Abstract
Exposure to estrogenic compounds during critical periods of fetal development could result in adverse effects on the development of reproductive organs that are not apparent until later in life. Bisphenol A (BPA), which is employed in the manufacture of a wide range of consumer products, is a prime candidate for endocrine disruption. We examined BPA to address the question of whether in utero exposure affects the uterus of the offspring and studied the expression and distribution of the estrogen receptors alpha (ERα) and beta (ERβ), because estrogens influence the development, growth, and function of the uterus through both receptors. Gravid Sprague-Dawley dams were administered by gavage either 0.1 or 50 mg/kg per day BPA or 0.2 mg/kg per day 17α-ethinyl estradiol (EE2) as reference dose on gestation days 6 through 21. Female offspring were killed in estrus. Uterine morphologic changes as well as ERα and ERβ distribution and expression were measured by immunohistochemistry and Western blot analysis. Striking morphologic changes were observed in the uterine epithelium of postpubertal offspring during estrus of the in utero BPA-treated animals (the thickness of the total epithelium was significantly reduced). ERα expression was increased in the 50-mg BPA and EE2-treated group. In contrast, we observed significantly decreased ERβ expression in all BPA- and EE2-treated animals when compared with the control. In summary, these results clearly indicate that in utero exposure of rats to BPA promotes uterine disruption in offspring. We hypothesize that the uterine disruption could possibly be provoked by a dysregulation of Erα and ERβ.
Keywords: Bisphenol A, low dose, estrogen receptor, rat uterus, estrogen
Introduction
Endocrine-disrupting industrial chemicals (EDCs) are released into the environment and interfere with normal hormonal processes. Many researchers hypothesize that inadvertent and untimely exposure to these EDCs during critical periods of development, i.e., early postnatal or in utero, may adversely affect the reproductive and general health, growth, and development in both wildlife and humans [1,2].
The origins of the endocrine disrupter hypothesis may be traced to reports on adolescent daughters born to women who had taken the highly potent synthetic estrogen diethylstilbestrol (DES) during pregnancy. The negative consequences of this practice began to emerge when studies reported that these daughters developed a wide range of reproductive tract abnormalities, including a rare form of vaginal cancer, vaginal adenocarcinoma, and uterine malformations, including hypoplasia and a T-shaped uterus [3,4].
Bisphenol A (BPA) is a monomer composed of two unsaturated phenolic rings that resemble DES. In vitro studies demonstrated that BPA binds to the estrogen receptors, induces estrogen-dependent gene expression/responses, and is weakly estrogenic when compared with 17β-estradiol or DES [5–8]. BPA is among those estrogenic industrial compounds that are in widespread use. BPA is used in the production of epoxy, polyester-styrene, and polycarbonate resins, which are used for the manufacture of dental fillings, baby bottles, and food packaging. The ability of BPA to migrate from polymer to food has been described [9–11]. Leaching of BPA increases with repeated use or exposure to high heat of the polycarbonate products [9,11–14]. These data indicated a likely exposure of wildlife and humans to BPA. Indeed, we detected parent BPA in pregnant women and their fetuses [15]. Exposure levels of parent BPA were found within a range typical of those used in recent animal studies [16] and which were shown to be toxic to reproductive organs of male and female offspring. Furthermore, BPA was present in human serum and follicular fluid, as well as in full-term amniotic fluid [17]. BPA has been widely discussed as a prime candidate for endocrine disruption.
Minuscule amounts of EDCs were shown to alter the reproductive organs of developing mice, sparking alarm within the scientific community and regulatory agencies. Particularly, studies have shown that low doses of BPA could alter reproductive organs of developing rodents [18–28]. Additional relevant studies reported findings where BPA is a potent meiotic aneugen [29], and at very low doses BPA induces proliferation of human prostate cancer cells through binding to a mutant form of the androgen receptor found in some prostate tumors [30]. Alarmed about the implications of these results, some laboratories, mainly industrial ones, tried to reproduce these data but failed [31–36]. However, in addition to finding no low-dose effects of BPA, no effects of their positive control chemicals, DES, estradiol, and ethinyl estradiol, were found. These discrepancies between the studies may be attributable to variable sensitivity to estrogenic chemicals by laboratory animals as well as the type of feed used in the experiment [37]. For example, one study demonstrated that rodent strains can vary dramatically in their response to estrogenic compounds [38]. Furthermore, the issues of dose and binding affinities to the estrogen receptors (ERs) seem to be the heart of the controversy regarding xenoestrogens.
Pointing to these uncertainties [39] and the intense public interest in the concept that inadvertent and untimely exposure to BPA may adversely affect the reproductive and general health. We thus started to investigate the mechanisms of estrogen action in fetal rodents, because the earliest life stages are the most sensitive to EDCs, and prenatal exposure to EDCs leads to developmental effects that may not be detectable until sexual maturity.
For that reason, we examined the effects of in utero treatment with BPA [26,40] because prenatal exposure of rodents to EDCs causes a variety of abnormalities in the reproductive tract, specifically on the uterus, which are similar to the abnormalities in humans. In our previous studies gravid Sprague-Dawley dams were administered by gavage either 50 or 0.1 mg/kg per day BPA on gestation days 6 through 21 [26,40]. We used these two different doses of BPA to treat our animals because, for risk-assessment purposes, the Society of the Plastic Industry has recommended using 50 mg/kg per day as the no effect dose level (NOEL), and the reproductive and offspring toxicity no adverse effect dose level (NOAEL) was recently identified as 750 ppm (50 mg/kg per day) of BPA [33]. Doses below 50 mg/kg per day would thus be considered to fall within the “low-dose” range.
Here, we specifically addressed the question of whether in utero exposure to BPA alters the uterus of the offspring because the uterus is a major target organ for circulating hormones. The uterus is composed of different cell types (stroma, epithelial, and smooth muscle cells) that undergo continuous changes of differentiation and proliferation in response to changes of circulating estrogens [41,42]. We hypothesize, therefore, that in utero exposure of the developing fetus to exogenous estrogens might have a major impact on the uterus leading to long-term deleterious effects. We studied, especially, expression and distribution of the estrogen receptors alpha (ERα) and beta (ERβ) because estrogens influence the development, growth, and function of the uterus through both receptors. Although it has been demonstrated that ERα plays a major role in the differentiation and proliferation of the uterine epithelium, it has recently been demonstrated that ERβ can modulate the effects of the uterine dominant ERα and, therefore, has an antiproliferative function in the uterus [43].
Materials and Methods
Female Sprague-Dawley rats with sperm-positive vaginal smears were treated with either 2% cornstarch (Mondamin) at 10 ml/kg per day, BPA at 0.1 or 50 mg/kg per day, or 17α-ethinyl estradiol (EE2) at 0.2 mg/kg per day. Cornstarch served as the vehicle for BPA and pharmacological-grade peanut oil was used as the vehicle for EE2. The gravid dams were treated by gavage on gestation days 6 through 21.
Intact female offspring were maintained on a 12:12-hour light-dark cycle (light turned on at 6:00 A.M.), and beginning at approximately 3 months of age, estrous-cycle stage was determined by vaginal swabbing for 3 weeks. Each estrus group contained 6 offspring in the cornstarch group, 6 offspring in the 0.1 mg/kg per day and 6 offspring in the 50 mg/kg per day BPA group, as well as 6 offspring in the 0.2 mg/kg per day EE2 group. At approximately 4 months of age, female offspring were killed by decapitation in estrus between 9:30 and 16:00 hours. Body and reproductive organ weight were determined. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals by the Physiological Society of Germany. BPA was purchased from Sigma-Aldrich Chemicals GmbH (Steinheim, Germany) and 17-α ethinyl estradiol from Aldrich Chemical Company (Milwaukee, WI).
Histology
Uterine tissue was fixed in methacarn solution. The tissue sections (3 µm thick) were deparaffinized and rehydrated in distilled water. For histochemical stains, the dewaxed sections were stained with 1.0% wt/vol Mayer's hematoxylin and 0.5% wt/vol eosin.
Immunohistochemistry
Uterine tissue was fixed in methacarn solution. The tissue sections (3 µm thick) were deparaffinized, mounted on Superfrost glass slides, and rehydrated in distilled water. Antigenic epitopes were demasked by boiling sections for 20 minutes in citrate buffer (0.01 M, pH 6.0) in a conventional pressure cooker. Briefly, the sections were treated with 0.5% hydrogen peroxide in methanol for 30 minutes, blocked with 2.5% of normal horse serum (Alexis, Gruenberg, Germany) for 30 minutes and incubated with avidin and biotin (Vectorshield; Alexis) for 15 minutes. Sections were reacted with specific primary polyclonal antibodies against ERα (MC-20, sc-542; Santa Cruz, Heidelberg, Germany) at a 1:150 dilution or ERβ (PA1-311, Affinity Bioreagents, Golden, CO) at a 1:500 dilution for 2 hours in a humid chamber at room temperature, followed by incubation with biotinylated polyclonal antibodies (Vector Staining Kits; Alexis). Then, the reaction products were visualized according to manufacturer's instructions (R.T.U. Vectastain Universal Elite Kit, Alexis) using 3-amino-9-ethylcarbazole as the chromagen (Sigma Immuno Chemicals, Munich, Germany) and covered with Mayer's hematoxylin for counterstaining.
Negative control reactions for ERα or ERβ immunostaining were conducted on uterine tissue either with substitution of PBS or preabsorbtion of the primary antibodies with an excess of ERα or ERβ peptide (MC-20, sc-542P; Santa Cruz; or P-011, Affinity Bioreagents, 1:1 competition for 30 minutes at room temperature).
Image Analysis
The thickness of the uterine epithelium, the number of uterine luminal epithelial cell layers, epithelial cell nuclei, and epithelial cell nuclei with condensed chromatin, and the appearance of cavities within the epithelial cells were measured with an image analyzing system [26]. Nine fields were analyzed from each section (three sections per uterus) in six rats from each group. In order to more accurately estimate the expression of ERα in the epithelium of the uterus, again, the same image analysis system was used. Quantification was performed on the digitized images of systematic randomly selected representative fields (a Dplan 20x objective) of stromal and luminal epithelial cells of the endometrium. Nine fields were analyzed from each section (two sections per uterus) in six rats from each group. All ERα-immunostained luminal epithelial nuclei, regardless of intensity, were scored as positive. The number of stained (brown-red reaction product + blue hematoxylin) and unstained nuclei (blue hematoxylin) per measuring field in sections from control and treated animals was determined, and also the percentage of ERα-immunostained nuclei expressed as ratio of ERα-immunostained to the total number of epithelial cell nuclei.
Western Blot Analyses
Western blot analyses were performed from uterine tissue of each in utero-treated offspring [26]. Briefly, 15 µg protein was separated by SDS-PAGE using 10% gels and electro-transferred to nitrocellulose membranes. The quality as well as equal loading of protein blots was determined by Ponceau S staining of nitrocellulose using a monoclonal antibody against β-actin (Sigma) at 1:15,000 dilution. Blots were incubated overnight with polyclonal antibodies against ERα (MC-20, sc-542; Santa Cruz) at 1:100 dilution or against ERβ (PA1-311, Affinity Bioreagents) at 1:1000 dilution. However, the commercial antibodies for ERβ have been variable in value; therefore, our data were strengthened by using positive (heart and liver) and negative (testis) tissue controls, particularly for the Western analysis of ERβ. We compared the specificity of the ERβ immunoreactive bands by using an additional monoclonal antibody raised against ERβ (GR39, Oncogene Research Products, Darmstadt, Germany) at a dilution of 1 µg/ml.
The Mr of the immunoreactive bands was determined by using molecular weight marker protein stocks SDS-PAGE 7b (Sigma) and a Biotinylated Protein Ladder Detection Pack (7727S, Cell Signaling Technology, Frankfurt am Main, Germany).
Specificity of the obtained immunoreactive bands was assessed by using peptide preabsorbed antiserum against ERα peptide (MC-20, sc-542P; Santa Cruz, 1:1 competition for 30 minutes at room temperature) or ERβ peptide (P-011, Affinity Bioreagents, 1:1 competition for 30 minutes at room temperature) or substituting Tris-buffered saline (TBS, pH 7.5) containing 0.5% nonfat dried milk (NFDM) instead of primary antibody for ERα and ERβ.
A semiquantitative Western blot approach was chosen to quantify the abundance of ERβ. Immunobands of the Western blot analyses were digitized using the raytest digital camera image analyzing system (raytest, Stranbenhardt, Germany). The optical density of the ERβ and β-actin immunobands was measured by integrating the average 8-bit gray-scale value of each immunoband using AIDA image analyzing software (raytest). To standardize for differences in background intensity between Western blots, the background 8-bit gray-scale value was subtracted from each immunoband average 8-bit gray-scale value. The amount of the ERβ message per tissue sample of each case was expressed as the relative ERβ abundance normalized with that of β-actin expression (ERβ/β-actin ratio).
Statistical Analysis
Data analysis was performed using the Statistical Package for Social Sciences, versions 11.0 for Windows (SPSS, Chicago, IL) and Sigma Plot 2002 for Windows Version 8.0 (Systat Software GmbH, Erkrath, Germany). Values are given as means ± SD if not otherwise indicated. The statistical difference of the thickness of uterine luminal epithelium, number of epithelial cell nuclei, number of epithelial cell nuclei with condensed chromatin, and the appearance of cavities within the epithelial cells, as well as the percentage of ERα-immunostained epithelial nuclei, ERα gene expression, and ERβ gene expression level between the groups were determined by Mann-Whitney test. Differences were regarded as significant when the P value was less than .05.
Results
Changes in Morphology of Uterus at Estrus
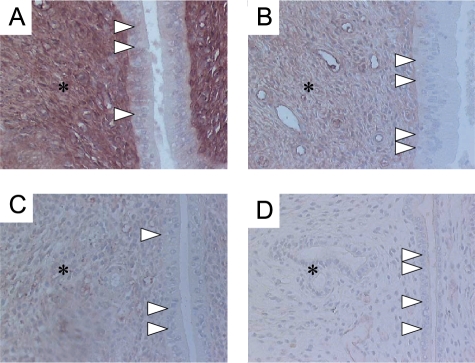
The differential ability of in utero treatment with BPA and EE2 to disrupt endometrial histomorphology in offspring after puberty is shown in Figure 1, A–D. Striking morphologic changes in the differentiation and stratification of the uterine epithelium could be observed during estrus from the in utero BPA-treated animals (Figure 1, C and D) when compared with the negative control group (Figure 1A). The thickness of the total epithelium was significantly reduced after exposure to 50 mg/kg per day BPA (Figure 1, D and E) when compared with the control group (Figure 1, A and E). The 0.1-mg BPA dose caused a similar effect, but was less pronounced (Figure 1, C and E) and not statistically significant to the control group. Within the 50-mg BPA-treated group the luminal endometrial epithelium was significantly riddled with cavities containing nuclei with condensed chromatin (Figures 1D and 2, B and C). The luminal endometrial epithelium of the 0.1-mg BPA-exposed group had a foamy appearance with significantly increased cavities containing nuclei with condensed chromatin when compared with the control group (Figures 1C and 2, B and C). Nuclei were less orderly and basally located (Figure 1C).
Figure 1.
(A–D) Representative high-magnification histology of the rat offspring uterus at estrus. (A) Control (cornstarch-treated animals) group. Typical thickened uterine epithelium at estrus stage (*) with orderly and basally located nuclei. Original magnification, x400. (B) 0.2 mg/kg EE2 (positive control). Pseudostratified hyperplastic epithelium (*, columnar cells with less orderly and basally located nuclei) harboring cavities (arrows). Original magnification, x400. (C) 0.1 mg/kg per day BPA. Decreased luminal endometrial epithelium thickness with a foamy appearance (*) and cavities (arrows). Nuclei are less orderly and basally located. Original magnification, x400. (D) 50 mg/kg per day BPA. Significantly reduced thickness of the total epithelium (*). The luminal endometrial epithelium is riddled with cavities containing nuclei with condensed chromatin (arrows). Original magnification, x400. (E) Statistical analysis of height (µm) of the luminal epithelial cell layers from rat offspring after in utero treatment with BPA or EE2 at estrus stage. Values are based on analysis of three sections for each uterine specimen (six from each group). Quantification was performed on the digitized images of 10 systematic, randomly selected, representative fields and are reported as the mean ± SD. Co, control group, 31.0 ± 3.9 µm, n = 6; EE2, 0.2 mg/kg per day 17α-ethinyl estradiol group, 33.2 ± 9.4 µm, n = 6; BPA0.1, 0.1 mg/kg per day BPA, 27.8 ± 1.8 µm, n = 6; BPA50, 50 mg/kg per day BPA, 19.2 ± 6.0 µm, n = 6.
Figure 2.
(A–C) Statistical analysis of epithelial cell nuclei, epithelial cell nuclei with condensed chromatin, and the appearance of cavities within the epithelial cells from rat offspring after in utero treatment with BPA or EE2 at estrus stage. Values are based on analysis of three sections for each uterine specimen (six from each group). Quantification was performed on the digitized images of nine systematic, randomly selected, representative fields and are reported as the mean ± SD. (A) Number of epithelial cell nuclei: Co, control group, 18.0 ± 4.0, n = 6; EE2, 0.2 mg/kg per day 17α-ethinyl estradiol group, 33.5 ± 12.78, n = 6; BPA0.1, 0.1 mg/kg per day BPA, 30.5 ± 6.8, n = 6; BPA50, 50 mg/kg per day BPA, 33.8 ± 3.7, n = 6. (B) Number of epithelial cell nuclei with condensed chromatin: Co, control group, 8.5 ± 3.9, n = 6; EE2, 0.2 mg/kg per day 17 α-ethinyl estradiol group, 20.0 ± 12.4, n = 6; BPA0.1, 0.1 mg/kg per day BPA, 22.7 + 6.1, n = 6; BPA50, 50 mg/kg per day BPA, 26.2 ± 7.6, n = 6. (C) Appearance of cavities within the epithelial cells: Co, control group, 7.7 ± 3.2, n = 6; EE2, 0.2 mg/kg per day 17α-ethinyl estradiol group, 15.2 ± 3.9, n = 6. BPA0.1, 0.1 mg/kg per day BPA, 16.2 ± 2.3, n = 6; BPA50, 50 mg/kg per day BPA, 14.4 ± 3.4, n = 6.
In contrast, the luminal endometrium of the reference group (EE2-exposed uteri) was mostly characterized by hypertrophic (columnar cells with less orderly and basally located nuclei), elongated epithelial cells significantly harboring cavities containing nuclei with condensed chromatin when compared with the control group (Figures 1B and 2, B and C).
Expression and Localization of Estrogen Receptors in the Uterus
ERα Figure 3, A–D, demonstrate representative ERα immunostainings within the uterine tissue of rat offspring after in utero treatment with EE2 and 0.1 and 50 mg BPA compared with the negative controls. Brown-red color, which indicates ERα immunoreaction, was recognized in nuclei of both epithelium and stromal cells. The percentage of ERα-immunostained, endometrial, luminal epithelial cell nuclei was significantly increased after EE2 and 50-mg BPA treatment (Figure 3, B, D, and E). However, in the 0.1-mg BPA-treated group no significant difference of the ERα immunostaining in the uteri was observed compared with the control group (Figure 3, A, C, and E). In summary, within the EE2- and 50-mg BPA-treated group (Figure 3, B and D) the intensity of the immunostaining increased in nuclei of the epithelial and stromal cells compared with the control (Figure 3A) and 0.1-mg BPA-treated (Figure 3C) uteri.
Figure 3.
(A–D) Representative high-magnification ERα immunostaining within the uterine tissue of rat offspring after in utero treatment with EE2 and 0.1 and 50 mg BPA compared with the negative controls. ERα immunoreaction was recognized in nuclei of both epithelium (arrowheads) and stromal cells (arrows). (A) Control (cornstarch-treated animals) group. Weak ERα immunoreaction in nuclei of both epithelium and stromal cells. Less ERα-immunostained luminal epithelium cell nuclei. Original magnification, x400. (B) 0.2 mg/kg per day EE2 (positive control). Significantly increased population of ERα-immunostained uterine luminal epithelial cell nuclei, as well as a strongly immunostained stromal cell pattern frequently describing a uniform, thick mesenchymal cell layer underlying the luminal epithelium. Original magnification, x400. (C) 0.1 mg/kg per day BPA. Weak ERα immunoreaction in nuclei of both epithelium and stromal cells. Less ERα-immunostained luminal epithelium cell nuclei. Original magnification, x400. (D) 50 mg/kg per day BPA. Significantly increased population of ERα-immunostained uterine luminal epithelial cell nuclei, as well as strongly immunostained stromal cells, which are not organized in a uniform cell layer underlying the epithelium. Stronger immunostaining was also recognized in the cytoplasm of luminal epithelial cells. Original magnification, x400. (E) Image analysis score of positive ERα-immunostained uterine luminal epithelial cells. Shown is the percentage of ERα-immunostained uterine epithelial cell nuclei. Values are based on analysis of nine fields from each section (two sections per uterus) in six rats from each group and are reported as the mean ± SD. Co, control (cornstarch-treated animals) group, 57 ± 19%, n = 6; EE2, 0.2 mg/kg per day 17α-ethinyl estradiol group, 90 ± 4%, n = 6; BPA0.1, 0.1 mg /kg / day BPA, 67 ± 7%, n = 6; BPA50, 50 mg/kg per day BPA, 95 ± 15%, n = 6.
However, stronger immunostaining was also recognized in the cytoplasm of luminal epithelial cells of the BPA 50-mg-dose group (Figure 3D). This cytoplasmic staining is possibly due to the staining of newly synthesized ERα. It is interesting to note that the mesenchymal tissues of the 50-mg and 0.1-mg BPA dose group displayed a disorganization of the ERα-immunostained stroma cells. They are not organized in a uniform cell layer underlying the epithelium (Figure 3, C and D), whereas the ERα-staining pattern frequently describes a uniform, thick, mesenchymal cell layer underlying the luminal epithelium within the stroma of the EE2-treated group (Figure 3B). Control reactions for ERα immunostaining using an excess of ERα peptide-preabsorbed primary antibody or PBS with normal serum instead of antibody first were used to evaluate the specificity of the ERα immunoreactivity and revealed no more ERα immunoreactivity (data not shown).
The protein expression of ERα in uteri was compared by Western blot analysis. We clearly demonstrate that the full-length ERα expression at 64 kDa is increased during estrus in the uterus of all female offspring exposed to the 50-mg dose of BPA and EE2 compared with the negative control group, whereas ERα expression does not differ between the 0.1-mg dose of BPA and the control group (Figure 4A). Within the 0.1-mg dose of BPA and the control group we could detect only very weak but specific ERα immunobands of the full-length ERα variant at 64 kDa (Figure 4A). Only two immunoreactive bands at 56 and 42 kDa from homogenates of rat uteri from control and 0.1-mg BPA-treated animals showed strong staining (Figure 4A). Indeed, the anti-ERα antibody specifically reacted with three bands at 64, 56, and 42 kDa from homogenates of rat uteri (Figure 4, A–C) because binding to all immunopositive bands was eliminated when the antibody was preincubated with antigen ERα peptide (Figure 4B). Substituting TBS containing 0.5% NFDM instead of primary antibody for ERα led to no more immunoreactivity (Figure 4C).
Figure 4.
Representative Western blot analyses of ERα expression of uterine protein at estrus stage of female Sprague-Dawley offspring exposed to 17α-ethinyl estradiol and bisphenol A in utero. Gravid dams were fed by gavage on gestation days 6 through 21 with either 2% cornstarch (negative control; CO) at 10 ml/kg per day, 0.2 mg/kg per day EE2 (EE2), used as a positive control, or 0.1 mg/kg per day BPA (BPA0.1) or 50 mg/kg per day BPA (BPA50). The female offspring were then sacrificed in estrus at 4 months of age. (A) The full-length ERα expression at 64 kDa is increased in all female offspring exposed to EE2 and the 50-mg dose of BPA compared with the negative control group. Within the 0.1-mg dose of BPA and the control group only very weak but specific ERα immunobands of the full-length ERα variant at 64 kDa could be detected. Only two immunoreactive bands at 56 and 42 kDa from homogenates of rat uteri from all treated animals showed strong staining. Protein loading was normalized to β-actin using a monoclonal primary antibody at a 1:15,000 dilution (Sigma), which was specific for a band at 42 kDa. The anti-ERα antibody specifically reacted with three bands at 64, 56, and 42 kDa from homogenates of rat uteri. (B) Binding to all immunopositive bands was eliminated when the antibody was preincubated with antigen Erα peptide. (C) Substituting TBS containing 0.5% NFDM (∅ 1.Ab) instead of primary antibody (+ 1.Ab) for ERα led to no more immunoreactivity. Protein, in the amounts of 14, 30, and 15 µg, was loaded.
ERβ Figure 5, A–D, demonstrates representative ERβ immunostainings within the uterine tissue of rat offspring after in utero treatment with EE2 and 0.1 and 50 mg BPA compared with the negative controls (Figure 5A). The brown-red color, which indicates ERβ immunoreaction, was recognized dominantly in stromal cells of the mesenchyme. ERβ immunostaining was significantly decreased after EE2 (Figure 5B) and BPA treatment (0.1 mg BPA, Figure 4C; 50 mg BPA, Figure 4D) when compared with the control group (Figure 5A).
Figure 5.
(A–D) Representative high magnification ERβ immunostaining within the uterine tissue of rat offspring after in utero treatment with EE2 and 0.1 and 50 mg BPA compared with the negative controls. The brown-red color, which indicates ERβ immunoreaction, was recognized dominantly in stromal cells of the mesenchyme (asterisks). (A) Control (cornstarch-treated animals) group. Distinct ERβ immunoreaction in stromal cells of the mesenchyme. Original magnification, x200. (B) 0.2 mg/kg per day EE2 (positive control). Weak immunostained mesenchyme. Original magnification, x200. (C) 0.1 mg/kg per day BPA. Decreased ERβ immunoreactions in stromal cells. Original magnification, x200. (D) 50 mg/kg per day BPA. No ERβ immunoreactions within the uterus. Original magnification, x200. Epithelium (arrow heads).
Control reactions for ERβ immunostaining using an excess of ERβ peptide-preabsorbed primary antibody or PBS with normal serum instead of antibody first were used to evaluate the specificity of the ERβ immunoreactivity and revealed no more ERβ immunoreactivity (data not shown).
Again, the protein expression of ERβ in uteri was compared by Western blot analysis. We clearly demonstrate that the ERβ expression at 53 kDa is decreased during estrus at the protein level in the uterus of all female offspring exposed to EE2 and the 0.1- and 50-mg dose of BPA compared with the negative control group (Figure 6, A and C). Within the 0.1- and 50-mg dose of BPA we could detect only a very weak ERβ immunoband.
Figure 6.
Representative semiquantitative Western blot approach of ERβ expression of uterine protein at estrus stage of female Sprague-Dawley offspring exposed to 17α-ethinyl estradiol and bisphenol A in utero. Gravid dams were fed by gavage on gestation days 6 through 21 with either 2% cornstarch (negative control; CO) at 10 ml/kg per day, 0.2 mg/kg per day EE2 (EE2), used as a positive control, or 0.1 mg/kg per day BPA (BPA0.1) or 50 mg/kg per day BPA (BPA50). The female offspring were then sacrificed in estrus at 4 months of age. (A) We clearly demonstrate that the ERβ expression at 53 kDa is decreased during estrus at the protein level in the uterus of all female offspring exposed to EE2 and the 0.1- and 50-mg dose of BPA compared with the negative control group. Within the 0.1- and 50-mg dose of BPA, we could detect only a very weak ERβ immunoband. Protein loading was normalized to β-actin using a monoclonal primary antibody at 1:15,000 dilution (Sigma), which was specific for a band at 42 kDa. (B) Additional control experiments investigated the specificity of the immunoreactions against ERβ at 53 kDa within the Western analysis by comparing two different commercial antibodies (PA1-311, Affinity Bioreagents, and GR39, Oncogene Research Products). Both antibodies raised against ERβ revealed the same specific immunobands in positive (uterus, liver, and heart) and negative (testis) tissue controls. (C) Statistical analysis of the semiquantitative Western blot approach of ERβ expression of uterine protein at estrus stage of all female Sprague-Dawley offspring. The optical density of the ERβ and β-actin immunobands was measured by integrating the average 8-bit gray-scale value of each immunoband. To standardize for differences in background intensity between Western blots, the background 8-bit gray scale value was subtracted from each immunoband average 8-bit gray-scale value. The amounts of the ERβ message per tissue sample of each case were expressed as the relative ERβ abundance normalized with that of β-actin expression (ratio ERβ/β-actin) and are reported as the mean ± SD. The statistical analysis revealed that the ERβ expression is decreased during estrus at the protein level in the uterus of all female offspring exposed to EE2 and the 0.1- and 50-mg dose of BPA compared with the negative control group.
The anti-ERβ antibody (PA1-311, Affinity Bioreagents) specifically reacted with one band at 53 kDa from homogenates of rat uteri (Figure 6, A and B), because binding to all immunopositive bands at 53 kDa was eliminated when the antibody was preincubated with antigen ERβ peptide (data not shown). Substituting TBS containing 0.5% NFDM instead of primary antibody for ERβ led to no more immunoreactivity (data not shown).
Additional control experiments investigated the specificity of the immunoreactions against ERβ at 53 kDa within the Western analysis by comparing two different commercial antibodies. Both antibodies raised against ERβ revealed the same specific immunoreactions in positive (uterus, liver, and heart) and negative (testis) tissue controls (Figure 6B).
Discussion
In the uterus, estrogens stimulate uterine epithelium proliferation in vivo [44] and play a critical role in uterine epithelial growth, morphogenesis, cytodifferentiation, and secretory activity [45].
Little is known about the deleterious effects in the uterus after prenatal exposure to BPA. This lack of data might exist because most traditional endpoints of toxicity may not be sensitive enough or not detectable until sexual maturity [39]. Indeed, we previously observed that the reduction in absolute uterine weight in the 0.1-mg BPA-treated group during estrus was qualitatively similar to that seen with the reference estrogen, whereas the absolute uterine weight of the 50-mg/kg group was similar to negative controls [40]. The decreases were no longer evident after calculation of the relative uterine weights (corrected for body weight), indicating that the effect of low doses of BPA on the uterus is very modest [40]. We demonstrated that birth weight was not affected by in utero exposure to either dose of BPA, which is in contrast to a decreased birth weight noted in offspring exposed to the reference estrogen [40].
Additionally, we recently reported finding a greater percentage of cycles with longer estrus phases in the 50-mg/kg BPA dose group. This effect was qualitatively similar to that observed with the reference estrogen, which caused almost persistent estrus. Likewise, the high dose of BPA led to a greater percentage of longer estrous cycles. These findings stimulated us to conduct the present experiments.
For the first time, we demonstrate striking morphologic changes of the uterine epithelium from the in utero BPA-treated animals during estrus (Figure 1, C–D, and Figure 2, A–C), which are similar to DES-specific disruption patterns [46]. In accordance with previous reports studying the effects of DES [46], we noted uterine disorganization, irregular nuclei, and the appearance of cavities in the uteri of all BPA-treated animals [47]. Normally, the uterine luminal epithelium is the tallest at estrus [46]. The thickness of the total epithelium was significantly reduced following exposure to 50 mg/kg BPA (Figure 1, D and E). The 0.1-mg BPA dose caused a similar effect, but was less pronounced (Figure 1, C and E). These abnormalities in the uterus of rat offspring are similar to the abnormalities found in rodents and humans after DES treatment [47]. Hypoplastic uteri were observed in human offspring after DES exposure [48–50]. Salle et al. [51] demonstrated by transvaginal ultrasound that particularly in the luteal phase, the thickness of the human endometrium was decreased significantly in patients with prenatal DES-exposed uteri. Neonatal DES exposure inhibited endometrial gland development and elicited a hypotrophic/hypoplastic response in the rat uterus [52–54]. In contrast, in utero treatment with EE2 led to a hypertrophic response in the uterus (Figure 1B). In the EE2-exposed uteri, a characteristic histopathologic profile [43,55–57], including hypertrophic elongated luminal endometrium epithelial cells with less orderly and basally located nuclei and cavities (Figures 1B and 2, A–C), was detected.
The observed morphologic differences between the EE2- and BPA-treated animals are not unexpected because previous studies already demonstrated similar differences of uterine disruption at the histologic level when exposed to DES or E2 [58–64]. Therefore, by summarizing all of these results from a number of studies, including our work, we have to question the unitary view of estrogen action. There are substantial differences in the potencies of different estrogens that result from complex biological and pharmacokinetic dynamics, such as their receptor-binding affinities and absorption, including method (e.g., oral route vs. subcutaneous injection) and time point (developmental stage) of administration, first-pass metabolism, plasma protein binding, and elimination [64,65].
Because of the striking morphologic changes of the uterine epithelium after in utero treatment with BPA and EE2, we studied the expression of ERα and ERβ to elucidate the possible mechanisms for the cellular effects. Most of the estrogenic effects on the uterus are mediated by ERα, which is the predominant subtype in the normal uterus and which regulates epithelial morphogenesis, cytodifferentiation, and secretory activity. ERα is fundamental for development and growth of the uterus. Recently, however, it has been demonstrated that ERβ acts as a modulator of the ERα-mediated effects in the uterus. ERβ can modulate ERα-mediated gene transcription leading to antiproliferative function in the uterus [43]. The importance of both ERs for the uterus was also indicated using the ER knockout mice, αERKO, βERKO, and αβERKO [66–68].
In comparison with recent studies [69], ERα immunoreactivity was confined to the nuclei of both endometrial epithelium and stromal cells in the rat uterus at estrus stage. We clearly demonstrate that the anti-ERα antibody specifically reacted with three bands at 64, 56, and 42 kDa from homogenates of rat uteri (Figure 4, A–C), which is in accordance with our previous study [26]. These shorter bands (56 and 42 kDa) are derived from the alternative usage of initiation ATG or splicing [70, 71]. Specificity of immunoreactivity was analyzed by using peptide-preabsorbed antibodies (Figure 4B) and substituting TBS instead of primary antibody for ERα (Figure 4C). In the EE2- and 50-mg BPA-treated groups, at the level of protein expression, the intensity of the ERα immunostaining of the full-length ERα variant at 64 kDa increased (Figures 3, B, D, and E, and 4A) in comparison with the control (Figures 3, A and E, and 4A) and 0.1-mg BPA-treated uteri (Figure 3, C and E, and 4A), where only very weak immunoreactions of the full-length ERα variant at 64 kDa (Figure 4A) could be detected by Western blot analysis and immunohistochemistry, respectively. These results clearly confirm data from previous studies in neonatal and immature rodents where 17β-estradiol, BPA, or DES treatment increased ERα mRNA expression, the number of positively ERα-immunostained epithelial cells, and the intensity of ERα-immunostaining within the uterus [43,72–75]. Stronger immunostaining was also recognized in the cytoplasm of luminal epithelial cells of the 50-mg BPA dose group (Figure 3D), which is possibly due to the staining of newly synthesized ERα that has been proven by a semiquantitative RT-PCR analysis (G. Schönfelder, unpublished data).
Nevertheless, in the present study it was unclear what caused the differences of uterine disruption at the histologic level between BPA, especially the 50-mg dose group, and the reference dose (EE2). There could be many explanations. One explanation is related to a dysregulation of the ERβ. As already stated, ERβ acts as a transdominant repressor on ERα transcriptional activity at subsaturating concentrations of endogenous 17β-estradiol (E2) in the uterus [76] by forming heterodimers with the ERα [77,78]. Thus, ERβ can oppose ERα effects on epithelial cell growth. This information stimulated us to study the expression and localization of ERβ in all uteri. Immunostaining revealed decreased immunoreactivity against ERβ after EE2 (Figure 5B) and BPA treatment (0.1 mg BPA, Figure 5C, and 50 mg BPA, Figure 5D) when compared with the control group (Figure 5A). The protein expression of ERβ in uteri was again compared by Western blot analysis. Western blot analysis clearly demonstrated downregulation of ERβ during estrus in the uterus of all female offspring exposed to EE2, the 0.1-mg and the 50-mg dose of BPA compared with the negative control group (Figure 6, A and C). The downregulation of ERβ within the in utero EE2-exposed rats would explain our morphologic observation of the uterine epithelium hypertrophy (Figure 1B). Indeed, these results are consistent with previous studies in rodents where estrogen treatment decreased ERβ expression in the uterus [43,79]. ERβ was downregulated and ERα becomes fully functional, leading to increased cell proliferation and enhanced responsiveness to E2 [43]. In contrast to the observed morphologic changes in the EE2 group we found significantly reduced thickness of the total epithelium and similar effects after exposure to 50 mg/kg per day BPA (Figure 1, D and E), but less pronounced after exposure with 0.1 mg BPA (Figure 1, C and E). One likely explanation for the phenomenon within the 0.1 mg/kg per day BPA group is that ERα expression is not highly enough induced by the low dose of BPA (Figures 3, C and E, and 4A). Therefore, ERα is not fully functional and subsaturating concentrations of the ovarian estradiol cannot stimulate uterine cell proliferation, even if ERβ expression is decreased (Figures 5C and 6, A and C). However, within the 50 mg/kg per day BPA group we would assume the same morphologic changes as in the EE2 dose group because of its ER's expression pattern. ERα is highly upregulated and ERβ was much more downregulated compared with the EE2 group. Nevertheless, we observed a hypoplastic epithelium. Until now, we have only one suggestion to explain this controversial effect.
We suggest that differences in sensitivity of the uterus to different estrogens and xenoestrogens may be attributed to differences in ERα and ERβ distribution and regulation over developmental periods. It has been demonstrated, e.g., that ERα and ERβ are highly regulated molecules whose transcriptional activity can be regulated by the nature of the bound ligand. Thus, different compounds induce different structural alterations within the estrogen receptors [80,81]. After activation, the ERα forms a dimer with/without the ERβ; nuclear receptor coactivators associate in a ligand-dependent manner with estrogen receptors, and they enhance ligand-dependent transcriptional activation. This activation will alter the differentiation and proliferation of the uterine epithelial cell by influencing target gene transcription, such as the progesterone receptor. Expression levels of the coactivators determine whether a given ER-ligand complex will manifest antagonist or agonistic activity in a particular cell [81]. Therefore, hormonal treatment could possibly represent an induction of uterine epithelial coactivators, perhaps in selected cells, or repression of stromal coactivator mRNA, which influence expression of genes important for uterine epithelial growth.
In summary, these results clearly indicate that in utero exposure (not neonatal) [74] of rats to BPA promotes uterine disruption by influencing expression and distribution of the estrogen receptors ERα and ERβ. BPA might influence the development, growth, and function of the uterus through both receptors. Although it has been demonstrated that ERα plays a major role in the differentiation and proliferation of the uterine epithelium, we can demonstrate that ERβ can modulate the effects of the uterine dominant ERα. Nevertheless, in the present study it remains unclear what caused the differences of uterine disruption at the histologic level between BPA, especially the 50-mg dose group, and the reference dose (EE2). Therefore, further studies for clarifying the effects of BPA and EE2 on the regulation of uterine progesterone receptor and coactivators are needed.
Acknowledgements
The authors thank Andrea Klau, Helga Stürje, and Heike Marburger for technical assistance and Doris Webb for critical reading of this manuscript.
Abbreviations
- BPA
Bisphenol A
- DES
diethylstilbestrol
- EDC
endocrine-disrupting industrial chemical
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- EE2
17α-ethinyl estradiol
- E2
estradiol
- NFDM
nonfat dried milk
Footnotes
This work was supported by a grant from the Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit (Federal Ministry for Environmental Protection and Radiation Security).
References
- 1.Loder N. Royal society warns on hormone disrupters. Nature. 2000;406:4. doi: 10.1038/35017709. [DOI] [PubMed] [Google Scholar]
- 2.Triendl R. Genes may solve hormone-disrupter debate. Nature. 2001;409:274. doi: 10.1038/35053303. [DOI] [PubMed] [Google Scholar]
- 3.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg JM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertil Steril. 1999;72:1–7. doi: 10.1016/s0015-0282(99)00153-3. [DOI] [PubMed] [Google Scholar]
- 5.Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ, McDonnell DP. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997;143:205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- 6.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vomSaal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong W, Perkins R, Xing L, Welsh WJ, Sheehan DM. QSAR models for binding of estrogenic compounds to estrogen receptor alpha and beta subtypes. Endocrinology. 1997;138:4022–4025. doi: 10.1210/endo.138.9.5487. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T, Yasuhara A. Quantities of bisphenol A leached from plastic waste samples. Chemosphere. 1999;38:2569–2576. doi: 10.1016/s0045-6535(98)00464-0. [DOI] [PubMed] [Google Scholar]
- 10.Howe SR, Borodinsky L. Potential exposure to bisphenol A from food-contact use of polycarbonate resins. Food Addit Contam. 1998;15:370–375. doi: 10.1080/02652039809374653. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T, Horie M, Hoshino Y, Nakazawa H. Determination of bisphenol A in canned vegetables and fruit by high performance liquid chromatography. Food Addit Contam. 2001;18:69–75. doi: 10.1080/026520301446412. [DOI] [PubMed] [Google Scholar]
- 12.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae B, Jeong JH, Lee SJ. The quantification and characterization of endocrine disruptor bisphenol-A leaching from epoxy resin. Water Sci Technol. 2002;46:381–387. [PubMed] [Google Scholar]
- 14.Takao Y, Lee HC, Kohra S, Arizono K. Release of bisphenol A from food can lining upon heating. J Health Sci. 2002;48:331–334. [Google Scholar]
- 15.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zalko D, Soto AM, Dolo L, Dorio C, Rathahao E, Debrauwer L, Faure R, Cravedi JP. Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect. 2003;111:309–319. doi: 10.1289/ehp.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 18.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vomSaal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 19.vomSaal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV. A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior. Toxicol Ind Health. 1998;14:239–260. doi: 10.1177/074823379801400115. [DOI] [PubMed] [Google Scholar]
- 20.Welshons WV, Nagel SC, Thayer KA, Judy BM, vomSaal FS. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol Ind Health. 1999;15:12–25. doi: 10.1177/074823379901500103. [DOI] [PubMed] [Google Scholar]
- 21.Dessi-Fulgheri F, Porrini S, Farabollini F. Effects of perinatal exposure to bisphenol A on play behavior of female and male juvenile rats. Environ Health Perspect. 2002;110(Suppl 3):403–407. doi: 10.1289/ehp.110-1241190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109:55–60. doi: 10.1289/ehp.0110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002;16:117–122. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 24.Palanza PL, Howdeshell KL, Parmigiani S, vomSaal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110(Suppl 3):415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schonfelder G, Flick B, Mayr E, Talsness C, Paul M, Chahoud I. In utero exposure to low doses of bisphenol A lead to long-term deleterious effects in the vagina. Neoplasia. 2002;4:98–102. doi: 10.1038/sj.neo.7900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos JG, Varayoud J, Sonnenschein C, Soto AM, Munoz DT, Luque EH. Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol Reprod. 2001;65:1271–1277. doi: 10.1095/biolreprod65.4.1271. [DOI] [PubMed] [Google Scholar]
- 28.Ramos JG, Varayoud J, Kass L, Rodriguez H, Costabel L, Munoz-De-Toro M, Luque EH. Bisphenol A induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology. 2003;144:3206–3215. doi: 10.1210/en.2002-0198. [DOI] [PubMed] [Google Scholar]
- 29.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 30.Wetherill YB, Petre CE, Monk KR, Puga A, Knudsen KE. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther. 2002;1:515–524. [PubMed] [Google Scholar]
- 31.Cagen SZ, Waechter JM, Dimond SS, Breslin WJ, Butala JH, Jekat FW, Joiner RL, Shiotsuka RN, Veenstra GE, Harris LR. Normal reproductive organ development in CF-1 mice following prenatal exposure to bisphenol A. Toxicol Sci. 1999;50:36–44. doi: 10.1093/toxsci/50.1.36. [DOI] [PubMed] [Google Scholar]
- 32.Tinwell H, Haseman J, Lefevre PA, Wallis N, Ashby J. Normal sexual development of two strains of rat exposed in utero to low doses of bisphenol A. Toxicol Sci. 2002;68:339–348. doi: 10.1093/toxsci/68.2.339. [DOI] [PubMed] [Google Scholar]
- 33.Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci. 2002;68:121–146. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- 34.Ashby J, Tinwell H, Lefevre PA, Joiner R, Haseman J. The effect on sperm production in adult Sprague-Dawley rats exposed by gavage to bisphenol A between postnatal days 91–97. Toxicol Sci. 2003;74:129–138. doi: 10.1093/toxsci/kfg093. [DOI] [PubMed] [Google Scholar]
- 35.Cagen SZ, Waechter JM, Dimond SS, Breslin WJ, Butala JH, Jekat FW, Joiner RL, Shiotsuka RN, Veenstra GE, Harris LR. Normal reproductive organ development in Wistar rats exposed to bisphenol A in the drinking water. Regul Toxicol Pharmacol. 1999;30:130–139. doi: 10.1006/rtph.1999.1340. [DOI] [PubMed] [Google Scholar]
- 36.Ashby J, Tinwell H, Haseman J. Lack of effects for low dose levels of bisphenol A and diethylstilbestrol on the prostate gland of CF1 mice exposed in utero. Regul Toxicol Pharmacol. 1999;30:156–166. doi: 10.1006/rtph.1999.1317. [DOI] [PubMed] [Google Scholar]
- 37.Thigpen JE, Haseman JK, Saunders HE, Setchell KD, Grant MG, Forsythe DB. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med. 2003;53:607–615. [PubMed] [Google Scholar]
- 38.Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- 39.Daston GP, Cook JC, Kavlock RJ. Uncertainties for endocrine disrupters: our view on progress. Toxicol Sci. 2003;74:245–252. doi: 10.1093/toxsci/kfg105. [DOI] [PubMed] [Google Scholar]
- 40.Talsness C, Fialkowski O, Gericke C, Merker HJ, Chahoud I. The effects of low and high doses of Bisphenol A on the reproductive system of female and male rat offspring. Congenit Anom. 2000;40:94–107. [Google Scholar]
- 41.Li S. Relationship between cellular DNA synthesis, PCNA expression and sex steroid hormone receptor status in the developing mouse ovary, uterus and oviduct. Histochemistry. 1994;102:405–413. doi: 10.1007/BF00268912. [DOI] [PubMed] [Google Scholar]
- 42.Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol. 1973;56:133–144. doi: 10.1677/joe.0.0560133. [DOI] [PubMed] [Google Scholar]
- 43.Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci USA. 2000;97:5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galand P, Leroy F, Chretien J. Effect of oestradiol on cell proliferation and histological changes in the uterus and vagina of mice. J Endocrinol. 1971;49:243–252. doi: 10.1677/joe.0.0490243. [DOI] [PubMed] [Google Scholar]
- 45.Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod. 2001;65:1311–1323. doi: 10.1095/biolreprod65.5.1311. [DOI] [PubMed] [Google Scholar]
- 46.Sato T, Fukazawa Y, Kojima H, Enari M, Iguchi T, Ohta Y. Apoptotic cell death during the estrous cycle in the rat uterus and vagina. Anat Rec. 1997;248:76–83. doi: 10.1002/(SICI)1097-0185(199705)248:1<76::AID-AR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida A, Newbold RR, Dixon D. Effects of neonatal diethylstilbestrol (DES) exposure on morphology and growth patterns of endometrial epithelial cells in CD-1 mice. Toxicol Pathol. 1999;27:325–333. doi: 10.1177/019262339902700308. [DOI] [PubMed] [Google Scholar]
- 48.Kaufman RH, Noller K, Adam E, Irwin J, Gray M, Jefferies JA, Hilton J. Upper genital tract abnormalities and pregnancy outcome in diethylstilbestrol-exposed progeny. Am J Obstet Gynecol. 1984;148:973–984. doi: 10.1016/0002-9378(84)90540-4. [DOI] [PubMed] [Google Scholar]
- 49.Pillsbury SG., Jr Reproductive significance of changes in the endometrial cavity associated with exposure in utero to diethylstilbestrol. Am J Obstet Gynecol. 1980;137:178–182. doi: 10.1016/0002-9378(80)90772-3. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Baruch G, Menczer J, Mashiach S, Serr DM. Uterine anomalies in diethylstilbestrol-exposed women with fertility disorders. Acta Obstet Gynecol Scand. 1981;60:395–397. doi: 10.3109/00016348109154132. [DOI] [PubMed] [Google Scholar]
- 51.Salle B, Sergeant P, Awada A, Bied-Damon V, Gaucherand P, Boisson C, Guibaud S, Benchaib M, Rudigoz RC. Transvaginal ultrasound studies of vascular and morphological changes in uteri exposed to diethylstilbestrol in utero. Hum Reprod. 1996;11:2531–2536. doi: 10.1093/oxfordjournals.humrep.a019153. [DOI] [PubMed] [Google Scholar]
- 52.Medlock KL, Sheehan DM, Nelson CJ, Branham WS. Effects of postnatal DES treatment on uterine growth, development, and estrogen receptor levels. J Steroid Biochem. 1988;29:527–532. doi: 10.1016/0022-4731(88)90188-4. [DOI] [PubMed] [Google Scholar]
- 53.Medlock KL, Branham WS, Sheehan DM. Long-term effects of postnatal exposure to diethylstilbestrol on uterine estrogen receptor and growth. J Steroid Biochem Mol Biol. 1992;42:23–28. doi: 10.1016/0960-0760(92)90007-6. [DOI] [PubMed] [Google Scholar]
- 54.Branham WS, Zehr DR, Chen JJ, Sheehan DM. Alterations in developing rat uterine cell populations after neonatal exposure to estrogens and antiestrogens. Teratology. 1988;38:271–279. doi: 10.1002/tera.1420380311. [DOI] [PubMed] [Google Scholar]
- 55.Hendry WJ, DeBrot BL, Zheng X, Branham WS, Sheehan DM. Differential activity of diethylstilbestrol versus estradiol as neonatal endocrine disruptors in the female hamster (Mesocricetus auratus) reproductive tract. Biol Reprod. 1999;61:91–100. doi: 10.1095/biolreprod61.1.91. [DOI] [PubMed] [Google Scholar]
- 56.Sheehan DM, Branham WS, Medlock KL, Olson ME, Zehr DR. Uterine responses to estradiol in the neonatal rat. Endocrinology. 1981;109:76–82. doi: 10.1210/endo-109-1-76. [DOI] [PubMed] [Google Scholar]
- 57.Branham WS, Zehr DR, Chen JJ, Sheehan DM. Uterine abnormalities in rats exposed neonatally to diethylstilbestrol, ethynylestradiol, or clomiphene citrate. Toxicology. 1988;51:201–212. doi: 10.1016/0300-483x(88)90150-3. [DOI] [PubMed] [Google Scholar]
- 58.Hendry WJ, III, Zheng X, Leavitt WW, Branham WS, Sheehan DM. Endometrial hyperplasia and apoptosis following neonatal diethylstilbestrol exposure and subsequent estrogen stimulation in both host and transplanted hamster uteri. Cancer Res. 1997;57:1903–1908. [PubMed] [Google Scholar]
- 59.Leavitt WW, Evans RW, Hendry WJ., III Etiology of DES-induced uterine tumors in the Syrian hamster. Adv Exp Med Biol. 1981;138:63–86. doi: 10.1007/978-1-4615-7192-6_4. [DOI] [PubMed] [Google Scholar]
- 60.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- 61.Newbold RR, McLachlan JA. Vaginal adenosis and adenocarcinoma in mice exposed prenatally or neonatally to diethylstilbestrol. Cancer Res. 1982;42:2003–2011. [PubMed] [Google Scholar]
- 62.Ostrander PL, Mills KT, Bern HA. Long-term responses of the mouse uterus to neonatal diethylstilbestrol treatment and to later sex hormone exposure. J Natl Cancer Inst. 1985;74:121–135. [PubMed] [Google Scholar]
- 63.Iguchi T, Takasugi N. Postnatal development of uterine abnormalities in mice exposed to DES in utero. Biol Neonate. 1987;52:97–103. doi: 10.1159/000242690. [DOI] [PubMed] [Google Scholar]
- 64.Hendry WJ, III, Branham WS, Sheehan DM. Diethylstilbestrol vs. estradiol as neonatal disruptors of the hamster (Mesocricetus auratus) cervix. Biol Reprod. 2004;70(05):1306–1316. doi: 10.1095/biolreprod.103.024992. [DOI] [PubMed] [Google Scholar]
- 65.Hyder SM, Chiappetta C, Stancel GM. Synthetic estrogen 17alpha-ethinyl estradiol induces pattern of uterine gene expression similar to endogenous estrogen 17beta-estradiol. J Pharmacol Exp Ther. 1999;290:740–747. [PubMed] [Google Scholar]
- 66.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Couse JF, Korach KS. Contrasting phenotypes in reproductive tissues of female estrogen receptor null mice. Ann NY Acad Sci. 2001;948:1–8. doi: 10.1111/j.1749-6632.2001.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Eriksson H, Sahlin L. Estrogen receptors alpha and beta in the female reproductive tract of the rat during the estrous cycle. Biol Reprod. 2000;63:1331–1340. doi: 10.1095/biolreprod63.5.1331. [DOI] [PubMed] [Google Scholar]
- 70.Friend KE, Ang LW, Shupnik MA. Estrogen regulates the expression of several different estrogen receptor mRNA isoforms in rat pituitary. Proc Natl Acad Sci USA. 1995;92:4367–4371. doi: 10.1073/pnas.92.10.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friend KE, Resnick EM, Ang LW, Shupnik MA. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol Cell Endocrinol. 1997;131:147–155. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 72.Yamashita S, Newbold RR, McLachlan JA, Korach KS. The role of the estrogen receptor in uterine epithelial proliferation and cytodifferentiation in neonatal mice. Endocrinology. 1990;127:2456–2463. doi: 10.1210/endo-127-5-2456. [DOI] [PubMed] [Google Scholar]
- 73.Shupnik MA, Gordon MS, Chin WW. Tissue-specific regulation of rat estrogen receptor mRNAs. Mol Endocrinol. 1989;3:660–665. doi: 10.1210/mend-3-4-660. [DOI] [PubMed] [Google Scholar]
- 74.Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141:4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- 75.Nephew KP, Long X, Osborne E, Burke KA, Ahluwalia A, Bigsby RM. Effect of estradiol on estrogen receptor expression in rat uterine cell types. Biol Reprod. 2000;62:168–177. doi: 10.1095/biolreprod62.1.168. [DOI] [PubMed] [Google Scholar]
- 76.Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 77.Pettersson K, Grandien K, Kuiper GG, Gustafsson JA. Mouse estrogen receptor beta forms estrogen response element-binding heterodimers with estrogen receptor alpha. Mol Endocrinol. 1997;11:1486–1496. doi: 10.1210/mend.11.10.9989. [DOI] [PubMed] [Google Scholar]
- 78.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 79.Kang JS, Lee BJ, Ahn B, Kim DJ, Nam SY, Yun YW, Nam KT, Choi M, Kim HS, Jang DD, et al. Expression of estrogen receptor alpha and beta in the uterus and vagina of immature rats treated with 17-ethinyl estradiol. J Vet Med Sci. 2003;65:1293–1297. doi: 10.1292/jvms.65.1293. [DOI] [PubMed] [Google Scholar]
- 80.Diel P, Schulz T, Smolnikar K, Strunck E, Vollmer G, Michna H. Ability of xeno- and phytoestrogens to modulate expression of estrogen-sensitive genes in rat uterus: estrogenicity profiles and uterotropic activity. J Steroid Biochem Mol Biol. 2000;73:1–10. doi: 10.1016/s0960-0760(00)00051-0. [DOI] [PubMed] [Google Scholar]
- 81.Diel P. Tissue-specific estrogenic response and molecular mechanisms. Toxicol Lett. 2002;127:217–224. doi: 10.1016/s0378-4274(01)00503-3. [DOI] [PubMed] [Google Scholar]