Abstract
It has been observed previously that plasma selenium and glutathione levels are subnormal in HIV-infected individuals, and plasma glutathione peroxidase activity is decreased. Under these conditions the survival rate of AIDS patients is reduced significantly. In the present study, using 75Se-labeled human Jurkat T cells, we show that the levels of four 75Se-containing proteins are lower in HIV-infected cell populations than in uninfected cells. These major selenoproteins migrated as 57-, 26-, 21-, and 15-kDa species on SDS/PAGE gels. In our earlier studies, the 57-kDa protein was purified from T cells and identified as a subunit of thioredoxin reductase. The 26- and 21-kDa proteins were identified in immunoblot assays as the glutathione peroxidase (cGPX or GPX1) subunit and phospholipid hydroperoxide glutathione peroxidase (PHGPX or GPX4), respectively. We recently purified the 15-kDa protein and characterized it as a selenoprotein of unknown function. In contrast to selenoproteins, low molecular mass [75Se]compounds accumulated during HIV infection and migrated as a diffuse band near the front of SDS/PAGE gels.
Keywords: selenocysteine, thioredoxin reductase, glutathione peroxidase, 15-kDa protein
Selenium is a trace element that is an essential micronutrient for many forms of life. It is a natural component of selenium-dependent enzymes, and in most of these the selenium occurs in the amino acid selenocysteine (1, 2), which is located in the catalytic centers of the proteins. There also are a few bacterial selenium-dependent enzymes that have a dissociable cofactor form of selenium in the active center instead of selenocysteine (3, 4). Additionally, there are selenocysteine-containing proteins of unknown function (1, 2), and one of these, selenoprotein P, is unusual in that 10 selenocysteine residues are present in the 43-kDa polypeptide of the glycoprotein (5). Under conditions of selenium deficiency the levels of selenoproteins are depressed to varying degrees depending on efficiency of biosynthesis, relative turnover rates, mRNA stability, enzyme stability, and many other factors. Their resulting lowered catalytic and regulatory activities can upset delicate metabolic balances, lead to cretinism by interfering with normal embryonic development (6), and exacerbate numerous diseases such as prostate, colon, and lung cancer (7) and HIV infection (8). Dietary supplementation with selenium offers a potentially effective and inexpensive means of ameliorating these and many other disorders. In fact, it was reported recently that there is a direct correlation between a low dietary selenium intake and a 20-fold increase in risk of developing AIDS in HIV-infected individuals (8). This negative correlation between selenium status and progression of AIDS noted in a number of studies supports a beneficial role of selenium for HIV-infected individuals. Several hypotheses have been advanced to explain the effects of selenium on AIDS development. Among these are increased expression of antioxidant selenoproteins (9) and incorporation of selenium by viral selenoproteins (10). The latter theory predicts that during HIV infection several viral selenoproteins should be expressed, either by ribosomal frame shifting and/or by suppression of termination codons, and synthesis of these viral selenoproteins would deplete the selenium pool of the host. To determine the biochemical basis of the protective effect of selenium on the host in HIV infection we have investigated selenium metabolism in infected and uninfected Jurkat T cells labeled with 75Se. The results of these studies are reported herein.
MATERIALS AND METHODS
Materials.
[75Se]Selenious acid (1,000 Ci/mmol) was obtained from the Research Reactor Facility, University of Missouri (Columbia); precast polyacrylamide gels and molecular weight standards were from NOVEX (San Diego); phosphatase substrate immunoblotting system was from Kirkegaard & Perry Laboratories; ECL immunoblotting system was from Amersham; heat-inactivated fetal bovine serum and RPMI medium 1640 were from Mediatech (Herndon, VA); and human glyceraldehyde-3-phosphate dehydrogenase (GPDH), bovine glutathione peroxidase 1 (GPX1), and polyclonal antibodies to human GPDH were from Sigma. Rat liver GPX1 and rabbit polyclonal antibodies to this protein and to rat liver thioredoxin were kindly provided by Ho Zoon Chae and Sue Goo Rhee (National Institutes of Health, Bethesda, MD). Rabbit polyclonal antibodies to human phospholipid hydroperoxide GPX (PHGPX) were kindly provided by Donna Driscoll (Cleveland Clinic Foundation). All other reagents were of the highest grade available.
Cell Growth and HIV Infection.
Infectious HIV-1 was generated from the molecular clone pNL4–3 by plasmid transfection of adherent HeLa cells (11). The amount of virus produced was quantitated by 32P-reverse-transcriptase assay (in our assays one HIV-1 particle contributes approximately 20 cpm). A human T-cell line, JPX9, (12) was propagated in RPMI medium 1640 with 10% fetal bovine serum and sodium [75Se]selenite (2 μCi/ml) as described (13). Primary human T cells were freshly isolated from whole blood and activated by incubation for 2 days with phytohemagglutinin (750 ng/ml) as described (14) and then incubated an additional 24 hr after addition of [75Se]selenite. To study the effect of HIV infection on selenoproteins, JPX9 T cells were infected with HIV-1 for 3 days and then 75Se was added and cultures were incubated an additional 24 hr. Uninfected cells were incubated in parallel as controls. Jurkat HIV-infected and uninfected T cells also were labeled with 35S by incubation with [35S]methionine and [35S]cysteine (1 μCi of each/ml) in place of 75Se (15). All cells were harvested by centrifugation, washed twice with PBS, and resuspended in reducing SDS/PAGE sample buffer. For infected cells these steps were carried out in virus containment facilities, and samples were subjected to analyses only after inactivation of HIV by heating for 5 min at 100°C in sample buffer. Virus isolation and immunoprecipitations of viral proteins were performed as described (15, 16).
Nucleic acid fractions from 75Se-labeled HIV-infected and uninfected cells were prepared by proteinase K digestion (10 μg/ml) followed by phenol/chloroform (1:1 vol/vol) extraction. The 75Se contents of ethanol-precipitated nucleic acids were determined with a Beckman 5500 γ counter.
Analysis and Distribution of Selenoproteins.
SDS/PAGE analysis was performed according to standard procedures using 12%, 16%, 18%, or gradient 4–20% polyacrylamide gels. Gels were stained with Coomassie blue R-250. For detection and quantification of 75Se in gels, a PhosphorImager (Molecular Dynamics) was used. For separation of Se-containing proteins, 75Se-labeled cells were mixed with unlabeled cells and disrupted by sonication in 30 mM Tris⋅HCl, pH 7.5/1 mM EDTA/2 mM DTT/1 mM phenylmethylsulfonyl fluoride. The supernatant obtained after centrifugation of the disrupted cells was applied to a DEAE-Sepharose anion-exchange column, equilibrated with 30 mM Tris⋅HCl, pH 7.5/2 mM DTT/1 mM EDTA. Proteins were eluted by application of a linear gradient from 0 to 400 mM NaCl in equilibrating buffer. The radioactivity of fractions containing 75Se was determined in a Beckman 5500 γ counter, and the peak Se-containing fractions were analyzed by SDS/PAGE. The fractions corresponding to the 57-, 26-, 21-, and 15-kDa selenoproteins were purified by phenyl-Sepharose column chromatography (13). The resulting phenyl-Sepharose fractions were used for immunoblot assays. The 26- and 21-kDa GPX proteins, estimated to be 20–40% enriched by visual inspection of Coomassie blue-stained gels, were not purified further. Additional purification of the 57- and 15-kDa proteins (13, 17) resulted in an apparently homogeneous preparation of the 57-kDa protein and a 50% pure preparation of the 15-kDa protein.
Immunoblot Analysis.
An immunoblotting procedure with a phosphatase substrate system was used for the detection of T-cell GPDH and thioredoxin in crude extracts and for the detection of partially purified human T-cell GPX1 by polyclonal antibodies raised against human GPDH, rat liver thioredoxin, and rat liver GPX1, respectively. Human GPDH and bovine and rat liver GPX1 served as controls. An immunoblotting procedure with an ECL detection system was used to study the cross reactivity of the T-cell thioredoxin reductase, the 15-kDa protein, and PHGPX (GPX4) to polyclonal rabbit antibodies raised against rat liver thioredoxin reductase, the C-terminal peptide of the human 15-kDa protein, and an internal peptide of the human GPX4, respectively.
RESULTS AND DISCUSSION
Detection of Selenoproteins in T Cells.
Human T cells, Jurkat JPX9 line, labeled with 75Se during growth in media supplemented with [75Se]selenite, were subjected to SDS/PAGE analyses followed by PhosphorImager detection of labeled proteins. A typical profile of selenium-containing proteins in these T cells is shown in Fig. 1B, lane 1. It can be seen that six radiolabeled bands corresponding to masses ranging from 6 to 57 kDa were separated with the 15-, 21-, 26-, and 57-kDa protein bands being the most prominent. This pattern of 75Se-labeled protein bands in the JPX9 cell line isolated from acute lymphocytic T-cell anemia did not differ significantly from the profile of 75Se-containing protein bands detected in activated primary human T cells labeled under comparable conditions (data not shown).
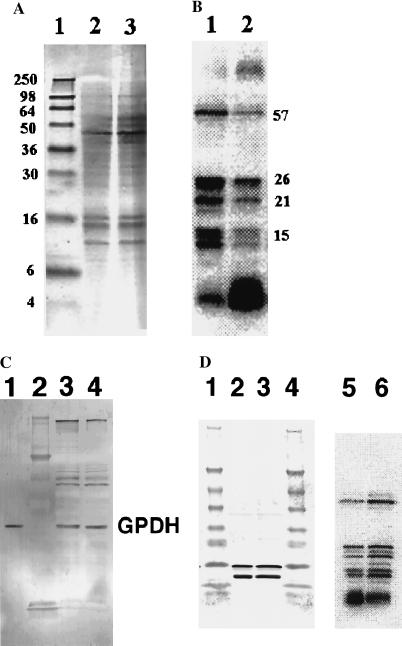
Figure 1.
Effect of HIV infection on [75Se]selenoproteins in JPX9 T cells. HIV-1-infected JPX9 T cells were cultured in parallel with control uninfected cells, labeled with 75Se, and prepared for SDS/PAGE analysis as described in Materials and Methods. (A) Coomassie blue-stained 16% polyacrylamide gel. Lane 1, molecular mass standards (sizes in kDa on left); lane 2, uninfected cell proteins, and lane 3, infected cell proteins. (B) PhosphorImager detection of 75Se-labeled protein bands. Lane 1, uninfected cells and lane 2, HIV-infected cells. Molecular masses of selenoproteins (in kDa) are shown. (C) Immunoblot detection of GPDH after 12% SDS/PAGE. Lane 1, human GPDH standard; lane 2, protein standards; lane 3, infected cell sample; lane 4, uninfected cell sample. (D) Immunoblot detection of thioredoxin after 4–20% gradient gel SDS/PAGE. Lanes 1 and 4, molecular size standards; lanes 2 and 5, infected cell sample; lanes 3 and 6, uninfected cell sample. Lanes 5 and 6, 75Se-labeled proteins in lanes 2 and 3, respectively, detected by PhosphorImager analysis. Two immunoreactive bands detected with the antibodies in lanes 2 and 3 indicate the presence of native and truncated species.
HIV Infection Affects the Level of Detected Selenoproteins.
Human Jurkat JPX9 cells that were infected with HIV for 3 days and then incubated for an additional 24 hr with 75Se-labeled selenite showed a marked reduction in 75Se contents of labeled selenoprotein bands detected in SDS/PAGE gels (Fig. 1B, lane 1) as compared with the corresponding profile of “mock infected cells” [i.e., uninfected cells resuspended initially in the same buffer with no added virus (Fig. 1B, lane 1)]. The protein bands with decreased 75Se-contents that migrated as 15-, 21-, 26-, and 57-kDa species correspond to known selenoproteins (see below). Visual inspection of the numerous Coomassie blue-stained protein bands in the gels did not reveal any marked differences in the protein compositions of uninfected and infected T cell populations (Fig. 1A, lanes 2 and 3). Likewise, comparison of 35S-labeled proteins from infected and “mock-infected” JPX9 cells that had been cultured in media containing [35S]methionine and [35S]cysteine, after separation by SDS/PAGE and quantification of major radioactive bands on a PhosphorImager, showed no apparent differences (data not shown). However, decreases in nonselenium containing proteins during HIV infection might not be detected because many protein bands from crude cell extracts are incompletely resolved on SDS gels. GPDH, a tetrameric enzyme with one essential sulfhydryl group per 44-kDa subunit at the active center, is sensitive to oxygen inactivation in the presence of iron and the oxidized enzyme then is susceptible to proteolysis (18). Thioredoxin is a 12-kDa protein containing a redox active disulfide center. Examination of GPDH and thioredoxin in HIV-infected and uninfected T cells by immunoblotting revealed no detectable differences in immunoreactive protein levels under these conditions (Fig. 1 C and D), suggesting that decreases in 75Se-labeled selenoproteins during HIV-1 infection might be the result of a selective rather than a generalized process. However, in those experiments in which only the selenium contents of the selenoprotein bands on the gels were measured, the actual amount of immunoreactive protein present is unknown. If loss of selenium from oxidized selenocysteine residues in the proteins occurs in HIV-infected cells, then removal of the oxidatively damaged proteins depends on subsequent proteolytic degradation. A relatively rapid rate of 75Se elimination from selenoproteins, which can be measured with precision and a slower rate of proteolysis of inactive oxidized proteins, could account for the observed results. In a study in which the major oxidatively damaged proteins in Escherichia coli cells exposed to oxidative stress were identified (19), there were no apparent differences in stained protein bands on gels that corresponded to immunodetected oxidized proteins with high carbonyl group contents. Even in iron-supplemented cultures that showed especially high oxidative damage and loss of enzyme activity there were no discernable changes in stained protein bands of crude extracts. Thus, unless inactivated enzyme species subsequently are degraded by proteolysis, there is little chance of detecting any changes by visualization of stained protein bands in SDS/PAGE gels or by the usual immunoblot procedures that do not distinguish inactivated from active enzymes.
HIV Infection Affects Low Molecular Mass Selenium Compounds.
Another observed difference between 75Se-labeled HIV-infected and uninfected T cells is a significant increase in the amounts of low molecular mass selenium species detected as a diffusely radiolabeled band near the gel front (Fig. 1B, lane 2). This fraction represented about 60–90% of the 75Se in infected cell extracts whereas in uninfected cell extracts the selenoproteins that migrated as 15-, 21-, 26-, and 57-kDa bands accounted for 60–80% of the total 75Se (Fig. 1B, lane 1).
Nucleic acids were isolated from HIV-infected and uninfected cells after labeling with 75Se as described in Materials and Methods to detect any selenium-modified tRNA species that might occur in T cells. Less than 15% of the radioactivity in the extracts was recovered in the nucleic acid fractions from either infected or uninfected cells, indicating that most of the low molecular mass anionic species detected on the SDS/PAGE gels were derived from other sources. To directly verify that the low molecular mass selenium species bind to cellular proteins, infected and uninfected samples were analyzed by nonreducing SDS/PAGE. The relatively greater amount of 75Se detected in protein bands throughout the gels in the HIV-infected samples (data not shown) suggested that the low molecular mass selenium species initially were associated with proteins. Among such compounds are selenosulfide derivatives of protein thiols or of glutathione-protein adducts and anionic polyselenide species.
Identification of Thioredoxin Reductase, GPX1, GPX4, and the 15-kDa Protein as Major T-Cell Selenoproteins.
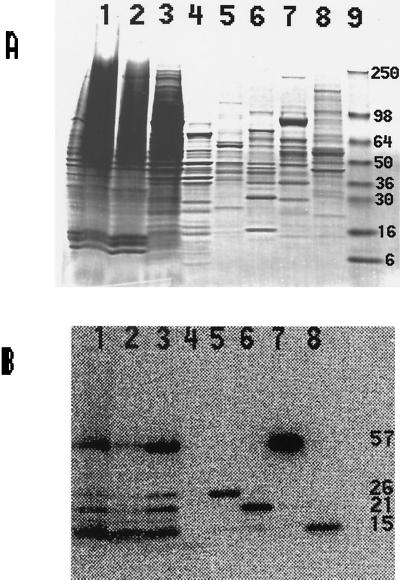
The supernatant fraction after centrifugation of the sonicated crude extract from uninfected T cells grown in the presence of [75Se]selenite was fractionated on a DEAE-Sepharose anion-exchange column to resolve the radioactive bands shown in Fig. 1. The radioactive protein components were eluted in the NaCl gradient applied to the column in four successive 75Se-containing peak fractions (Fig. 2, lanes 5–8). The last two radioactive peak fractions eluted from the DEAE column contained the 57- and 15-kDa proteins (Fig. 2, lanes 7 and 8); these were purified previously from JPX9 cells and identified as thioredoxin reductase (13) and a 15-kDa selenoprotein of unknown function (17), respectively. No other [75Se]-containing proteins with similar migration properties on SDS gels were detected in DEAE fractions.
Figure 2.
SDS/PAGE analysis of T cell Se-proteins separated by DEAE-Sepharose chromatography. 75Se-labeled T cells were sonicated and centrifuged, and soluble selenoproteins were chromatographed on a DEAE-Sepharose column (see Materials and Methods). The peak radioactive fractions were analyzed by SDS/PAGE. Lane 1, sonic extract; lane 2, sedimented pellet; lane 3, supernatant fraction; lane 4, proteins not retained on DEAE column (flow through fraction); lane 5, 26-kDa protein (eluted with 150 mM NaCl); lane 6, 21-kDa protein (eluted with 200 mM NaCl); lane 7, 57-kDa protein (eluted with 250 mM NaCl); lane 8, 15-kDa protein (eluted with 350 mM NaCl); lane 9, mass standards. (A) Coomassie blue-stained gel. (B) PhosphorImager detection of 75Se-labeled proteins in lanes 1–8 of A.
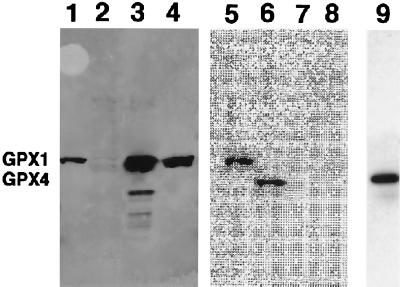
The first 75Se-labeled protein fraction eluted from the DEAE column contained a labeled protein migrating as a 26-kDa species and the next contained a 21-kDa species (Fig. 2, lanes 5 and 6, respectively). The 26-kDa protein was identified as GPX1 based on its comigration in SDS gels with bovine and rat liver cytosolic GPXs (GPX1) and on its reactivity with anti-rat liver GPX1 polyclonal antibodies (Fig. 3). The smaller 21-kDa 75Se-labeled protein was identified as GPX4 on the basis of immunoblot assays of partially purified enzyme with rabbit polyclonal antibodies elicited to human GPX4 (Fig. 3).
Figure 3.
Immunoblotting and imaging of GPX1 and PHGPX. Lanes 1 and 5, partially purified 75Se-labeled human T-cell GPX1; lanes 2, 6, and 9, partially purified 75Se-labeled human T-cell PHGPX; lanes 3 and 7, rat liver GPX1; lanes 4 and 8, bovine GPX1. Lanes 1–4, immunoblot assay with anti-GPX (GPX1) antibodies; lanes 5–8, PhosphorImaging of the immunoblot shown in lanes 1–4; lane 9, immunoblot detection with the anti-PHGPX antibodies. The locations of GPX1 and PHGPX (GPX4) are shown on the left.
Does HIV Encode Viral Selenoproteins?
Generation of new HIV-1 ORFs containing sporadic UGA codons by ribosomal frame shifting and translation to give novel virus encoded selenocysteine-containing proteins has been suggested as a mechanism to explain selenium deficiency in HIV-infected individuals (10). However, synthesis of HIV-encoded selenoproteins in amounts sufficient to deplete the host cell of selenium should require elevated expression of the frame-shift mutants, whereas the putative UGA codons that may not be effectively introduced by this process are a potential limiting factor. From our studies, it appears that if any HIV-encoded selenoproteins are expressed they are produced in amounts too low to be detected by radiolabeling and therefore the possibility that the host selenium supply is sequestered in viral proteins is highly unlikely. A comparison of lanes 1 and 2 in Fig. 1B shows no discrete 75Se-labeled protein bands present exclusively in infected cells. An unidentified slower migrating faint band in lane 2 may be aggregated protein. We attempted to isolate 75Se-labeled HIV and to immunoprecipitate putative viral selenoproteins from HIV-infected T cells using hyperimmune sera from patients to further test the hypothesis. However, no 75Se-containing material was found in isolated virions nor was any 75Se label immunoprecipitated specifically from HIV-infected cells (data not shown). These results clearly indicate that selenium deficiency in HIV-infected individuals cannot be attributed to selective utilization of the selenium pool for viral selenoprotein synthesis.
To date there is only one example of a virus-encoded selenoprotein, which is a GPX from Molluscum contagiosum, a specific human pox virus that causes skin lesions and compromises the immune system. The viral GPX gene exhibits 80% homology to human GPX1 and when expressed in HeLa cells it conferred protection against H2O2 and UV damage (20). The possibility that the viral gene is of human origin is considered likely.
Is There an Effect of Viral Tat Protein on Levels of 75Se-Labeled Proteins in T Cells?
The HIV-1 tat gene encodes a 12-kDa nuclear protein, Tat, which is secreted by HIV-1 infected cells and this protein can be taken up by noninfected cells (21). In addition to its role as a transcriptional transactivator of the long terminal repeat region of HIV-1, Tat has been reported to inhibit the proliferation of antigen-specific T lymphocytes (22) and to induce apoptosis in these cells (23). A T-cell line that was stably transfected with the HIV-1 tat gene showed the characteristic DNA fragmentation pattern of apoptosis. The extent of apoptosis was markedly greater in cells that were grown in serum-deficient media as compared with cells cultured in the presence of adequate growth factors supplied as 10% fetal calf serum. Selenium, known to be one of the essential factors for cell growth supplied by the fetal calf serum, was not tested directly for a possible role in apoptosis in this study. Speculation as to a possible relationship of HIV Tat to selenium metabolism was based on a recent report that the human protein that recognizes the selenocysteine insertion sequence (SECIS) in selenoprotein mRNAs and HIV Tat contain similar predicted arginine-rich domains (24). These homologous domains comprise the predicted amino acid residues 51–67 of HIV-1 Tat and residues 241–256 of the human SECIS-binding protein (originally designated as DNA-binding protein B, dbnB). The human protein specifically binds to the stem-loop structure of the SECIS element in the nontranslated region of human GPX1 mRNA and in so doing allows UGA in the ORF to be translated as selenocysteine instead of termination. The arginine-rich region comprising residues 51–67 of the HIV-1 Tat protein is essential for binding to the TAR element, also a putative stem-loop structure found at the 5′ ends of all nascent HIV-1 transcripts. Whether the similarity of these two arginine-rich regions of HIV-1 Tat and SECIS-binding protein is sufficient to cause interference in recognition of their respective stem-loop structures is not known. However, the SECIS-binding protein from COS-1 cells did not recognize the HIV-1 TAR element in gel retardation assays (25).
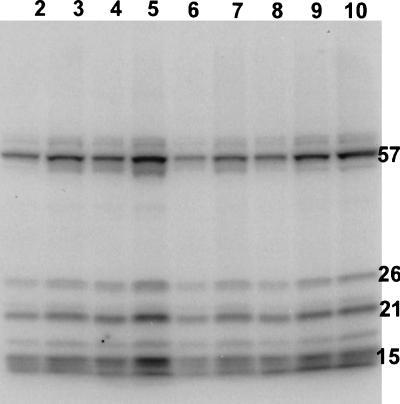
To examine directly a possible relationship of viral Tat protein to the observed decreased levels of selenoproteins in HIV-infected T cells, Jurkat T cells first were cultured in media containing added Tat protein and then allowed to grow further after 75Se supplementation. The amount of 75Se present in the SDS extracts of T cells that had been treated initially with Tat protein was more than double the amount present in the corresponding control cell extracts (Table 1). However, comparison of the distribution of radioactivity in protein bands in gels revealed that the major selenoproteins from Tat-treated cells contained a much lower percentage of the total 75Se applied to each gel lane than the corresponding selenoproteins from control cells (Fig. 4 and Table 2). The relative amount of 75Se that either migrated off the gel or appeared as three diffuse bands at the front appeared greater in the case of the Tat-treated cells, but exact amounts were not measured. These effects on 75Se distribution in T-cell extracts resulting from treatment of T cells with Tat protein alone thus mimic to some degree the decreased levels of 75Se-labeled selenoproteins and the higher amounts of low molecular mass compounds found in HIV-infected T cells. In the case of the Tat protein-treated T-cells, a fixed amount of a Tat fusion protein (10 μg) was added at the start to each 6-ml culture whereas in HIV-infected cells Tat protein presumably is synthesized and excreted into the medium throughout the growth period. If cells undergo apoptosis during the period of incubation with 75Se and under these conditions there is rapid loss of reduced glutathione as has been reported for Jurkat T cells induced to undergo apoptosis by treatment with anti-Fas/APO-1 antibody (26), then oxidative stress conditions induced by the lowered glutathione levels would lead to nonreversible oxidation and elimination of selenium from oxygen labile selenocysteine residues in thioredoxin reductase (27), GPXs, and other selenoenzymes, which could not be regenerated efficiently to their active selenol forms.
Table 1.
Effect of added Tat protein on incorporation of 75Se by Jurkat T cells
| Incubation with 75Se, hr | Total 75Se present/0.6 ml of extract
|
|||
|---|---|---|---|---|
| Control T cells,
|
Tat-treated T cells,
|
|||
| cpm × 106 | % | cpm × 106 | % | |
| 28 | 0.3 | 0.5 | 1.02 | 1.73 |
| 50 | 1.2 | 2.05 | 2.85 | 4.85 |
Jurkat T cells were propagated in 6-ml cultures containing RPMI medium 1640 with 10% fetal bovine serum and either maltose-binding protein (42 kDa), 10 μg, (controls) or maltose-binding-Tat fusion protein (52 kDa), 10 μg, (Tat-treated cells) for 24 or 48 hr at 37°C in a CO2 incubator. Then 58.8 × 106 cpm 75SeO32− (ca. 3 nmoles; Los Alamos National Laboratory, NM) were added to each 6-ml culture and incubation was continued for 28 hr (24-hr culture) or 50 hr (48-hr culture). Cells were harvested, washed twice, and suspended in SDS/PAGE sample buffer with mercaptoethanol as described in Materials and Methods. Extracts (0.6 ml) were heated at 100°C for 10 min.
Figure 4.
Effect of treatment of Jurkat T cells with Tat protein on levels of 75Se-labeled proteins. SDS/12% gel PAGE analysis of extracts of Tat-treated and untreated T cells incubated with 75Se032- for 28 and 50 hr. Size of the aliquot and amount of radioactivity applied to each well from the indicated cell extract are as follows: 28 hr, control cells (5,000 cpm/10 μl), lane 2, 10 μl and lane 3, 15 μl; 28 hr, Tat-treated cells (17,000 cpm/10 μl) lane 6, 5 μl and lane 7, 10 μl; 50 hr, control cells (20,000 cpm/10 μl), lane 4, 5 μl and lane 5, 10 μl; 50 hr, Tat-treated cells (47,500 cpm/10 μl), lane 8, 4 μl, lane 9, 8 μl, and lane 10, 10 μl.
Table 2.
Effect of Tat protein treatment on 75Se content of individual selenoproteins
| Selenoprotein |
75Se content of protein band, arbitrary density units
|
|
|---|---|---|
| Control T cells | Tat-treated T cells | |
| Thioredoxin reductase (57-kDa band) | 1,572 | 1,304 |
| GPX4 (21-kDa band) | 975 | 598 |
| 15-kDa proteins (2 bands) | 1,766 | 1,095 |
The proteins from control T cells and Tat-treated T cells, incubated 50 hr with 75Se, are shown in lanes 5 and 10, respectively, of Fig. 4. Aliquots (10 μl) applied to the gel contained 20,000 cpm for the control cell extracat and 47,500 cpm for the Tat-treated cell extract. The arbitrary density units are not directly convertable to cpm 75Se.
Another explanation for our data is that oxidative stress, which is known to be characteristic of HIV infection, could result in decreased amounts of selenophosphate, the oxygen-labile highly reactive selenium donor compound, that is required for selenocysteyl-tRNASec formation. In this case, biosynthesis of specific selenoenzymes would be inhibited because of lack of the required selenocysteine precursor whereas synthesis of nonselenoproteins might be relatively unaffected. Alternatively, if the HIV-1-encoded Tat protein and/or the TAR stem-loop structure indeed can interfere with selenoprotein synthesis in infected T cells, then decreased levels of 75Se-labeled proteins could be expected.
In the studies reported here, both with HIV-1-infected and with Tat-treated T cells, only the 75Se contents of protein bands on gels were measured and protein concentrations were not determined. Because the levels of the major selenoenzymes in T cells generally are low, especially sensitive immunoblotting procedures are needed for reliable quantitation in crude extracts and for calculation of specific radioactivity values. Although the presence of low selenium content proteins would suggest that elimination of selenium had occurred subsequent to oxidative damage, decreased levels of normal selenium content selenoproteins could be indicative of suppression of selenoenzyme biosynthesis. Detailed investigation of the effects of Tat protein on T cell selenium metabolism may serve to distinguish these possibilities and provide further clues concerning the pathogenicity of HIV-1.
Acknowledgments
We thank Drs. Donna Driscoll, Sue Goo Rhee, and Ho Zoon Chae for gifts of proteins and antibodies.
ABBREVIATIONS
- GPX
glutathione peroxidase
- GPX1
glutathione peroxidase 1
- PHGPX or GPX4
phospholipid hydroperoxide GPX
- GPDH
glyceraldehyde-3-phosphate dehydrogenase
- SECIS
selenocysteine insertion sequence
References
- 1.Stadtman T C. Annu Rev Biochem. 1996;65:83–100. doi: 10.1146/annurev.bi.65.070196.000503. [DOI] [PubMed] [Google Scholar]
- 2.Hatfield D L, Gladyshev V N, Park J, Park S I, Chittum H S, Baek H J, Carlson B A, Yang E S, Moustafa M E, Lee B J. Comp Nat Products Chem. 1999;4:353–380. [Google Scholar]
- 3.Dilworth G L. Arch Biochem Biophys. 1982;219:30–38. doi: 10.1016/0003-9861(82)90130-8. [DOI] [PubMed] [Google Scholar]
- 4.Gladyshev V N, Khangulov S V, Stadtman T C. Proc Natl Acad Sci USA. 1994;91:232–236. doi: 10.1073/pnas.91.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill K E, Burk R F. In: Selenium in Biology and Human Health. Burk R F, editor. New York: Springer; 1994. pp. 117–132. [Google Scholar]
- 6.Contempre B, Vanderpas J, Dumont J E. Mol Cell Endocrinol. 1991;81:C193–C195. doi: 10.1016/0303-7207(91)90197-z. [DOI] [PubMed] [Google Scholar]
- 7.Clark L C, Combs G F, Turnbull B W, Slate E H, Chalker D K, Chow J, Davis L S, Glover R A, Graham G F, Gross E G, et al. J Am Med Assoc. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 8.Baum M K, Shor-Posner G, Lai S, Zhang G, Lai H, Fletcher M A, Sauberlich H, Page J B. J AIDS Hum Retrovirol. 1997;15:370–374. doi: 10.1097/00042560-199708150-00007. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin B M, Rosenthal W S, Wormser G P, Weiss L, Nunez M, Joline C, Herp A. Biol Trace Elem Res. 1988;20:86–96. doi: 10.1007/BF02990135. [DOI] [PubMed] [Google Scholar]
- 10.Taylor E W, Ramanathan C S, Jalluri R K, Nadimpalli R G. J Med Chem. 1994;37:2637–2654. doi: 10.1021/jm00043a004. [DOI] [PubMed] [Google Scholar]
- 11.Neuveut C, Jeang K-T. J Virol. 1996;70:5572–5581. doi: 10.1128/jvi.70.8.5572-5581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata K, Ohtani K, Nakamura M, Sugamura K. J Virol. 1989;63:3220–3226. doi: 10.1128/jvi.63.8.3220-3226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladyshev V N, Jeang K-T, Stadtman T C. Proc Natl Acad Sci USA. 1996;93:6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L-M, Joshi A, Willey R, Orenstein J, Jeang K-T. EMBO J. 1994;13:2886–2896. doi: 10.1002/j.1460-2075.1994.tb06583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun R F, Semmes O J, Neuveut C, Jeang K T. J Virol. 1998;72:2615–2629. doi: 10.1128/jvi.72.4.2615-2629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeang K-T, Chiu R, Santos E, Kim S-J. Virology. 1991;181:218–227. doi: 10.1016/0042-6822(91)90487-v. [DOI] [PubMed] [Google Scholar]
- 17.Gladyshev V N, Jeang K-T, Wootton J C, Hatfield D L. J Biol Chem. 1998;273:8910–8915. doi: 10.1074/jbc.273.15.8910. [DOI] [PubMed] [Google Scholar]
- 18.Fucci L, Oliver C N, Coon M J, Stadtman E R. Proc Nat Acad Sci USA. 1983;80:1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamarit J, Cabiscol E, Ros J. J Biol Chem. 1998;273:3027–3032. doi: 10.1074/jbc.273.5.3027. [DOI] [PubMed] [Google Scholar]
- 20.Shisler J L, Senkevich T G, Berry M J, Moss B. Science. 1998;279:102–105. doi: 10.1126/science.279.5347.102. [DOI] [PubMed] [Google Scholar]
- 21.Vaishnav Y N, Wong-Staal F. Annu Rev Biochem. 1991;60:577–630. doi: 10.1146/annurev.bi.60.070191.003045. [DOI] [PubMed] [Google Scholar]
- 22.Viscidi R P, Mayur K, Lederman H M, Frankel A D. Science. 1989;246:1606–1608. doi: 10.1126/science.2556795. [DOI] [PubMed] [Google Scholar]
- 23.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 24.Shen Q, Wu R, Leonard J L, Newburger P E. J Biol Chem. 1998;273:5443–5446. doi: 10.1074/jbc.273.10.5443. [DOI] [PubMed] [Google Scholar]
- 25.Shen Q, McQuilkin P A, Newburger P E. J Biol Chem. 1995;270:30448–30452. doi: 10.1074/jbc.270.51.30448. [DOI] [PubMed] [Google Scholar]
- 26.van den Dobbelsteen D J, Nobel C S I, Schlegel J, Cotgreave I A, Orrenius S, Slater A F G. J Biol Chem. 1996;271:15420–15427. doi: 10.1074/jbc.271.26.15420. [DOI] [PubMed] [Google Scholar]
- 27.Gorlatov S N, Stadtman T C. Proc Natl Acad Sci USA. 1998;95:8520–8525. doi: 10.1073/pnas.95.15.8520. [DOI] [PMC free article] [PubMed] [Google Scholar]