-JNK1/2 in CD40-stimulated or 2-h TNF-α (positive control)–stimulated hepatocytes (A) and IHECs (B) and/or treatment with specific inhibitors to ERK (50 μM PD98059) and JNK (100 μM DMAP). Aliquots of cytoplasmic extracts (40 μg of protein) from cultured and stimulated primary human hepatocytes, and IHECs were subjected to Western blot analysis. In hepatocytes, increased levels of phospho-ERK1/2 and phospho-JNK1/2 were detected at 2 and 24 h after CD40 activation (A) (top and bottom, lanes 3 and 4), whereas in IHECs no activation of these phospho-MAPKs in response to CD40 was detected at either time point (B) (top and bottom). Use of the selective inhibitors to ERK or JNK in CD40-stimulated hepatocytes prevented activation of phospho-ERK1/2 and phospho-JNK1/2, respectively (A) (top and bottom, lanes 7 and 8). (A) Top, hepatocyte phospho-ERK1/2: lane 1, unstimulated control; lane 2, 2-h TNF-α stimulation; lane 3, 2-h CD40 stimulation; lane 4, 24-h CD40 stimulation; lane 5, unstimulated control + ERK inhibitor; lane 6, 2-h TNF-α stimulation + ERK inhibitor; lane 7, 2-h CD40 stimulation + ERK inhibitor; and lane 8, 24-h CD40 stimulation + ERK inhibitor. Bottom, hepatocyte phospho-JNK1/2: lane 1, unstimulated control; lane 2, 2-h TNF-α stimulation; lane 3, 2-h CD40 stimulation; lane 4; 24-h CD40 stimulation; lane 5, unstimulated control + JNK inhibitor; lane 6, 2-h TNF-α stimulation + JNK inhibitor; lane 7, 2-h CD40 stimulation + JNK inhibitor; and lane 8, 24-h CD40 stimulation + JNK Inhibitor. (B) Top, IHEC phospho-ERK1/2: lane 1, unstimulated control; lane 2, 2-h CD40 stimulation; lane 3, 24-h CD40 stimulation; and lane 4, 2-h TNF-α stimulation. Bottom, IHEC phospho-JNK1/2: lane 1, unstimulated control; lane 2, 2-h CD40 stimulation; lane 3, 24-h CD40 stimulation; and lane 4, 2-h TNF-α stimulation.
Assessment of Apoptosis
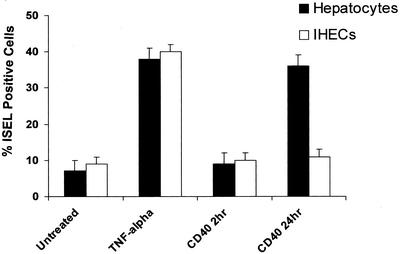
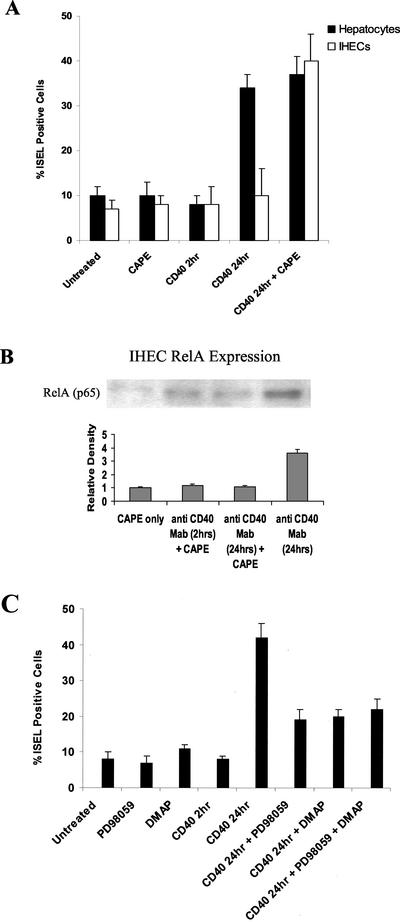
The viability of untreated hepatocytes and IHECs at 2 h was >99% and remained high beyond the duration of the experiment (viability at 4 d was 98.5 ± 0.3% for hepatocytes and 96.7 ± 0.6% for IHECs). Activation of CD40 for 24 h induced apoptosis in hepatocytes as did direct stimulation with TNF-α (10 ng/ml) (Figure 8). CD40 engagement led to a threefold increase in the number of ISEL-positive apoptotic hepatocytes at 24 h, although no increase was detected at 2 h. Stimulation of IHECs via CD40 for 2 or 24 h resulted in no significant induction of apoptosis.
Figure 8.
Levels of apoptosis in primary cultures of hepatocytes and IHECs stimulated via CD40 or TNF-α. Histogram shows the percentage of ISEL-positive apoptotic hepatocytes or IHECs after CD40 or TNF-α stimulation. Black bars show results for hepatocytes and the white bars show results for IHECs (n = 5 for each cell type). Although TNF-α triggered similar levels of increased apoptosis in both cell types, CD40 activation only caused an increase in apoptosis in hepatocytes.
Assessment of Proliferation
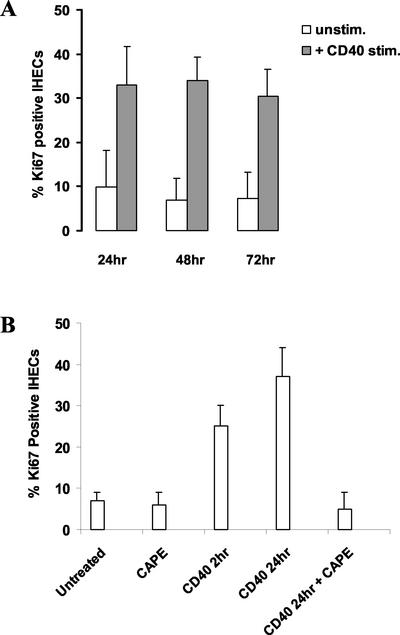
CD40 ligation did not result in proliferation of hepatocytes (our unpublished data). However, after 24 h there was a marked increase in IHEC proliferation (control 9.9%; Ki67-positive cells ± SD, 8.2–37% Ki67-positive cells ± SD 8.7), which was maintained throughout the culture period (Figure 9A). Pretreatment of IHECs with CAPE, a selective inhibitor of NF-κB (see below), resulted in the reduction of CD40-induced proliferation to basal levels (Figure 9B).
Figure 9.
Effect of CD40 ligation and NF-κB inhibition on IHEC proliferation. (A) Histogram shows the proliferative index expressed as the percentage of Ki67-positive cells up to 72 h for either unstimulated IHECs or after CD40 stimulation (n = 8). CD40 stimulation led to a marked increase in cell proliferation in the endothelial cells. (B) Histogram shows the proliferative index expressed as the percentage of Ki67-positive cells for unstimulated IHECs or cells stimulated via CD40 in the presence or absence of the specific and potent NF-κB inhibitor, CAPE (25 μg/ml). The addition of CAPE completely inhibited the endothelial cell proliferation in response to CD40 activation. The results represent data from at least three separate experiments.
Effect of Specific Inhibitors on Apoptosis
Specific inhibitors of NF-κB, JNK, and ERK were used to investigate whether NF-κB and AP-1 signaling pathways determined cell fate of hepatocytes and IHECs after CD40 ligation. CAPE is a specific and potent inhibitor of NF-κB (Natarajan et al., 1996), which prevents the translocation of the RelA (p65) subunit to the nucleus. Incubation of cells with 25 μg/ml CAPE for the duration of the experiments resulted in a fourfold increase in the numbers of IHECs undergoing apoptosis after 24-h CD40 stimulation, suggesting that NF-κB activation was protecting the IHECs from apoptosis (Figure 10A). The addition of CAPE to hepatocytes did not enhance the levels of CD40-induced apoptosis. The specificity of CAPE to inhibit NF-κB was assessed by examining levels of nuclear RelA (p65) in IHECs by using Western blotting (Figure 10B). Little RelA (p65) was detected in IHECs with CAPE treatment alone, and this was comparable with cells stimulated with 2-h/24-h CD40 and CAPE. IHECs stimulated with CD40 treatment alone (24 h) was used as a positive control.
Figure 10.
Effects of specific NF-κB, ERK, and/or JNK inhibitors on levels of apoptosis in hepatocytes or IHECs after CD40 stimulation. (A) Histogram shows the percentage of ISEL-positive apoptotic cells in either unstimulated or CD40-stimulated primary human hepatocytes or IHECs in the presence or absence of the NF-κB inhibitor, CAPE (25 μg/ml). The addition of CAPE to IHECs allowed CD40 to trigger similar levels of apoptosis to those induced in the hepatocytes. The results summarize data from at least four separate experiments. (B) Western immunoblot showing inhibition of nuclear RelA levels by a specific NF-κB inhibitor in CD40-stimulated IHECs. Aliquots of nuclear extracts (40 μg of protein) from cultured and stimulated primary human IHECs were subjected to Western blot analysis. Relative changes in RelA levels as determined by densitometric analysis, and various experimental conditions are shown in the histograms (n = 3). Use of the selective inhibitor to NF-κB, CAPE (25 μg/ml), in CD40-stimulated IHECs prevented increase in nuclear RelA (p65) levels. (C) Histogram shows the percentage of CD40-stimulated and unstimulated primary human hepatocytes positive by ISEL staining in the presence or absence of the selective ERK and/or JNK inhibitors PD98059 (50 μM) and DMAP (100 μM), respectively. The inhibitors reduced hepatocyte apoptosis in response to CD40 activation, however, levels of apoptosis were still above baseline. The results summarize data from at least four separate experiments.
Incubation of hepatocytes with 100 μM DMAP, a selective inhibitor of JNK (Marino et al., 1996; Cheng and Chen, 2001), resulted in a 50% reduction in the number of cells going into apoptosis at 24 h (Figure 10C). Similar findings were observed with the specific ERK inhibitor PD98059 (50 μM) (Alessi et al., 1995), which blocks activation of mitogen-activated protein kinase kinase 1. Combining ERK- and the JNK-specific inhibitors had no additive effect on apoptosis. To examine the specificity of the selective inhibitors to ERK and JNK, we used phospho-Western immunoblotting (Figure 7A, top and bottom, lanes 5–8). Both PD98059 and DMAP were shown to inhibit levels of hepatocyte pERK1/2 and pJNK1/2, respectively, after 2-h/24-h CD40 stimulation (Figure 7A, top and bottom, lanes 7 and 8).
DISCUSSION
TNFR family members deliver signals that regulate diverse cellular responses from proliferation and differentiation to growth suppression and apoptosis (Smith et al., 1994; Cleveland and Ihle, 1995). Many of these receptors, including Fas, TNFR1, and death receptor 3, share a death domain homology in their cytoplasmic tails through which they transmit apoptotic signals. However, certain receptors such as CD40, TNFR2, and CD30, which lack the death domain, have also been reported to suppress cell growth and survival (Eliopoulos et al., 1996; Hess and Engelmann, 1996; Young et al., 1998). We have shown previously that human hepatocytes and cholangiocytes undergo apoptosis as a result of a cooperative interaction between CD40 and Fas receptors (Afford et al., 1999, 2001). In this model CD40 activation triggers FasL expression by the epithelial cells, leading to autocrine or paracrine activation of Fas and consequent cell death. Despite expressing CD40, Fas, and FasL (our unpublished data), IHECs do not undergo apoptosis in response to CD40 activation but instead proliferate (Figure 9A). Although we have yet to elucidate the mechanisms responsible for CD40-mediated proliferation of IHEC, results from our present study (Figure 9B) indicate that NF-κB does play a key role in regulating this process. Indeed, the NF-κB family of transcription factors has been shown to regulate proliferation in several cell types (Hoshi et al., 2000; Brantley et al., 2001; Lim et al., 2001). Endothelial cell proliferation in response to CD40 could be important not only in chronic inflammation but also in angiogenesis and tumor vascularization where 60% of hepatocellular carcinomas express high levels of CD40 (Sugimoto et al., 1999). Our recent data (Russell, Adams, and Afford, unpublished data) suggest that CD40 ligation on IHECs can modulate other endothelial cell functions, including expression of adhesion molecules and chemokines in a manner similar to that reported with endothelial cells from other tissues (Van Kooten, 2000). This indicates that CD40 expression by IHECs may have multiple roles beyond regulation of cell fate.
Our present study shows for the first time that after CD40 ligation, differences in activation of the transcription factors NF-κB and AP-1, key components of intracellular apoptotic signaling cascades, occur in primary human hepatocytes compared with endothelial cells, providing a possible mechanism for the apparent cell-specific effects of CD40 ligation. The use of selective inhibitors also demonstrated the contribution of these signaling pathways to cell fate. Confirmation of the activation of NF-κB and AP-1 and the effects of inhibitors on their modulation was also sought and confirmed by studying selected downstream signaling events to CD40.
NF-κB is activated by CD40 and other members of the TNFR family and has a potentially important function in regulating inflammation (Schwabe et al., 2001) and apoptosis (Foo and Nolan, 1999). In addition, NF-κB is critical for regulating liver regeneration and development (Iimuro et al., 1998). In the current study, we report that CD40 activation leads to a transient activation of NF-κB in hepatocytes, but to a sustained up-regulation of NF-κB activity in IHECs that lasts for >24 h. The fact that inhibiting NF-κB with CAPE leads to marked increases in endothelial cell apoptosis at 24 h suggests that this prolonged NF-κB activation is protecting the IHECs from apoptosis.
Many antiapoptotic genes contain NF-κB binding sites (Baeuerle and Henkel, 1994), including inhibitor of apoptosis protein 2, A20, and TRAFs 1 and 2, and it is likely that CD40 engagement on IHECs leads to the activation of antiapoptotic genes in a NF-κB–dependent manner. NF-κB also binds to promoters of proinflammatory genes such as interleukin-8, monocyte chemoattractant protein-1, intercellular adhesion molecule, and vascular cell adhesion molecule, and the sustained NF-κB up-regulation in IHECs could explain why chemokine and adhesion molecule expression are up-regulated in endothelial cells during CD40 ligation (Van Kooten, 2000). In agreement with our data, several studies have also shown persistent NF-κB activation after stimulation of the CD40 receptor on B cells (Berberich et al., 1994; Lee et al., 1995). It is possible that the sustained NF-κB activity in IHECs could be a consequence of the NF-κB1/RelA dimer being less susceptible to inhibition by IκB proteins. Alternatively, IκBβ can be differentially regulated such that IκBβ produced in response to the initial wave of NF-κB activity is hypophosphorylated and acts as a competitive inhibitor of IκBα but not of NF-κB, leading to persistent NF-κB activity in the nucleus (Phillips and Ghosh, 1997).
We observed a transient up-regulation of NF-κB in hepatocytes after CD40 ligation, which peaked at 2 h and was back at baseline by 4 h. Many proapoptotic genes also have NF-κB binding sites on their promoters, for example, FasL (Kasibhatla et al., 1999), Fas receptor (Gil et al., 1999; Kuhnel et al., 2000), and p53 (Wu and Lozano, 1994), and it is possible that the transient induction of NF-κB may be critical in up-regulating FasL expression (Holtz-Heppelmann et al., 1998).
Several studies have demonstrated a relationship between activation of AP-1 and apoptosis (Xia et al., 1995; Le-Niculescu et al., 1999; Fan et al., 2001). The main components of AP-1 are encoded by two families of genes related to the protooncogenes c-jun and c-fos, the products of which form homo- and heterodimers some of which bind promoters of genes involved in apoptosis (i.e., FasL, caspase 3, p53). Induction of AP-1 activity can occur either as a consequence of increased abundance of AP-1 (Angel and Karin, 1991) or secondary to increased activity. Our results show that the CD40-induced sustained rise in AP-1 activity in hepatocytes is associated with a simultaneous rise in c-Jun and c-Fos protein levels, suggesting that CD40 ligation regulates phosphorylation and expression of the AP-1 family members in these cells. However, it remains to be determined whether the increased protein expression of c-Jun and c-Fos is the result of enhanced synthesis. The sustained rise in AP-1 activity in the absence of sustained NF-κB activity in hepatocytes could allow AP-1 to act unopposed to promote apoptosis. Our previous study has shown increased FasL expression in cultured hepatocytes after CD40 ligation (Afford et al., 1999), and the present study suggests that this is associated with an increase in AP-1 activity. However, our study also indicates that the AP-1/JNK pathway may not be solely responsible for CD40-induced apoptosis in the epithelial cells because selective ERK and JNK inhibitors failed to completely abrogate CD40-induced apoptosis. It is possible that stage of progression through the cell cycle, or other transcription factors such as signal transducer and activator of transcription 3 (STAT3), may be involved. STAT3 has been implicated in apoptosis (Akira, 2000; Chapman et al., 2000), and CD40 ligation in B cells results in tyrosine phosphorylation of Janus tyrosine kinase3, leading to the phosphorylation and subsequent activation of STAT3 (Hanissian and Geha, 1997). We have previously reported NF-κB and AP-1 activation in response to CD40 ligation in primary human cholangiocytes (Afford et al., 2001), and further experiments have confirmed that both hepatocytes and cholangiocytes show similar signaling responses to CD40 activation (our unpublished data for cholangiocytes). These data suggest that in both liver epithelial cell types a common mechanism exists for regulation of cell survival, which is distinct from the response of hepatic endothelial cells.
Recent studies provide possible explanations for the different cellular fates of epithelial and endothelial cells in response to CD40 ligation. We have reported that differences in response to CD40 death signal transduction in carcinoma cells may be due to the differential signals emanating from two distinct CD40 cytoplasmic tail domains: the membrane proximal domain and the TRAF-interacting PXQXT motif (Eliopoulos et al., 2000). Tsukamoto et al. (1999) also demonstrated differential NF-κB regulation by these two CD40 domains. Another recent study reports the existence of CD40 isoforms generated through posttranscriptional and posttranslational alternative splicing (Tone et al., 2001), providing another potential mechanism for differential signaling and hence cell fate in response to CD40 activation.
We conclude that CD40 activation in primary human hepatic epithelial and endothelial cells is associated with differential activation of the NF-κB and AP-1 transcription factors and disruption of these signaling pathways can alter cell fate in primary human hepatocytes or IHECs in vitro. These events could explain why hepatocytes are lost through apoptosis in inflammatory liver disease, whereas endothelial cells are not, even though they all show increased expression of CD40 (Afford et al., 1999). Such differential responses of hepatocytes and endothelial cells make teleological sense in the context of inflammation where the endothelium needs to actively recruit effector cells by expressing adhesion molecules and chemokines, whereas infected epithelial cells need to be destroyed to control either viral or bacterial pathogens. Therapeutic strategies targeted at modulation of CD40-mediated NF-κB/AP-1–dependent mechanisms are of potential importance. This study illustrates that such approaches will need to take into account the wide range of functional consequences that can occur in primary cells after CD40 ligation.
ACKNOWLEDGMENTS
We thank members of the Clinical Transplant and Hepatobiliary Unit (Queen Elizabeth Hospital) for procurement of human liver tissue. This study was supported by Biotechnology and Biological Sciences Research Council project grant 6/C11113.
Abbreviations used:
- AP-1
activator protein-1
- CAPE
caffeic acid phenethyl ester
- DMAP
6-dimethylaminopurine
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-related kinase
- FasL
Fas ligand
- IHEC
intrahepatic endothelial cell
- IκB
inhibitor κB
- ISEL
in situ DNA end labeling
- JNK
c-Jun NH2-terminal protein kinase
- mAb
monoclonal antibody
- MAPK
mitogen activated protein kinase
- NF-κB
nuclear factor-κB
- pERK1/2
phospho-extracellular signal-related kinase 1/2
- pJNK1/2
phospho-c-Jun NH2-terminal protein kinase 1/2
- STAT
signal transducer and activator of transcription
- TNF-α
tumor necrosis factor-α
- TNFR
tumor necrosis factor receptor
- TRAF
tumor necrosis factor receptor-associated factor
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–07–0378. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–07–0378.
REFERENCES
- Abmayr SM, Worman JL. Preparation of nuclear and cytoplasmic extracts from mammalian cells. In: Asubel, Brent FM, Kingston R, Moore RE, Seidman DD, Struhl JG, editors. Current Protocols in Molecular Biology. 1991. K. John Wiley & Sons, New York, 12.1.1–12.1.9. [DOI] [PubMed] [Google Scholar]
- Afford SC, Ahmed-Choudhury J, Randhawa S, Russell C, Youster J, Crosby HA, Eliopoulos A, Hubscher SG, Young LS, Adams DH. CD40 activation-induced, Fas-dependent apoptosis and NF-κB/AP-1 signaling in human intrahepatic biliary epithelial cells. FASEB J. 2001;15:2345–2354. doi: 10.1096/fj.01-0088com. [DOI] [PubMed] [Google Scholar]
- Afford SC, Hubscher S, Strain AJ, Adams DH, Neuberger JM. Apoptosis in the human liver during allograft rejection and end-stage liver disease. J Pathol. 1995;176:373–380. doi: 10.1002/path.1711760408. [DOI] [PubMed] [Google Scholar]
- Afford SC, Rhandawa S, Eliopoulos AG, Hubscher SG, Young LS, Adams DH. CD40 activation induces apoptosis in cultured human hepatocytes via induction of cell surface FasL expression and amplifies Fas mediated hepatocyte death during allograft rejection. J Exp Med. 1999;189:441–446. doi: 10.1084/jem.189.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S. Roles of STAT3 defined by tissue-specific gene targeting. Oncogene. 2000;19:2607–2611. doi: 10.1038/sj.onc.1203478. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Ansari B, Coates PJ, Greenstein BD, Hall PA. In situ end-labeling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993;170:1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA. The inducible transcription activator NF-κB: regulation by distinct protein subunits. Biochim Biophys Acta. 1991;1072:63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Berberich I, Shu GL, Clark EA. Cross-linking CD40 on B cells rapidly activates nuclear factor-κB. J Immunol. 1994;153:4357–4366. [PubMed] [Google Scholar]
- Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- Brantley DM, Chen CL, Muraoka RS, Bushdid PB, Bradberry JL, Kittrell F, Medina D, Matrisian LM, Kerr LD, Yull FE. Nuclear factor-κB (NF-κB) regulates proliferation and branching in mouse mammary epithelium. Mol Biol Cell. 2001;12:1445–1455. doi: 10.1091/mbc.12.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RS, Lourenco P, Tonner E, Flint D, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. The role of Stat3 in apoptosis and mammary gland involution. Conditional deletion of Stat3. Adv Exp Med Biol. 2000;480:129–138. doi: 10.1007/0-306-46832-8_16. [DOI] [PubMed] [Google Scholar]
- Cheng N, Chen J. Tumor necrosis factor-α induction of endothelial ephrin A1 expression is mediated by a p38 MAPK- and SAPK/JNK-dependent but nuclear factor-κB-independent mechanism. J Biol Chem. 2001;276:13771–13777. doi: 10.1074/jbc.M009147200. [DOI] [PubMed] [Google Scholar]
- Cleveland JL, Ihle JN. Contenders in FasL/TNF death signaling. Cell. 1995;81:479–482. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Denfeld RW, Hollenbaugh D, Fehrenbach A, Weiss JM, von Leoprechting A, Mai B, Voith U, Schopf E, Aruffo A, Simon JC. CD40 is functionally expressed on human keratinocytes. Eur J Immunol. 1996;26:2329–2334. doi: 10.1002/eji.1830261009. [DOI] [PubMed] [Google Scholar]
- Eliopoulos AG, Davies C, Knox PG, Gallagher NJ, Afford SC, Adams DH, Young LS. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol. 2000;20:5503–5515. doi: 10.1128/mcb.20.15.5503-5515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos AG, et al. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr Virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- Fan M, Goodwin ME, Birrer MJ, Chambers TC. The c-Jun NH(2)-terminal protein kinase/AP-1 pathway is required for efficient apoptosis induced by vinblastine. Cancer Res. 2001;61:4450–4458. [PubMed] [Google Scholar]
- Foo SY, Nolan GP. NF-κB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- Galle PR, Hofmann WJ, Walczak H, Schaller H, OTTO G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, Alcami J, Esteban M. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the α subunit of eukaryotic translation initiation factor 2 and NF-κB. Mol Cell Biol. 1999;19:4653–4663. doi: 10.1128/mcb.19.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. CD40 and its ligand: central players in B lymphocyte survival, growth, and differentiation. Blood Rev. 1995;9:53–56. doi: 10.1016/0268-960x(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Hanissian SH, Geha RS. Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997;6:379–387. doi: 10.1016/s1074-7613(00)80281-2. [DOI] [PubMed] [Google Scholar]
- Harada K, Ozaki S, Gershwin ME, Nakanuma Y. Enhanced apoptosis relates to bile duct loss in primary biliary cirrhosis. Hepatology. 1997;27:1399–1405. doi: 10.1002/hep.510260604. [DOI] [PubMed] [Google Scholar]
- Hess S, Engelmann H. A novel function of CD40: induction of cell death in transformed cells. J Exp Med. 1996;183:159–167. doi: 10.1084/jem.183.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbaugh D, Mischelpetty N, Edwards CP, Simon JC, Denfeld RW, Keiner PA, Aruffo A. Expression of functional cd40 by vascular endothelial-cells. J Exp Med. 1995;182:33–40. doi: 10.1084/jem.182.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz-Heppelmann CJ, Algeciras A, Badley AD, Paya CV. Transcriptional regulation of the human FasL promoter-enhancer region. J Biol Chem. 1998;273:4416–4423. doi: 10.1074/jbc.273.8.4416. [DOI] [PubMed] [Google Scholar]
- Hoshi S, Goto M, Koyama N, Nomoto K, Tanaka H. Regulation of vascular smooth muscle cell proliferation by nuclear factor-κB and its inhibitor, I-κB. J Biol Chem. 2000;275:883–889. doi: 10.1074/jbc.275.2.883. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NFκB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Genestier L, Green DR. Regulation of fas-ligand expression during activation-induced cell death in T lymphocytes via nuclear factor κB. J Biol Chem. 1999;274:987–992. doi: 10.1074/jbc.274.2.987. [DOI] [PubMed] [Google Scholar]
- Kondo T, Suda T, Fukuyama H, Adachi M, Nagata S. Essential roles of the Fas ligand in the development of hepatitis. Nat Med. 1997;3:409–413. doi: 10.1038/nm0497-409. [DOI] [PubMed] [Google Scholar]
- Kuhnel F, Zender L, Paul Y, Tietze MK, Trautwein C, Manns M, Kubicka S. NFκB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem. 2000;275:6421–6427. doi: 10.1074/jbc.275.9.6421. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transendothelial migration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169:983–992. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Bonfoco E, Kasuya Y, Claret FX, Green DR, Karin M. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol. 1999;19:751–763. doi: 10.1128/mcb.19.1.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Arsura M, Wu M, Duyao M, Buckler AJ, Sonenshein GE. Role of Rel-related factors in control of c-myc gene transcription in receptor-mediated apoptosis of the murine B cell WEHI 231 line. J Exp Med. 1995;181:1169–1177. doi: 10.1084/jem.181.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JW, Kim H, Kim KH. Nuclear factor-κB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab Invest. 2001;81:349–360. doi: 10.1038/labinvest.3780243. [DOI] [PubMed] [Google Scholar]
- Marino MW, et al. Inhibition of tumor necrosis factor signal transduction in endothelial cells by dimethylaminopurine. J Biol Chem. 1996;271:28624–28629. doi: 10.1074/jbc.271.45.28624. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Singh S, Burke TRJ, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peguet-Navarro J, Dalbiez-Gauthier C, Moulon C, Berthier O, Reano A, Gaucherand M, Banchereau J, Rousset F, Schmitt D. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J Immunol. 1997;158:144–152. [PubMed] [Google Scholar]
- Phillips RJ, Ghosh S. Regulation of IκBβ in WEHI 231 mature B cells. Mol Cell Biol. 1997;17:4390–4396. doi: 10.1128/mcb.17.8.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Schnabl B, Kweon YO, Brenner DA. CD40 activates NF-κB and c-Jun N-terminal kinase and enhances chemokine secretion on activated human hepatic stellate cells. J Immunol. 2001;166:6812–6819. doi: 10.4049/jimmunol.166.11.6812. [DOI] [PubMed] [Google Scholar]
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Strain AJ, Ismail T, Tsubouchi H, Arakaki N, Hishida T, Kitamura N, Daikuhara Y, McMaster P. Native and recombinant human hepatocyte growth factor are highly potent promoters of DNA synthesis in both human and rat hepatocytes. J Clin Invest. 1991;87:1853–1857. doi: 10.1172/JCI115207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Shiraki K, Ito T, Fujikawa K, Takase K, Tameda Y, Moriyama M, Nakano T. Expression of functional CD40 in human hepatocellular carcinoma. Hepatology. 1999;30:920–926. doi: 10.1002/hep.510300424. [DOI] [PubMed] [Google Scholar]
- Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci USA. 2001;98:1751–1756. doi: 10.1073/pnas.98.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto N, Kobayashi N, Azuma S, Yamamoto T, Inoue J. Two differently regulated nuclear factor κB activation pathways triggered by the cytoplasmic tail of CD40. Proc Natl Acad Sci USA. 1999;96:1234–1239. doi: 10.1073/pnas.96.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kooten C. Immune regulation by CD40-CD40-l interactions-2; Y2K update. Front Biosci. 2000;5:D880–D693. doi: 10.2741/kooten. [DOI] [PubMed] [Google Scholar]
- Van Kooten C, Bancherau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- von Leoprechting A, Van der Bruggen P, Pahl HL, Aruffo A, Simon JC. Stimulation of CD40 on immunogenic human malignant melanomas augments their cytotoxic T lymphocyte-mediated lysis and induces apoptosis. Cancer Res. 1999;59:1287–1294. [PubMed] [Google Scholar]
- Wu H, Lozano G. NF-κB activation of p53. A potential mechanism for suppressing cell growth in response to stress. J Biol Chem. 1994;269:20067–20074. [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Young LS, Eliopoulos AG, Gallagher NJ, Dawson CW. CD40 and epithelial cells: across the great divide. Immunol Today. 1998;19:502–506. doi: 10.1016/s0167-5699(98)01340-1. [DOI] [PubMed] [Google Scholar]