Abstract
The platelet-derived growth factor receptor (PDGFR) is a receptor tyrosine kinase overexpressed in a subset of solid tumors and therefore is the target of drugs inhibiting this function such as imatinib mesylate (Gleevec). Thus far, drug therapy has played a limited role in the treatment of localized prostate cancer (PCa). This study characterizes PDGFR-β expression in a wide spectrum of PCa samples to provide empirical data as part of a rational treatment strategy. A survey of five published prostate expression array studies, including 100 clinically localized PCa, did not identify tumors with increased PDGFR-β expression level. Protein expression of PDGFR-β, as determined by immunohistochemistry, revealed 5% of clinically localized PCa and 16% of metastatic PCa cases to show moderate or strong expression. To develop a strategy to detect patients most likely to profit from Gleevec treatment, we analyzed cDNA expression array data from 10,000 transcripts for PDGFR-β expression and divided tumors in groups based on PDGFR-β expression level. Performing a supervised analysis to identify potential comarkers of PDGFR-β in PCa, we identified a set of genes whose expression was associated with PDGFR-β status including early growth response 1 (Egr1), an upstream effector of PDGF (4.2-fold upregulation), α-methylacyl-CoA racemase, as well as v-Maf and neuroblastoma suppressor of tumorigenicity (both with a 2.2-fold downregulation). Taken together, this study suggests that only a small subset of PCas may be amenable to tyrosine kinase inhibitors specific for PDGFR.
Keywords: Platelet-derived growth factor receptor (PDGFR), prostate cancer, imatinib mesylate, tissue microarray, cDNA expression
Introduction
Prostate cancer (PCa) is the second leading cause of male cancer-related death after lung cancer in the United States [1]. Currently, there is no standard approach for the treatment of clinically localized and locally advanced PCa. Treatment options include radical prostatectomy, radiation therapy, and watchful waiting. Alternative treatment methods for higher-risk patients now include the development of specific drug inhibitors of tyrosine kinase receptors. The tyrosine kinase inhibitor imatinib mesylate (Geevec; Novartis, East Hanover, NJ) has been successfully used in the therapy of chronic myeloid leukemia (CML) and in a rare subtype of mesenchymal tumors called gastrointestinal stromal tumors (GISTs). A recent phase II trial demonstrated a high rate of response in CML at all stages and still continues to result in durable complete response [2,3]. In a phase II trial treating GISTs with imatinib mesylate, a partial response was described in 50% of the cases, compared to only a 10% response rate with the standard chemotherapy [4–6]. Imatinib mesylate inhibits the receptor tyrosine kinase (RTK) Bcr-Abl, the oncogene found in more than 90% of patients with CMLG13 and CMLG1314, and other Abl RTKs such as c-kit, the stem cell factor whose oncogenetic mutation is seen in 95% of the patients with GIST, and the platelet-derived growth factor (PDGF)-β receptor [7].
Binding of PDGF to its receptor leads to dimerization of two subunits, α and β, either as a homodimers or heterodimers resulting in autophosphorylation. The active receptor is able to recruit multiple signal transduction molecules containing SH2 domains, resulting in the initiation of various signal pathways [8,9]. Known target pathways include the phospholipase C-gamma (PLC) [10], src kinase [11,12], growth factor receptor-bound 2 (Grb2) [13], and phosphatidylinositol-3-kinase (PI3K) pathways [14]. Therefore, there is increased interest in using inhibitors of platelet-derived growth factor receptor (PDGFR) in other diseases where these pathways are implicated.
PDGFR was recently reported to be expressed in 88% of PCa samples tested by immunohistochemistry [15] and also in its precursor lesion, prostatic intraepithelial neoplasia (PIN), suggesting that PDGFR expression is seen early in PCa progression [16]. The success of treating cancer associated with overexpression or abnormal activation of RTKs like CML or GIST with inhibitors of RTK leads to the development of a new therapeutic approach for the treatment of PCa. Thus far, two phase II trials have investigated the benefit of PDGFR inhibitors in the treatment of PCa [15,17] and a third is currently being established with the purpose of developing strategies to identify patients with PDGFR expressing PCa prior to treatment [17]. In recent work, gene expression profiles containing PDGFR-β along with four other genes were found to be associated with a higher prostate-specific antigen (PSA) failure rate in men treated by radical prostatectomy for clinically localized PCa [18]. Therefore, due to the increasing interest in using the tyrosine kinase inhibitor imatinib mesylate as an adjunct to PCa treatment, we undertook the current study to characterize the frequency of PDGFR-β expression in a wide range of PCa samples, investigate associations between overexpression and other important pathologic and clinical parameters, and identify surrogate markers of PDGFR-β expression.
Methods
Prostate Sample Collection
Prostate tissues were taken from the radical prostatectomy series and the rapid autopsy program at the University of Michigan Prostate Cancer Specialized Program of Research Excellence (SPORE) Tissue Core. Institutional Review Board approval was obtained for the use of these samples. Clinically localized PCa samples used for this study were taken from a cohort of men, who underwent radical retropubic prostatectomy as a monotherapy (i.e., no hormonal or radiation therapy) for clinically localized PCa between the years 1995 and 2001. Tumors were staged using the Tumor-Node-Metastasis (TNM) system [19]. Tumors were graded using the Gleason grading system. The median age at time of surgery was 59 years (range 39–77 years) with a median pretreatment PSA of 5.1 ng/ml (range 0.09–47.3 ng/ml). Gleason scores ranged from 5 to 9, with 68% having either a Gleason score of 6 or 7. Processing of the prostatic tissue started within 20 minutes after surgical resection. The snap-frozen samples used for cDNA expression array analysis were all evaluated by the study pathologist. All samples were trimmed to ensure that >95% of the sample used represented the desired lesion. Areas of benign prostate tissue from prostates with PCa were used as normal adjacent tissue in these experiments. Metastatic PCa samples were collected from the rapid (“warm”) autopsy program as previously described [20]. In this study, metastatic PCa from 15 rapid autopsy cases performed from 1997 to 2000 were used. The patient's ages ranged from 40 to 84 years, with a median age of 65 years. All patients died with widely metastatic PCa after extensive treatment, which included antiandrogen and chemotherapy.
Tissue Microarray (TMA) Construction, Digital Image Capture, and Analysis
As previously described, high-density TMAs composed of samples from a wide range of prostate tissues were assembled using an automated tissue arrayer (Beecher Instruments, Silver Spring, MD) [20–23]. Three to four 0.6-mm tissue cores were taken from each targeted lesion (i.e., benign PCa, or metastatic PCa) and placed into a recipient block. Digital images were acquired from the 4 µm-thick hematoxylin and eosin (H&E) sections as well as all immunostained TMA slides using the BLISS Imaging System (Bacus Laboratory, Lombard, IL). Protein expression was evaluated in a blinded manner using an Internet-based TMA presentation tool, TMA Profiler (University of Michigan, Ann Arbor, MI). The tissue sample diagnosis was confirmed and immunostaining was scored by the study pathologist for protein expression intensity. All data entered into the TMA Profiler were stored in a relational database. In order to stratify the clinically localized PCa cases into two groups based on their PDGFR-β protein expression, a TMA was constructed with 60 cases, which had already been used for expression array analysis [21,24]. Three other validation TMAs were used, which contained benign prostatic tissue (118 cores from 51 cases), postatrophic hyperplasia (32 cores from 29 cases), high-grade PIN (8 cores from 5 cases), clinically localized PCa (287 cores from 109 cases), and metastatic PCa (116 cores of metastatic PCa from 30 cases).
cDNA Expression Array Analysis
As previously reported, the spotted glass cDNA microarray slides used in this study included approximately 10,000 genes from the Research Genetics human cDNA clone set [21,24]. Fluorescently labeled (Cy5) cDNA was prepared from total RNA from each of the prostate samples. The reference samples, a pool of benign prostate tissue, were labeled using a second distinguishable fluorescent dye (Cy3) using a previously established protocol (http:\\www.microarrays.org). After labeling, the cDNA samples were neutralized, washed, and then applied to the microarray chips. After remaining in a hybridization water bath at 65°C overnight, the microarray slides were processed and scanned with a Genepix 4000 scanner (Axon Instruments, Union City, CA). Primary analysis was done using the Genepix software package. Images of scanned microarrays were gridded and linked to a gene print list. Initially, data were viewed as a scatter plot of Cy3 versus Cy5 intensities. Cy3-to-Cy5 ratios are determined for the individual genes along with various other quality control parameters (e.g., intensity over local background). The Genepix software analysis package flags spots as absent based on spot characteristics. Furthermore, bad spots or areas of the array with obvious defects were manually flagged. Spots with small diameters (<50 µm) and spots with low signals strengths (<350 fluorescence intensity units) over local background in the more intense channel were discarded. Flagged spots were not included in subsequent analyses. Data are the ratio of the fluorescent cDNA probe signal hybridized against the reference pool.
Immunohistochemistry
After paraffin removal and hydration, the TMA slides were immersed in 10mMcitrate buffer placed in a pressure cooker chamber and microwaved for 10 minutes for optimal antigen retrieval. Immunostaining was performed using a Dako autostainer (Dako, Carpinteria, CA). Primary antibodies [anti-PDGFR-β monoclonal (18A2), sc-19995, Santa Cruz Biotechnology, Santa Cruz, CA; anti-PDGFR-α and anti-PDGFR-β monoclonal, Upstate Biotechnology, Inc. Lake Placid, NY] were incubated for 45 minutes at room temperature (RT) in a 1:50 dilution and a secondary biotin-labeled antibody for 30 minutes. Streptavidin LSA amplification method (Dako K0679) was carried out for 30 minutes followed by peroxidase/diaminobenzidine substrate/chromagen. The slides were counterstained with hematoxylin. Membranous (PDGFR-β) protein expression was determined by the study pathologist (M.A.R.) and immunohistochemistry was scored as negative (score = 1), weak (score = 2), moderate (score = 3), or strong (score = 4), by using a system that has been previously validated on several TMA studies [20,21,23,25].
Stimulation of PDGFR-β Phosphorylation in NIH-3T3 Cells
NIH-3T3 cells were incubated at 37°C and 5% CO2. To enhance phosphorylation of PDGFR-β, the cell line was stimulated with 100 ng/ml PDGF in serum-free DMEM for 10 min. Three 75-cm2 cell culture flasks were trypsinized, and the cells were washed in phophate-buffered saline (PBS) and fixed in 10% formalin for 1 hour. After another step of washing with PBS, the cell pellet was gradually dehydrated in increasing concentrations of ethanol (75-95%) and embedded in paraffin. Phosphorylated PDGFR-β was detected with a phospho-PDGFR-β-specific antibody (no. 3161; Cell Signaling, Beverly, MA) at a dilution of 1:50 following the same protocol as described above.
Western Blot Analysis for Phospho-PDGFR-β
To ensure that PDGFR-β was phosphorylated in the NIH-3T3 cells, the cells were incubated in the presence of 100 ng/ml PDGF in serum-free DMEM for 10 minutes. Cell lysis was performed with lysis buffer (1% NP-40, 50 mM Tris HCl, pH 8, 100 mM Na-fluoride, 30 mM pyrophosphate, 2 mM Na-molybdate, 5 mM EDTA, 2 mM Na-vanadate, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM PMSF, and 2 mM vanadate) on ice, and the cell lysates were homogenized by aspiration in a syringe. Protein estimation of the lysate was carried out using a protein quantification kit from Bio-Rad (Hercules, CA). Twenty micrograms of lysate (treated and untreated) was loaded on the gel and electrophoretically separated (12% precast sodium dodecyl sulfate polyacrylamide gel; Invitrogen, Carlsbad, CA). The protein was transferred on to a nitrocellulose membrane (Schleicher and Schuell, Riviera Beach, FL) and the membrane was stained with Ponceau red and washed for 5 minutes twice with phosphate-buffered saline with Tween 20 (PBST). Blocking was carried out in blocking buffer (10 ml PBST containing 5% wt/vol nonfat dry milk) for 2 hours at RT. The membrane was washed five times with PBST (5 minutes each) and incubated with the primary antibody (no. 3161; Cell Signaling) at 4°C overnight. After the membrane was washed five times with PBST (2 x 5 minutes, 3 x 15 minutes), it was incubated with secondary antibody (anti-goat HRP; Caltag Laboratories, Burlingham, CA) diluted 1:4000 in PBST containing 5% wt/vol BSA for 2 hours at RT and washed five times with PBST (2 x 5 minutes, 3 x 15 minutes). Detection was carried out using an enhanced chemoluminescence (ECL) reagent (Amersham Biosciences, Piscataway, NJ).
Statistical Analysis
Primary analysis of the cDNA expression data was done using the Genepix software (Axon Instruments). Cluster analysis (Stanford University, Palo Alto, CA) and generation of figures with TreeView were performed using software developed by Eisen et al. [26]. The significance analysis of microarray (SAM) software program (Stanford University) was used to assign a score to each gene on the basis of change in gene expression relative to the standard deviation of repeated measurements [27]. For genes with scores greater than an adjustable threshold, SAM uses permutations of the repeated measurements to estimate the percentage of genes identified by chance, referred to as the false discovery rate (FDR). SAM analysis was performed on cDNA expression array data that are now in the public domain [21]. Cluster, TreeView, and SAM software can be obtained at http://www.dnachip.org (Stanford University). Multiple public expression array data sets were interrogated using Oncomine (http://www.Oncomine.org). Additional statistical analysis was performed using SPSS (SPSS, Chicago, IL). At the univariate level, Kaplan-Meier analysis was used to evaluate the cumulative risk of PSA failure following prostatectomy. Multivariate analysis using Cox hazards regression was used to evaluate clinical pathology parameters and PDGFR-β expression.
Results
cDNA Microarray Analysis of PDGFR-α and PDGFR-β
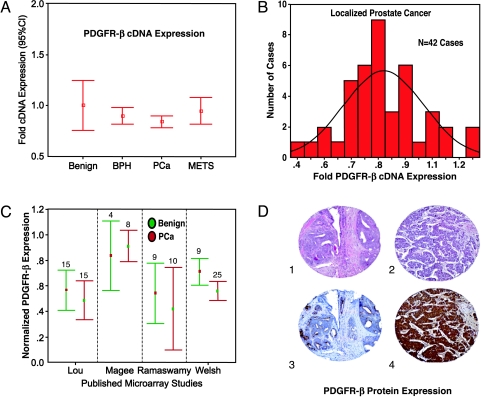
Examination of a range of prostate samples from benign to metastatic PCa demonstrated different expression patterns for PDGFR-α and PDGFR-β. PDGFR-α showed a nonsignificant decrease in expression (data not shown), whereas PDGFR-β showed no significant dysregulation with PCa progression (Figure 1A). For example, a modest but statistically insignificant difference was observed between localized PCa and metastatic PCa for PDGFR-β expression. We performed a focused evaluation concentrating on the localized PCa samples to determine if there is a subset of tumors, which overexpress PDGFR-β. Approximately 5% of clinically localized PCa demonstrated overexpression of PDGFR-β, suggesting that this is a rare phenomenon in clinically localized PCa (Figure 1B). Using Oncomine (http://www.oncomine.org), a newly developed database tool developed by one of the coauthors (A.M.C.), we were able to interrogate four other expression array datasets that contained information on PDGFR-β expression on clinically localized PCa [28–31]. After normalization of the expression array data, as previously described [32], the results of this analysis are presented in Figure 1C using error bars with 95% confidence intervals. None of the five studies containing 100 clinically localized PCa demonstrated a two-fold overexpression in any sample. Table 1 presents the median normalized expression levels.
Figure 1.
PDGFR-β cDNA expression and protein expression in PCa. (A) Expression array analysis was performed on a wide range of prostate samples: 10 histologically benign, 15 benign prostatic hyperplasia, 41 clinically localized PCas, and 27 metastatic prostate tumors. PDGFR-β demonstrated an increase in cDNA expression between normal and the remaining samples. The results represent trends and were not statistically significant. Expression levels were normalized for benign samples and the error bars represent 95% confidence intervals of the fold changes in cDNA expression for PDGFR-β. (B) The PDGFR-β cDNA expression distribution in 42 clinically localized PCa cases reveals only a small number of cases with increased PDGFR-β expression as measured by fold increase after normalization against benign prostate tissue samples. (C) The PDGFR-β cDNA expression array results from four previously published microarray studies. No significant differences between benign and PCa samples were seen. (D) PDGFR-β protein expression as determined by immunohistochemistry in tissue microarray samples demonstrated negative to weak expression in the majority of localized PCa samples (1 = H&E stain and 3 = weak immunostaining, x200). Strong PDGFR-β protein expression was seen in approximately 1% of the localized PCa samples (2 = H&E stain, 4 = strong immunostaining, x200).
Table 1.
Analysis of Normalized PDGFR-b (Unigene Hs.76144) Expression in Benign Prostate Tissue and Localized Prostate Cancer from Five Published Studies.
PDGFR-β Is Overexpressed in a Fraction of PCa at the Protein Level
As the cDNA data demonstrated an upregulation solely for the β-subunit of PDGFR and this subunit has previously been reported to be associated with cancer progression [33–35], we focused the analysis on the β-subunit. In order to characterize PDGFR-β protein expression and evaluate its potential association with PCa progression, PDGFR-β protein expression was evaluated on a large number of clinical samples from men with either localized or metastatic PCa. Figure 1D demonstrates examples of weak (staining score = 2 of 4) and strong (staining score = 4 of 4) protein expression as determined by immunohistochemistry. Using a system that is reproducible and tested [21,23–25,36,37], protein staining intensity evaluated as either negative, weak, moderate, or strong was assigned a numeric score of 1, 2, 3, or 4, respectively, for purposes of analysis. Table 2 summarizes these results. For clinically localized PCa, approximately 5% (8 of 169) of patient samples demonstrated moderate to strong PDGFR-β expression. Metastatic PCa showed an increased fraction with 16% positive patient cases (5 of 30). PDGFR-β expression was not present in benign prostate tissues examined (0 of 92), although two benign tissue cores demonstrated a weak staining (staining score = 2). Similar results were obtained with a second antibody against the PDGFR-α and PDGFR-β subunits (results not shown). In order to evaluate whether PDGFR-β protein expression is associated with PCa progression, we performed a univariate analysis with standard pathology and clinical parameters. This analysis did not reveal any significant associations between PDGFR-β protein expression and clinically relevant parameters including Gleason score (i.e., tumor grade), tumor size, or preoperative serum PSA. No association was identified between the development of postsurgical PSA or biochemical failure as defined by an elevation of PSA >0.2 ng/ml.
Table 2.
PDGFR-b Protein Expression in Prostate Tissue and Cancer Evaluated Using High-Density Tissue Microarray.
| Prostate Tissue | Cases | Staining Intensity (Percentage) | |||
| Absent | Weak | Moderate | Strong | ||
| Benign | 92 | 90 (98%) | 2 (2%) | 0 | 0 |
| Atrophy | 27 | 27 (100%) | 0 | 0 | 0 |
| PIN | 7 | 7 (100%) | 0 | 0 | 0 |
| Localized PCa | 169 | 129 (76%) | 32 (19%) | 6 (4%) | 2 (1%) |
| Metastatic PCa | 30 | 23 (76%) | 2 (7%) | 1 (3%) | 4 (13%) |
Localized PCa, clinically localized prostate cancer.
Detection of Phosphorylated PDGFR-β in Formalin-Fixed, Paraffin-Embedded Tissue
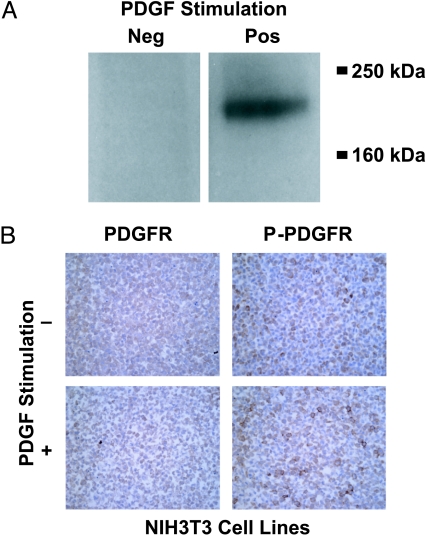
The expression array analysis, consistent with previous studies on PDGFR-β expression in PCa [15,38], examined the expression of the receptor and not specifically its activated or phosphorylated form. The initial study objective was to evaluate the activated form of PDGFR-β in formalinfixed, paraffin-embedded tissues. Multiple protocols and phospho-specific antibodies were tested before concluding that the phosphorylation status could not be reliably determined in formalin-fixed samples. These results are summarized in the following experiments. NIH-3T3 cells, a fibroblast cell line, were stimulated with PDGF-β to increase the presence of phosphorylated PDGFR-β as has been previously described [39]. Figure 2A shows immunoblot detection of phospho-PDGFR-β in stimulated—but not unstimulated—NIH-3T3 cells. Both stimulated and unstimulated NIH-3T3 cells were fixed and embedded in paraffin. PDGFR-β expression was detectable at the weak to moderate levels in both stimulated and unstimulated cells with the anti-phospho-PDGFR-β antibody (Figure 2B). Anti-PDGFR-b detected the receptor in both samples at similar levels (Figure 2B). This experiment demonstrates that antibodies against the phosphorylated form of PDGFR-β do not appear to specifically detect phosphorylated PDGFR-β in paraffin-embedded tissue. As we intended to characterize the fraction of patients with PCa that would be suitable for RTK inhibitor treatment, we chose to use a nonphospho-PDGFR-β antibody. Therefore, taking this approach, we most probably are overestimating the number of PCa that are suitable for treatment targets.
Figure 2.
Failure to detect phosphorylated PDGFR-β in formalin-fixed, paraffin-embedded tissue. NIH-3T3 cells were stimulated with 100 ng/ml PDGF-BB for 10 minutes to increase the amount of phosphorylated PDGFR-β. (A) Immunoblot analysis demonstrates the presence of phospho- PDGFR (190 kDa) in the stimulated cell line and the absence in the unstimulated cell line. (B) The same antibody was used to detect phospho-PDGFR-β in paraffin blocks generated from the same cell line (same stimulation). No substantial differences between both samples can be identified. Similar lack of discrimination is seen with anti-PDGFR-β antibodies between activated and nonactivated NIH-3T3 cells (magnification, x400).
DNA Microarray Characterization of PDGFR-β-Positive PCa
Hierarchical clustering of the PCa samples did not stratify the tumors based on their PDGFR-β expression. Therefore, a supervised approach was used. PDGFR-β overexpression, even in a small subset of PCa, suggests that targeted treatment would be aimed at those tumors that differ in their expression profile. Patients with high PDGFR-β expression might be the most suitable candidates for drug treatment. Therefore, in order to characterize PDGFR-β overexpressing tumors from low-expressing tumors, we used two analytic strategies. We performed a supervised analysis using SAM [27] by stratifying cases based either on their PDGFR-β transcript or protein expression level with the goal of finding other genes or pathways associated with overexpression. In the first analysis, we divided the PCa cases into those with high PDGFR-β transcript level as determined by cDNA expression analysis and those with low PDGFR-β transcript levels. In the second analysis, we used the TMA results from the same tumors represented on the cDNA arrays to create two classes of localized PCa based on PDGFR-β protein expression (i.e., absent versus present at any level).
Both methods led to similar sets of dysregulated genes. As demonstrated in Tables 3 and 4, PDGFR-β overexpression was associated with a 3.8-fold increase in early growth response 1 (Egr1). Egr1, a 59-kDa polypeptide with several DNA-binding domains, acts as a transcriptional activator and its expression is induced by several agents including tissue damage, hypoxia, and growth factors including PDGF [40]. Connective tissue growth factor (CTGF) was also found to be upregulated 3.1-fold in the PDGFR-β-overexpressing cases. CTGF is a downstream effector of TGF and physiologically supports cell growth, cell development, and cellular differentiation. As a member of a family of immediate early genes, it is involved in cellular proliferation, embryogenesis, and wound healing, and also in inducing the synthesis of extracellular matrix. Activating transcription factor 3 (ATF3) was upregulated three-fold. In its homodimeric form, ATF3 acts as a transcriptional repressor and, in heterodimeric form (e.g., with c-Jun), as a transcriptional activator. This gene was previously seen to be upregulated in PCa [40]. α-Methylacyl-CoA racemase (AMACR), an enzyme for peroxisomal β oxidation of branched chain fatty acid molecules, known to be overexpressed in the majority of PCa was 2.1-fold upregulated [23,25,37,41].
Table 3.
Upregulated Genes in Localized Prostate Cancer Associated with PDGFR-β Overexpression*.
| Gene Name | Gene ID† | Fold Upregulation |
q Value |
| Early growth response 1 | 840944 | 3.8 | 0.15 |
| Connective tissue growth factor | 898092 | 3.1 | 0.1 |
| Activating transcription factor 3 | 51448 | 3.0 | 0.1 |
| Activated leukocyte cell adhesion molecule | 26617 | 2.7 | 0.1 |
| Ribosomal protein L15 | 837904 | 2.4 | 0.1 |
| SPARC-like 1 (mast9, hevin) | 823871 | 2.2 | 0.05 |
| α-Methylacyl-CoA racemase | 133130 | 2.1 | 0.1 |
| Zinc finger protein 36, C3H type, homolog (mouse) | 23804 | 2.1 | 0.17 |
| Lumican | 813823 | 1.8 | 0.24 |
Overexpression of PDGFR-β was determined by immunohistochemistry of localized prostate cancer cases with associated cDNA array data. A supervised analysis was performed using SAM, which identified dysregulated genes.
Unigene designation.
Table 4.
Downregulated Genes in Localized Prostate Cancer Associated with PDGFR-β Overexpression*.
| Gene Name | Gene ID† | Fold Downregulation |
q Value |
| Down syndrome critical region gene 1-like 2 | 132828 | 2.9 | 0.1 |
| Myosin, light polypeptide 2, regulatory, cardiac, slow | 300051 | 2.8 | 0.1 |
| Neuroblastoma, suppression of tumorigenicity 1 | 898305 | 2.8 | 0.1 |
| Carbonic anhydrase III | 838856 | 2.5 | 0.1 |
| v-maf | 487793 | 2.4 | 0.1 |
| Solute carrier family 2 | 190732 | 2.3 | 0.1 |
| α-2-Glycoprotein 1, zinc | 1456160 | 2.3 | 0.1 |
| Growth arrest-specific 1 | 365826 | 2.2 | 0.1 |
| Stearoyl-CoA desaturase | 810711 | 2.1 | 0.1 |
| Synaptotagmin I | 971399 | 1.9 | 0.4 |
| Galectin-3 | 811000 | 1.9 | 0.4 |
| Developmentally regulated GTP binding protein 1 | 842980 | 1.8 | 0.2 |
Overexpression of PDGFR-β was determined by immunohistochemistry of localized prostate cancer cases with associated cDNA array data. A supervised analysis was performed using SAM, which identified dysregulated genes.
Unigene designation.
Downregulation of genes was not as dramatic as the upregulation in the clinically localized tumors. The most highly downregulated gene in PDGFR-β- expressing PCas was Down syndrome critical region 1, an evolutionary conserved protein that is located on chromosome 21 and found to be overexpressed in Down syndrome. This gene was 2.9-fold underexpressed in PDGFR-β-expressing PCa.
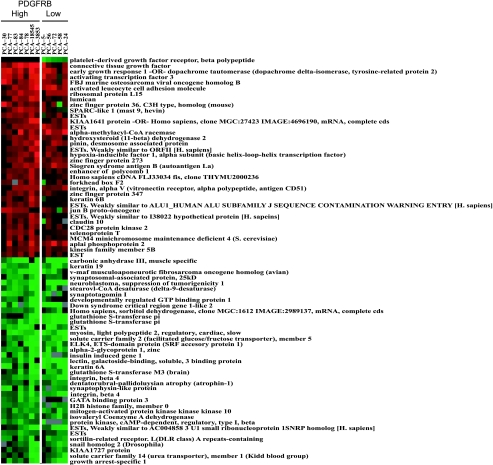
A heat map showing a gene expression profile of the five PCa cases from our dataset that expressed PDGFR-β at the lowest level and the seven cases that expressed PDGFR-β at the highest level, respectively, is presented in Figure 3 This map summarizes the dysregulated genes identified through the supervised analysis from the TMA data. PCa cases stratified as PDGFR-β-overexpressing all had a staining intensity from weak to strong, whereas the PCa cases stratified as PDGFR-β-underexpressing showed an absent staining. As the heatmap resulted from this supervised analysis of the expression of PDGFR-β at the protein level, the differences in the gene expression levels among all cases were subtle.
Figure 3.
Dysregulation of genes associated with PDGFR-β status in localized PCa. Supervised expression array analysis was performed using SAM. Cases were divided into high and low PDGFR-β expression based on protein expression using a high-density tissue microarray. This heat map demonstrates set of genes consistently over (red) and under (green) expressed in PCa, which either showed the highest (left) or the lowest PDGFR-β expression (right) at the protein level (see Tables 3 and 4 for details).
Discussion
The main goal of the current study was to characterize the expression of PDGFR in a broad range of prostate tissues and thus to determine the potential spectrum for the use of the tyrosine kinase inhibitor imatinib mesylate. As previous work suggested that the β-subunit of PDGFR is responsible for a myriad of cellular functions such as migration and proliferation and is also responsible for the angiogenic effect of PDGF [33–35], this study concentrated on the evaluation of expression of the β subunit.
Recent work suggested that PDGFR, a RTK, is expressed in a high fraction of PCa [15] and therefore may represent another possible target of imatinib mesylate or other RTK inhibitors such as SU101. Results from a phase II clinical trial, using the selective transient inhibitor of PDGFR autophosphorylation SU101 to target PDGFR as possible cause for development and progression of PCa in patients with hormone-refractory prostate carcinoma, found only modest clinical benefits [15].
Recent data linked PDGFR-β expression to PCa progression. Ko et al. [15] reported a significantly higher level of PDGFR expression in PCa describing PDGFR expression in 88% of primary and 80% of metastatic PCa. Chott et al. [38] also reported similar PDGFR expression levels in clinically localized and metastatic PCa. These levels of expression are significantly higher than those reported in the current study. There are a few explanations for these differences. Chott et al. focused solely on the expression of the α-subunit and analyzed a small cohort consisting of eight patients. In the study by Ko et al., both PDGFR subunits were detected in a cohort of 44 patients with hormone-refractory (metastatic) PCa. In the current study, we used a broad spectrum of PCa tissue ranging from high-grade PIN to localized PCa and hormone-refractory metastatic PCa in a cohort of 204 PCa patients. Therefore, an explanation for the vast differences of PDGFR expression levels in these studies compared to ours may reside in the use of different study populations. To get the widest perspective of PDGFR expression in PCa, we examined a significantly larger set of cases drawn from a wide range of patients with clinically localized PCa and advanced hormone-refractory PCa as compared to the previous work. Another possible explanation is that Ko et al. used different cutoffs for grading PDGFR expression. They considered weak staining as positive, whereas we considered moderate and strong staining as positive. At least at the transcript level, the cDNA microarray data demonstrated PDGFR-β to be expressed in only a small subset of PCa patients—a finding that was supported by the immunohistochemistry results for PDGFR-β, which demonstrated expression in only 5% of localized PCa and 16% metastatic PCa. Given only a moderate effect of PCa treatment with imatinib mesylate reported by Ko et al., one possible conclusion is that a nontargeted approach may not work as well as determining the PDGFR-β expression prior to treatment.
In the second phase of analysis, we also wanted to concentrate on the extreme cases, which demonstrated unquestionable protein expression. Ideally, we would have preferred to measure the activated form of the PDGFR-β. However, as we were not able to get these antibodies to work specifically in a large number of prostate tissues including positive controls, we performed a cell line experiment to convincingly demonstrate that reliably detecting activated PDGFR-β is not feasible with currently available reagents. In a cell line experiment, we detected phospho-PDGFR-β in Western blot analysis of cell lysates only from PDGF-stimulated NIH-3T3 cells but were unable to discriminate PDGFR-β activation in the same samples once they were fixed in formalin and embedded in paraffin. This limits the use of immunohistochemistry as a reliable method to distinguish PDGFR-β activation. The antibodies against phosphorylated PDGFR are presently not suitable for clinical use of PDGFR detection in PCa sample. The current study estimated that PDGFR-β in PCa is low. As few as 5% of patients with clinically localized PCa may benefit from RTK inhibitor treatment. We also recognize that this figure probably overestimates the percentage of tumors with activated PDGFR-β.
In the current study, PDGFR-β protein expression was not associated with poor prognostic indicators including elevated preoperative PSA, large tumor size, higher Gleason score, higher tumor stage, or PSA failure following surgery. Due to multiple clinical factors such as time of diagnosis, PDGFR-β expression may not always be associated with a higher risk. In recent work by Singh et al., although PDGFR-β was included in a multigene model for higher risk of biochemical failure following treatment, PDGFR-β alone was not determined to place the patient at higher risk of developing a biochemical failure [18].
The second goal of this study was to identify other genes that may be coexpressed in PDGFR-β-positive tumors, with the hope of identifying surrogate markers of PDGFR-β expression and/or alternate pathway targets. This was accomplished by stratifying cases not by tumor progression as we have done previously [24], but instead by evaluating the PDGFR-β expression at the transcript and protein levels and then dividing cases into high and low expressors. This approach examines extreme cases in order to identify important trends due to, or associated with, the PDGFR-β pathway. This process is an exercise in hypothesis generation with the goal of identifying more reliable and perhaps robust biomarkers that could serve as surrogate markers of activated PDGFR-β expression.
The majority of genes upregulated with PDGFR-β overexpression were involved in the induction of cell proliferation, migration, and angiogenesis. In addition, for most genes, an overexpression had been reported in various cancers previously. The gene that was upregulated to the highest level concurrently with PDGFR-β was the immediate early growth response gene Egr1, which acts as a transcriptional activator. Egr1 expression itself leads to an increase in growth factor expression including PDGF and, as such, induces cellular proliferation and contributes to tumor progression in PCa by accelerating tumor growth, also via accelerated angiogenesis [42]. Although Egr1 was shown to enhance the expression of the growth factors PDGF and TGFβ1 between 30-fold and 60-fold, we did not observe overexpression of these downstream effectors, or of the other known effectors such as IGF II, EGF, or FGF. PDGFR activation and Egr1 lead to many similar effects, such as cellular proliferation, differentiation, and also angiogenesis, indicating that there might be a closer connection of these two proteins. Overexpression of PDGFR-β was significantly concurrent with a greater than four-fold upregulation of Egr1, which also suggests that the use of this protein as a surrogate marker for PDGFR-positive PCa may be feasible.
Advances in targeted drug therapy will require identifying cohorts of patients most likely to benefit from treatment. In the example of imatinib mesylate, where there are minimal drug-related side effects, overtreatment is not as great a risk-to-benefits issue. However, other novel drugs may have more associated serious side effects, making their use only appropriate in cases suspected to respond. The vast majority of CML and GISTs demonstrate an overexpression or abnormal function of RTKs, and, therefore, treating all patients with imatinib mesylate is a reasonable strategy. In solid tumors, such as PCa, only a small fraction of patients is likely to benefit from imatinib mesylate treatment as demonstrated in the current study. Therefore, we anticipate that advancing molecular techniques will play an important future role in identifying these susceptible cohorts. This ideally needs to be done before treatment, and identifying PDGFR-β expression on prostate needle biopsy samples or performing wide-scale genome profiling on biopsy material may be the best way to determine treatment decisions. As suggested by Sauter (personal communication), TMAs may be useful in retrospectively determining which previously treated patients may benefit from newly developed adjuvant drugs. Using a TMA screening technique, as described in this study, one could identify (even if the overall numbers were small) patients most likely to benefit from treatment.
In summary, by cDNA microarray analysis, we were able to show that PDGFR-β expression was upregulated in considerably smaller subpopulations of localized and metastatic PCa than has been previously reported. No associations were identified between PDGFR-β expression and other pathologic or clinical parameters. A number of genes are upregulated and downregulated along with PDGFR-β including Egr1, AMACR, and neuroblastoma suppressor of tumorigenicity. The exact role of these genes in PDGFR-β-overexpressing tumors remains unclear and must still be determined but may characterize a subset of tumors that proliferate at least in part by PDGFR pathway. Our findings give experimental data for the limited likelihood of success in treating all PCa patients with drugs targeting PDGFR. Determining the PDGFR expression prior to treatment for selection of patients amenable for RTK inhibitor treatment should result in a higher percentage of treatment responders.
Abbreviations
- GIST
gastrointestinal stroma tumor
- PBST
phosphate-buffered saline with Tween 20
- PCa
prostate cancer
- PDGF
platelet-derived growth factor
- PDGFR
plateletderived growth factor receptor
- PIN
prostatic intraepithelial neoplasia
- PSA
prostate-specific antigen
- RTK
receptor tyrosine kinase
- SAM
significance analysis of microarray
- TMA
tissue microarray
Footnotes
This work was supported by the Specialized Program of Research Excellence for Prostate Cancer (SPORE) NCI grants P50CA69568 (M.A.R., A.M.C., and K.G.P.) and P50CA90381; NCI grant CA 97063 (A.M.C. and M.A.R.); and a Department of Defense Fellowship Award PC030214 (M.D.H. and M.A.R.).
These authors contributed equally to this work.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCRABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 4.Dagher R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, Rahman A, Chen G, Staten A, Griebel D, Pazdur R. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002;8:3034–3038. [PubMed] [Google Scholar]
- 5.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevik S, Druker BJ, Corless C, Flecher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 6.van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A, Sciot R, Van Glabbeke M, Silberman S, Nielsen OS. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358:1421–1423. doi: 10.1016/s0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, Demetri GD. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 8.Wennstrom S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 9.Wennstrom S, Siegbahn A, Yokote K, Arvidsson AK, Heldin CH, Mori S, Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF beta-receptor require the binding site for phosphatidylinositol 3′kinase. Oncogene. 1994;9:651–660. [PubMed] [Google Scholar]
- 10.Ronnstrand L, Mori S, Arridsson AK, Eriksson A, Wernstedt C, Hellman U, Claesson-Welsh L, Heldin CH. Identification of two C-terminal autophosphorylation sites in the PDGF beta-receptor: involvement in the interaction with phospholipase C-gamma. EMBO J. 1992;11:3911–3919. doi: 10.1002/j.1460-2075.1992.tb05484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori S, Ronnstrand L, Yokote K, Engstrom A, Courtneidge SA, Claesson-Welsh L, Heldin CH. Identification of two juxtamembrane autophosphorylation sites in the PDGF beta-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 1993;12:2257–2264. doi: 10.1002/j.1460-2075.1993.tb05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Twamley GM, Kypta RM, Hall B, Courtneidge SA. Src family tyrosine kinases and the response to platelet-derived growth factor. Adv Second Messenger Phosphoprot Res. 1993;28:187–194. [PubMed] [Google Scholar]
- 13.Arvidsson AK, Rupp E, Nanberg E, Downward J, Ronnstrand L, Wennstrom S, Schlessinger J, Heldin CH, Claesson-Welsh L. Tyr-716 in the platelet-derived growth factor beta-receptor kinase insert is involved in GRB2 binding and Ras activation. Mol Cell Biol. 1994;14:6715–6726. doi: 10.1128/mcb.14.10.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol- 3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 15.Ko YJ, Small EJ, Kabbinavar F, Chachoua A, Taneja S, Reese D, DePaoli A, Hannah A, Balk SP, Bubley GJ. A multi-institutional phase ii study of SU101, a platelet-derived growth factor receptor inhibitor, for patients with hormone-refractory prostate cancer. Clin Cancer Res. 2001;7:800–805. [PubMed] [Google Scholar]
- 16.Fudge K, Bostwick DG, Stearns ME. Platelet-derived growth factor A and B chains and the alpha and beta receptors in prostatic intraepithelial neoplasia. Prostate. 1996;29:282–286. doi: 10.1002/(SICI)1097-0045(199611)29:5<282::AID-PROS2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.George DJ. Receptor tyrosine kinases as rational targets for prostate cancer treatment: platelet-derived growth factor receptor and imatinib mesylate. Urology. 2002;60:115–121. doi: 10.1016/s0090-4295(02)01589-3. (discussion 122) [DOI] [PubMed] [Google Scholar]
- 18.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D'Amico AV, Richie JP, Lander ES, Loda M, Kantoff PW, Golub TR, Sellers WR. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1:203–209. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 19.Bostwick DG, Foster CS. Predictive factors in prostate cancer: current concepts from the 1999 College of American Pathologists Conference on Solid Tumor Prognostic Factors and the 1999 World Health Organization Second International Consultation on Prostate Cancer. Semin Urol Oncol. 1999;17:222–272. [PubMed] [Google Scholar]
- 20.Perrone EE, Theoharis C, Mucci NR, Hayasaka S, Taylor JM, Cooney KA, Rubin MA. Tissue microarray assessment of prostate cancer tumor proliferation in African-American and white men. J Natl Cancer Inst. 2000;92:937–939. doi: 10.1093/jnci/92.11.937. [DOI] [PubMed] [Google Scholar]
- 21.Dhanasekaran SM, Barrette TR, Ghosh D, Shah R, Varambally S, Kurachi K, Pienta KJ, Rubin MA, Chinnaiyan AM. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 22.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 23.Rubin MA, Zhou M, Dhanasekaran SM, Varambally S, Barrette TR, Sanda MG, Pienta KJ, Ghosh D, Chinnaiyan AM. alpha-Methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA. 2002;287:1662–1670. doi: 10.1001/jama.287.13.1662. [DOI] [PubMed] [Google Scholar]
- 24.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 25.Kuefer R, Varambally S, Zhou M, Lucas PC, Loeffler M, Wolter H, Mattfeldt T, Hautmann RE, Gschwend JE, Barrette TR, Dunn RL, Chinnaiyan AM, Rubin MA. alpha-Methylacyl-CoA racemase: expression levels of this novel cancer biomarker depend on tumor differentiation. Am J Pathol. 2002;161:841–848. doi: 10.1016/s0002-9440(10)64244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–51121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo JH, Yu YP, Cieply K, Lin F, Deflavia P, Dhir R, Finkelstein S, Michalopoulos G, Becich M. Gene expression analysis of prostate cancers. Mol Carcinog. 2002;33:25–35. doi: 10.1002/mc.10018. [DOI] [PubMed] [Google Scholar]
- 29.Magee JA, Araki T, Patil S, Ehrig T, True L, Humphrey PA, Catalona WJ, Watson MA, Milbrandt J. Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res. 2001;61:5692–5696. [PubMed] [Google Scholar]
- 30.Ramaswamy S, Tamayo P, Rifkin R, Mukherjee S, Yeang CH, Angelo M, Ladd C, Reich M, Latulippe E, Mesirov JP, Poggio T, Gerald W, Loda M, Lander ES, Golub TR. Multiclass cancer diagnosis using tumor gene expression signatures. Proc Natl Acad Sci USA. 2001;98:15149–15154. doi: 10.1073/pnas.211566398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF, Jr, Hampton GM. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974–5978. [PubMed] [Google Scholar]
- 32.Rhodes DR, Barrette TR, Rubin MA, Ghosh D, Chinnaiyan AM. Meta-analysis of microarrays: interstudy validation of gene expression profiles reveals pathway dysregulation in prostate cancer. Cancer Res. 2002;62:4427–4433. [PubMed] [Google Scholar]
- 33.Claesson-Welsh L. Platelet-derived growth factor receptor signals. J Biol Chem. 1994;269:32023–32026. [PubMed] [Google Scholar]
- 34.Crosby JR, Seifert RA, Soriano P, Bowen-Pope DF. Chimeric analysis reveals role of PDGF receptors in all muscle lineages. Nat Genet. 1998;18:385–388. doi: 10.1038/ng0498-385. [DOI] [PubMed] [Google Scholar]
- 35.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 36.Claeskens A, Ongenae N, Neefs JM, Cheyns P, Kaijen P, Cools M, Kutoh E. Hevin is down-regulated in many cancers and is a negative regulator of cell growth and proliferation. Br J Cancer. 2000;82:1123–1130. doi: 10.1054/bjoc.1999.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou M, Chinnaiyan AM, Kleer CG, Lucas PC, Rubin MA. Alpha-Methylacyl-CoA racemase: a novel tumor marker over-expressed in several human cancers and their precursor lesions. Am J Surg Pathol. 2002;26:926–931. doi: 10.1097/00000478-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Chott A, Sun Z, Morganstern D, Pan J, Li T, Susani M, Mosberger I, Upton MP, Bubley GJ, Balk SP. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. 1999;155:1271–1279. doi: 10.1016/S0002-9440(10)65229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villalonga P, Lopez-Alcala C, Chiloeches A, Gil J, Marais R, Bachs O, Agell N. Calmodulin prevents activation of Ras by PKC in 3T3 fibroblasts. J Biol Chem. 2002;277:37929–37935. doi: 10.1074/jbc.M202245200. [DOI] [PubMed] [Google Scholar]
- 40.Dash A, Maine IP, Varambally S, Shen R, Chinnaiyan AM, Rubin MA. Changes in differential gene expression because of warm ischemia time of radical prostatectomy specimens. Am J Pathol. 2002;161:1743–1748. doi: 10.1016/S0002-9440(10)64451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ernst T, Hergenhahn M, Kenzelmann M, Cohen CD, Bonrouhi M, Weninger A, Klaren R, Grone EF, Wiesel M, Gudemann C, Kuster J, Schott W, Staehler G, Kretzler M, Hollstein M, Grone HJ. Decrease and gain of gene expression are equally discriminatory markers for prostate carcinoma: a gene expression analysis on total and microdissected prostate tissue. Am J Pathol. 2002;160:2169–2180. doi: 10.1016/S0002-9440(10)61165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eid MA, Kumar MV, Iczkowski KA, Bostwick DG, Tindall DJ. Expression of early growth response genes in human prostate cancer. Cancer Res. 1998;58:2461–2468. [PubMed] [Google Scholar]