Abstract
Polycomb group (PcG) genes are responsible for maintenance of cellular identity and contribute to regulation of the cell cycle. Recent studies have identified several PcG genes as oncogenes, and a role for PcG proteins in human oncogenesis is suspected. We investigated the expression of BMI-1 and EZH2 PcG oncogenes in human bronchial squamous cell carcinomas (SCCs) and bronchial premalignant precursor lesions (PLs). Whereas normal bronchial epithelium was associated with widespread expression of BMI-1 in resting EZH2-negative cells, neoplastic cells in lung carcinomas displayed altered expression of both BMI-1 and EZH2. Two patterns of abnormal PcG expression were observed: increased expression of BMI-1 in dividing neoplastic cells of PLs and SCCs, and enhanced expression of EZH2 and Ki-67 in BMI-1-positive cells according to severity of the histopathologic stage. We propose that altered expression of BMI-1 and EZH2 is an early event that precedes high rates of proliferation in lung cancer. Because PcG complexes are normally involved in the maintenance of cell characteristics, abnormal PcG expression may contribute to loss of cell identity.
Keywords: Premalignant lesions, BMI-1, EZH2, polycomb genes, lung carcinoma
Introduction
Lung cancer is the leading cause of cancer-related deaths in the western world [1]. It is generally assumed that squamous cell carcinoma (SCC) in the central lung develops in a stepwise manner from normal bronchial epithelium to cancer through the accumulation of (epi)genetic alterations [2]. Our understanding of the carcinogenic process is continuously growing with new genes or classes of genes discovered that are involved in carcinogenesis. Polycomb group (PcG) genes form such a class of genes.
PcG genes have been identified in Drosophila melanogaster as repressors of homeobox genes throughout development [3]. Drosophila PcG genes are evolutionarily conserved and several vertebrate homologues have been identified. PcG proteins form heterogeneous multimeric protein complexes [4]. Two distinct and evolutionary conserved PcG complexes have been identified, consisting of various PcG proteins and non-PcG proteins. The polycomb repressive complex 1 (PRC1) maintenance complex contains the BMI-1, MEL-18, RING1, HPH, and HPC PcG proteins, and the PRC2 initiation complex contains the EZH2, EED, YY1, and SUZ [12] PcG proteins [4–16]. PcG complexes most likely regulate gene activity by epigenetic mechanisms because they are associated with histone deacetylases and methyltransferases involved in chromatin modification [15,17–20]. Although PcG genes are essential for embryogenesis, they continue to contribute to the regulation of various processes in the adult, including cell cycle and hematopoiesis [21–25].
The pivotal role of PcG genes in normal development is underscored by studies of mice with altered PcG gene expression, which indicated that PcG genes are involved in carcinogenesis. For instance, overexpression of Bmi-1 as a transgene results in a high predisposition for B-cell and T-cell lymphomas [26]. Similarly, binding partners of BMI-1 in the PRC1 complex, including RING1 and HPC2, have also been associated with malignant transformation [11,27]. PcG genes most likely contribute to oncogenesis by interfering with cell cycle control mechanisms. Bmi-1 was shown to be an upstream regulator of the INK4a-ARF locus, and Bmi-1 overexpression results in suppression of p16INK4a in various experimental settings [28–30]. Recent work on components of the PRC2 complex showed that EZH2 is capable of providing a proliferative advantage to primary cells [31]. EZH2 is regulated by the pRB-E2F pathway and appears essential for cellular proliferation [31]. Evidence is rapidly increasing that PcG genes are also associated with carcinogenesis in humans. Several studies showed that BMI-1 is overexpressed in malignant lymphomas, resulting in coexpression with EZH2 in cycling neoplastic cells [32–36]. The observation that altered PcG gene expression also occurs in solid tumors, including carcinomas from the lung [37], breast [38–41], prostate [42,43], and colorectum [44], suggests that aberrant PcG expression may be a mechanism of transformation that is shared by various tumors. Given the role of PcG complexes in epigenetic modification of target gene expression, altered expression of PcG genes may contribute to the observed epigenetic modifications that occur in cancer cells (recently reviewed in Ref. [45]).
In the current study, we aimed to find out at what stage the expression patterns of BMI-1 and EZH2 are altered during bronchial carcinogenesis. To that end, expression of these PcG genes was analyzed in bronchial SCC and various stages of premalignant lesions (PLs).
Materials and Methods
Human Tissues
Bronchial carcinomas and adjacent macroscopically normal tissues Neoplastic tissues and adjacent macroscopically normal bronchial tissues were obtained from patients who underwent a surgical resection of a primary SCC of the bronchus at the VU Medical Center. Tissue parts of the tumor and tumor-distant, macroscopically normal bronchial tissues were collected and bisected, after which one part was formalin-fixed and paraffin-embedded, and the other part snap-frozen. We selected seven patients who were not pretreated by chemotherapy or radiotherapy prior to surgery. At the time of surgical resection, patients with bronchial SCC were aged 71 years (median; range 47–76 years). The male:female ratio was 5:2. Consecutive 4-µm sections were cut from formalin-fixed and frozen tissues, for both histopathologic assessment and immunohistochemistry (IHC; formalin-fixed tissue) and immunofluorescence (IF; frozen tissue), respectively. After histologic examination, tumor samples were all classified as moderately or poorly differentiated SCC, and the normal samples as histomorphologically normal.
Premalignant endobronchial lesions Patients without clinically overt cancer who were at risk for developing bronchial carcinoma were investigated using autofluorescence bronchoscopy (AFB; Xillix Technologies Corporation, Richmond, BC, Canada) in combination with conventional white light bronchoscopy (WLB). Indications for performing AFB were suspicion of lung cancer or follow-up of treated cancer in the aerodigestive tract [46]. Paired biopsy materials were obtained from each area, revealing abnormal autofluorescence. Biopsies were formalin-fixed and used for routine histopathology, and snap-frozen in liquid nitrogen for IF. We randomly selected 29 samples of 19 patients, covering the spectrum of endobronchial PLs. At the time biopsies were obtained, patients were aged 63 years old (median; range 40–73 years). The male:female ratio was 16:3. Consecutive sections were stained in hematoxylin and eosin (H&E) and a single pathologist (E. R.) examined the slides to confirm their histopathologic diagnosis. The slides were categorized as follows: normal (N; n = 3), hyperplasia (H; n = 3), squamous metaplasia (SM; n = 2), mild dysplasia (MiD; n = 7), severe dysplasia (SD; n = 13), and carcinoma in situ (CIS; n = 1).
IHC
IHC was performed as described before [32,33,35,36,41,47–49]. Briefly, sections were deparaffinized and rehydrated, and endogenous peroxidase was inhibited. For antigen retrieval, slides were boiled in 0.01 M sodium citrate buffer (pH 6.0) for 15 minutes. Primary antibodies were applied following preincubation with normal swine serum (1/:10 for EZH2), normal rabbit serum (1:50 for BMI-1), and MIB-1/Ki-67) for 10 minutes. For IHC detection of the PcG proteins BMI-1 and EZH2, slides were incubated with, respectively, the 6C9 mouse monoclonal antiserum for 60 minutes at room temperature and the K538 rabbit polyclonal antiserum (1: 800) overnight at 4°C. The primary antibody (Ab) (MIB-1; DAKO, Glostrup, Denmark) was used against MIB-1/Ki-67 (further notified as Ki-67). Secondary antisera used were a biotinylated rabbit antimouse (1:200) or a biotinylated swine antirabbit (1:300). Following incubation in streptavidin-biotin complex/horseradish peroxidase (sABC-HRP) (1:1000), slides were incubated in biotinylated tyramine for 10 minutes to enhance the signal. For the detection of Ki-67, no signal enhancer was used. Incubating the slides in sABC-HRP (1:100) completed the detection. 3,3′-Diaminobenzidine tetrahydrochloride (DAB) was used for light microscopic visualization of the signal. Slides were counterstained in hematoxylin for 40 seconds, dehydrated in methanol, and finally covered. All washing steps of the slides were performed using phosphate-buffered saline (PBS). Slides processed in the same manner, using PBS instead of the primary Ab, were considered as a negative control. A tonsil specimen, in which both BMI-1 and EZH2 are present [49,50], was included as a positive control for the IHC procedure during each experiment. Photographs were taken with a Zeiss Axiophot microscope (Zeiss, Oberkochen, Germany) and digitized using an Agfa Duoscan scanner (Agfa, Mortsel, Belgium).
IF
Immunofluorescent stainings were performed as described before [32,33,35,41,48,50]. Briefly, 4-mm sections were cut from frozen tissues and mounted on poly-l-lysine-coated slides. Sections were fixed in 2% formaldehyde. Following inhibition of endogenous peroxidase, slides were preincubated with PBS containing 5% bovine serum albumin. For the detection of the PcG proteins, BMI-1, EZH2, and Ki-67 slides were incubated, respectively, with 6C9, a mouse IgG2b monoclonal Ab l K358 (1:100), a rabbit polyclonal Ab; and MiB-1 (IgG1) overnight at 4°C. The BMI-1 Ab was detected using biotinylated goat anti-mouse IgG2 antiserum (1:100) followed by rhodamine-thyramine (RT) in ethanol (1:1000). The EZH2 Ab was detected using swine anti-rabbit Ig fluorescein isothiocyanate (FITC) as secondary step, followed by streptavidin Cy3 (1:300). Ki-67 Ab was detected using biotinylated anti-IgG1 (1:100) followed by streptavidin allophycocyanin (1:1000) All washing steps were performed using PBS containing 0.1% saponin. Slides processed in the same manner, using PBS instead of the primary Ab, were considered as negative controls. A tonsil was included as a positive control for the staining procedure during each experiment [50]. After slides were covered, sections were analyzed with a Leica DMR Confocal Laserscan Microscope (Leica, Rijswijk, The Netherlands). Photographs were taken of representative areas of the slide; images were stored digitally at 1024 dpi and processed using Corel Photopaint 8.
Results
IHC on Bronchial SCCs and Adjacent Normal Tissues
We started our analysis by IHC staining of tumor tissues and morphologically normal tissues of tumor distant sites, obtained from seven patients with bronchial SCC. BMI-1, EZH2, and Ki-67 were exclusively detected as nuclear staining of the epithelial cells. Representative examples of BMI-1-positive and EZH2-positive immunostainings are shown in Figures 1 and 2. Normal bronchial epithelia of all seven tumor distant samples showed abundant expression of BMI-1 (Figures 1A and 2B) and sporadic staining for Ki-67 (Figure 2C), suggesting that BMI-1 expression was primarily associated with resting Ki-67-negative cells. Normal epithelium contained sporadic EZH2-positive cells, which were primarily associated with the basal layer (Figure 1B). The corresponding SCC samples revealed staining for Ki-67 in most of the nuclei of neoplastic cells (not shown). Six of the seven SCCs displayed diffuse nuclear staining for BMI-1 in a large proportion of the neoplastic cells. The remaining sample showed a weak nuclear staining for BMI-1 in the neoplastic cells. Furthermore, all except two SCCs showed diffuse clear nuclear staining for EZH2. The two exceptions included one sample with weak but diffuse nuclear EZH2 immunostaining and one sample that was EZH2-immunonegative. In one particular case (Figure 2), the transition from normal bronchial epithelium to invasive carcinoma could be observed. In this sample, expression of BMI-1 was abundant in both normal epithelium and SCC. By contrast, EZH2 and Ki-67 were not detected in the normal bronchial epithelium, whereas EZH2/Ki-67 expression became evident in adjacent, noninvasive dysplastic nuclei, as well as in malignant cells. These staining patterns collectively show that neoplastic cells in SCC display increased expression of EZH2, and that this coincides with the presence of Ki-67. In addition, the immunohistochemical staining patterns suggest that proliferating Ki-67-positive neoplastic cells in SCC have retained expression of BMI-1, and that this PcG gene is coexpressed with EZH2.
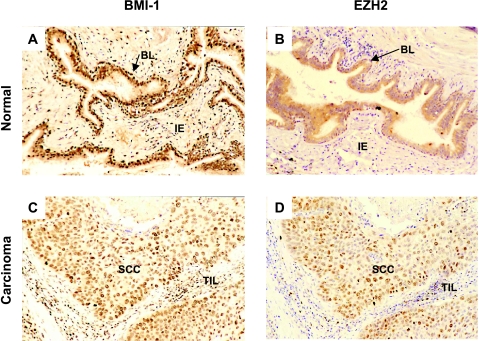
Figure 1.
Expression of BMI-1 and EZH2 in normal bronchial epithelium and bronchial carcinoma. (A and B) Normal bronchial epithelium. (C and D) Bronchial carcinoma. Normal bronchial epithelium displays widespread expression of BMI-1 (A) and sporadic expression of EZH2 in the basal layer (BL). By contrast, both BMI-1 (C) and EZH2 (D) are expressed in the majority of neoplastic cells in bronchial carcinoma. Note that healthy intraepithelial (IE) cells in normal epithelium (A and B) and tumor-infiltrating lymphocytes (TILs) in bronchial carcinoma (C and D) are BMI-1-positive and EZH2-negative.
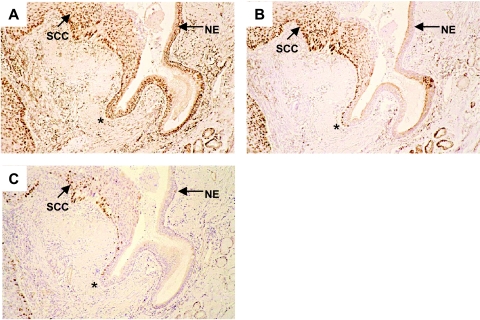
Figure 2.
Expression of BMI-1, EZH2, and Ki-67 at the transition from normal bronchial epithelium to invasive cancer. BMI-1 expression (A) is detected throughout the normal epithelium (NE) and in SCC. By contrast, EZH2 is undetectable in NE but widespread in SCC (B). A minority of the EZH2-positive cells in SCC appear to express the proliferation marker Ki-67 (C). The transition of normal epithelium to SCC is indicated by (*) and coincides with the appearance of EZH2 expression and the continued presence of BMI-1.
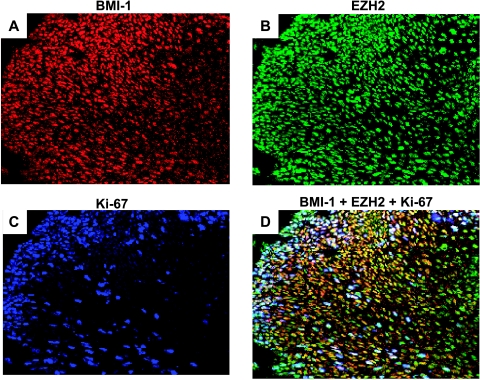
Figure 4.
Expression of BMI-1, EZH2, and Ki-67 in severe dysplasia. Neoplastic cells in severe dysplasia display widespread expression of BMI-1 (A; detected by red immunofluorescence), EZH2 (B; detected by green immunofluorescence), and Ki-67 (C; detected by blue fluorescence). Combination of signals for BMI-1, EZH2, and Ki-67 (D) displays a diverse population of neoplastic cells with extensive fluctuation in gene expression. Note that the majority of the cells coexpresses BMI-1 and EZH2, but that a subfraction of these appears to be in cycle (Ki-67-positive).
Immunofluorescent Staining on Bronchial SCCs and Adjacent Normal Tissues
To confirm the coexpression of BMI-1 and EZH2 in the nuclei of neoplastic cells of bronchial SCCs, IF double stainings for BMI-1 and EZH2 were performed on frozen cryosections that were available in six of the abovementioned SCCs (including five cases that revealed both BMI-1 and EZH2 expression, and the case that was BMI-1-positive but EZH2-negative). The IF staining patterns confirmed the results obtained by IHC: normal bronchial epithelial samples showed widespread BMI-1 expression, whereas EZH2 positivity was rarely observed (Table 1) and not associated with the presence of BMI-1. The IF staining on frozen tumor samples showed colocalization of BMI-1 and EZH2 in the same nuclei in four of six cases that were BMI-1-positive and EZH2-positive by IHC. In one of these cases, EZH2 staining was less intense, which was in accordance with IHC findings of this case. In two of the cases without colocalization, only BMI-1 was detected by IF. In one of those cases, EZH2 was detected by IHC immunostaining but not by IF immunostaining. Representative IF staining patterns for tumor samples are shown in Figure 3. We conclude that expression of BMI-1 and EZH2 are separated in normal bronchial epithelia (Table 1) and that BMI-1-positive neoplastic cells in SCC gain expression of EZH2.
Table 1.
Results of IHC and IF Stainings on SCC and IHC Stainings on Adjacent Normal Tissues.
| Age (years) | Histology | SCC (⊘; cm) | Sex | BMI-1/EZH2 Expression | ||
| IHC in SCC | IF in SCC | IHC and IF in Adjacent Normal Tissues | ||||
| 71 | SCC | 1 | Male | ±/+ | +/- | +/- |
| 67 | SCC | 4.5 | Female | +/+ | +/+ | +/- |
| 71 | SCC | 7 | Male | +/+ | +/+ | +/- |
| 73 | SCC | 4.5 | Male | +/- | +/- | +/- |
| 47 | SCC | 6 | Male | +/+ | +/+ | +/- |
| 48 | SCC | 4 | Female | +/± | +/± | +/- |
| 76 | SCC | 3 | Male | +/+ | ND | +/- |
IHC, immunohistochemistry; IF, immunofluorescence; SCC, squamous cell carcinoma; (+) positive staining; (±) weak staining; (-) negative staining.
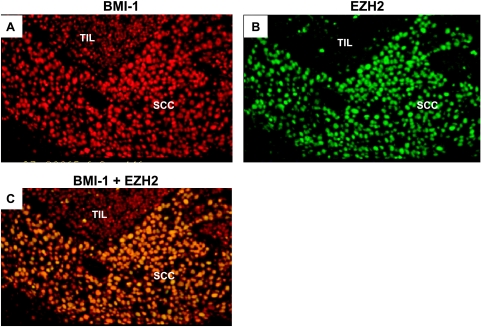
Figure 3.
Co expression of BMI-1 and EZH2 in bronchial carcinoma. Neoplastic cells in bronchial carcinoma (SCC) display widespread expression of BMI-1 (A; detected by red immunofluorescence) and EZH2 (B; detected by green immunofluorescence). Coexpression of BMI-1 and EZH2 in the same nucleus is indicated by yellow fluorescence (C), resulting from combination of the individual signals for BMI-1 and EZH2. Note that TILs are BMI-1-positive and EZH2-negative.
Immunofluorescent Staining on Premalignant Endobronchial Lesions
To determine at what stage in bronchial tumorigenesis the expression of BMI-1 and EZH2 is altered, we performed IF triple stainings for BMI-1 and EZH2 in conjunction with Ki-67 in an additional group of patients at varying stages of the disease (Table 2). The proliferation marker was added to identify the proliferative cell fraction in PLs. The biopsies containing only normal bronchial epithelium (n = 3) showed a similar staining pattern as the tumor distant normal samples described above (i.e., positive staining for BMI-1 and infrequent detection of EZH2-positive cells) and occasional detection of Ki-67 in cells that were negative for BMI-1 and EZH2. Lesions classified as hyperplasia (n = 3) showed positive staining for BMI-1 in two of three cases. In one case, a coexpression with EZH2 was evident, whereas the other two cases revealed absence of EZH2 IF staining. Ki-67 staining was detected only occasionally, and in none of the lesions could colocalization of the PcG proteins with Ki-67 be found. Two lesions that were classified as squamous metaplasia revealed no staining for BMI-1, but one of them was EZH2-positive. Ki-67 staining was detected in the basal cell layer only and revealed no colocalization with EZH2 in the single case that showed EZH2 expression. Five of seven lesions that were classified as mild dysplasia revealed staining for BMI-1, whereas all seven lesions were EZH2-positive. Four of the lesions showed clear BMI-1/EZH2 colocalization. Ki-67 was detected mainly in the lower third of the epithelial cell layer. All seven lesions showed colocalization of EZH2 with Ki-67, whereas a very few cells showed colocalization of BMI-1 and Ki-67 in only three lesions. These lesions revealed triple staining for BMI-1, EZH2, and Ki-67 in the same nuclei. All of the 14 lesions that were classified as severe dysplasia or carcinoma in situ had EZH2 expression, 12 of which also revealed positivity for BMI-1. All these 12 cases showed BMI-1/EZH2 colocalization. Ki-67 could be found throughout the whole epithelial layer in all 14 lesions, although its IF staining was more pronounced in the lower segments of the epithelium. Colocalization of EZH2 with Ki-67 was observed in all lesions, and coexpression of BMI-1 and Ki-67 in the same nuclei was found in 10 of 14 lesions. If lesions showed colocalization of BMI-1 with Ki-67, EZH2 was also expressed in the same nuclei. A representative example is shown in Figure 4. We conclude that EZH2 overexpression occurs at the level of PLs, which, in combination with disability to downregulate BMI-1, leads to coexpression. Furthermore, EZH2 expression appears to precede increased proliferation in PLs.
Table 2.
Results of IF Stainings on Biopsies of Histomorphologically Normal Bronchial Epithelial Samples and Premalignant Endobronchial Lesions (Positive Lesions Per Histopathologic Diagnosis).
| Histology | BMI-1 | EZH2 | BMI-1 x EZH2 | EZH2 x Ki-67 | BMI-1 x MIB | BMI-1 x EZH2 x Ki-67 |
| N (n = 3) | 3/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| H (n = 3) | 2/3 | 1/3 | 1/3 | 0/3 | 0/3 | 0/3 |
| SM (n = 2) | 0/2 | 1/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| MiD (n = 7) | 5/7 | 7/7 | 4/7 | 7/7 | 3/7 | 3/7 |
| SD/CIS (n = 14) | 12/14 | 14/14 | 12/14 | 14/14 | 10/14 | 10/14 |
N, normal; H, hyperplasia; SM, squamous metaplasia; MiD, mild dysplasia; SD/CIS, severe dysplasia and/or carcinoma in situ.
Discussion
PcG genes play a key role in maintenance of cell identity, and contribute to processes such as regulation of the cell cycle and hematopoiesis. Their essential function in normal cellular development is reflected by the fact that abnormal PcG gene expression is linked to carcinogenesis. In the current paper, we analyzed the expression of the BMI-1 and EZH2 PcG oncogenes, representing the PRC1 PcG and PRC2 PcG complex, respectively. We discovered that bronchial SCCs and their PLs are associated with two altered patterns of PcG expression: the presence of BMI-1 protein in dividing neoplastic cells, and increased expression of EZH2 resulting in coexpression with BMI-1.
BMI-1 is abnormally expressed in neoplastic cells of bronchial SCCs and PLs. Analysis of histologically normal bronchial epithelium showed abundant expression of BMI-1 in epithelial cells, and that BMI-1 was primarily expressed in resting Ki-67-negative cells. This pattern is reminiscent of earlier studies of lymphocytes [48–50] and breast epithelia [41], where resting cells preferentially express BMI-1. By contrast, we found that cycling neoplastic cells in PL and SCCs display an increasing frequency of BMI-1 expression: in 43% of the mild dysplasias and 71% of the severe dysplasias, BMI-1 was detected in proliferating cells. Given the association of BMI-1 with resting cells in the normal epithelium, we interpret this expression pattern as abnormal. Experimental model systems convincingly demonstrated that overexpression of BMI-1 is strongly associated with increased cell proliferation and malignant transformation through suppression of p16INK4a and upregulation of telomerase [28,29,38]. Our study fits well with abnormal BMI-1 expression patterns in lymphoid malignancies [32–36], breast carcinoma [38,41], and colorectal carcinoma [44], and supports the earlier observations that development of lung carcinomas is associated with BMI-1 overexpression and loss of p16INK4a expression [37]. The presence of BMI-1 in PLs suggests that BMI-1 expression in proliferating neoplastic cells is an early event in lung carcinogenesis.
Development of lung cancer is associated with enhanced expression of the EZH2 PcG gene. In addition to the abnormal expression pattern of BMI-1 in cycling neoplastic cells, we also found an increased expression of the EZH2 PcG gene in bronchial SCC and its PLs. Whereas EZH2 expression was rare in normal bronchial epithelia, EZH2 was present in nonproliferating and cycling cells of all mildly—and severely—dysplastic lesions. These findings are in line with a recent study by Bracken et al. [31], who demonstrated that EZH2 expression is moderately to strongly enhanced in over 30% of primary human tumors. We found that the proportion of EZH2-positive cells in proliferating cell fraction increased with the severity of the histopathologic stage, and that the majority of bronchial SCCs showed expression of EZH2. Increased expression of EZH2 appears to be an early event because EZH2 was detected in one hyperplasia and one squamous metaplasia, and became predominantly manifest in dysplastic tissues. EZH2 expression resulted in coexpression with the BMI-1 PcG gene in neoplastic cells—a pattern that was not observed in normal epithelia. These observations collectively suggest that EZH2 expression in BMI-1-positive neoplastic cells is associated with the development of lung cancer. Although EZH2 expression is linked to a variety of human epithelial tumors [31,40,42,43,51], the mechanism responsible for enhanced expression of EZH2 remains ill defined. EZH2 appears to be controlled by the pRB pathway [31], which is frequently deregulated in human tumors [52]. An additional possible mechanism that may contribute to deregulation of EZH2 expression is amplification of the EZH2 gene, which was recently reported in up to 15% of human cancers [31].
The question remains as to whether induction of EZH2 is responsible for cellular transformation, or whether it is mainly reflective of increased cell division. Current evidence supports both conclusions. Previous studies of lymphoid development demonstrated that EZH2 expression is primarily associated with cycling normal lymphocytes and proliferating neoplastic cells [32–36,48–50]. A direct link between EZH2 expression and proliferation is further supported by experimental suppression of EZH2, which resulted in diminished proliferation of cell lines [31], whereas overexpression of EZH2 resulted in enhanced proliferation rates [34]. In the current work, we found that the EZH2 expression pattern of histologically normal epithelia did not necessarily overlap with Ki-67, and that a considerable proportion of nuclei in PLs expressed EZH2 in the absence of Ki-67. These observations suggest that EZH2 expression in PLs precedes the induction of proliferation during bronchial carcinogenesis, as was recently observed in PLs of breast carcinomas [41]. To what extent EZH2 expression is directly linked to cellular transformation cannot be determined by the current study, but the recent identification of EZH2 as a bona fide oncogene suggests that EZH2 may directly contribute to lung carcinogenesis and loss of cell identity [31]. Aside from being essential for proliferation, our current work also suggests that EZH2 expression is related to differentiation because EZH2 was detected at the transition from normal and hyperplastic epithelium (both consisting of cylindrical type of epithelium) to squamous metaplasia. Interestingly, enhanced expression of EZH2 in breast carcinomas is primarily seen in ductal carcinoma in situ (DCIS) and carcinomas of poor differentiation [41]. In the present study, all bronchial SCCs were moderately or poorly differentiated, possibly explaining the high proportion of SCCs with frequent EZH2 expression. Functional and follow-up studies are needed to elucidate the precise role of EZH2 and other PcG genes in bronchial carcinogenesis.
In conclusion, we showed that neoplastic cells in lung carcinomas display altered expression of the BMI-1 and EZH2 PcG genes. We propose that altered expressions of BMI-1 and EZH2 are early events that precede high rates of proliferation. Because PcG complexes are normally involved in the maintenance of cell characteristics, abnormal expression of PcG genes may contribute to loss of cell identity. Several members of the PcG gene family have been identified as oncogenes, and recent studies in humans demonstrated that hematologic malignancies and solid tumors are all linked to altered PcG gene expression. Collectively, these studies strongly suggest that altered expression of PcG genes is an important contributing factor in the development of neoplasms in humans.
Acknowledgements
We thank Tjasso Blokzijl and Elly Fieret for expert technological assistance during immunological staining, and other members of the VUMC Polycomb research group for stimulating discussions.
Abbreviations
- PcG
polycomb group
- PRC
polycomb repressive complex
Footnotes
This study was supported by NKB grant no. VU-1748.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Rom WN, Hay JG, Lee TC, Jiang Y, Tchou-Wong KM. Molecular and genetic aspects of lung cancer. Am J Respir Crit Care Med. 2000;161:1355–1367. doi: 10.1164/ajrccm.161.4.9908012. [DOI] [PubMed] [Google Scholar]
- 3.Pirrotta V. Polycomb silencing and the maintenance of stable chromatin states. Results Probl Cell Differ. 1999;25:205–228. doi: 10.1007/978-3-540-69111-2_10. [DOI] [PubMed] [Google Scholar]
- 4.Otte AP, Kwaks TH. Gene repression by Polycomb group protein complexes: a distinct complex for every occasion? Curr Opin Genet Dev. 2003;13:448–454. doi: 10.1016/s0959-437x(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 5.Schoorlemmer J, Marcos-Gutierrez C, Were F, Martinez R, Garcia E, Satijn DP, Otte AP, Vidal M. Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 1997;16:5930–5942. doi: 10.1093/emboj/16.19.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satijn DP, Gunster MJ, van der Vlag J, Hamer KM, Schul W, Alkema MJ, Saurin AJ, Freemont PS, van Driel R, Otte AP. RING1 is associated with the polycomb group protein complex and acts as a transcriptional repressor. Mol Cell Biol. 1997;17:4105–4113. doi: 10.1128/mcb.17.7.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunster MJ, Satijn DP, Hamer KM, den Blaauwen JL, de Bruijn D, Alkema MJ, van Lohuizen M, van Driel R, Otte AP. Identification and characterization of interactions between the vertebrate polycomb-group protein BMI1 and human homologs of polyhomeotic. Mol Cell Biol. 1997;17:2326–2335. doi: 10.1128/mcb.17.4.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkema MJ, Bronk M, Verhoeven E, Otte A, van't Veer LJ, Berns A, van Lohuizen M. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian polycomb complex. Genes Dev. 1997;11:226–240. doi: 10.1101/gad.11.2.226. [DOI] [PubMed] [Google Scholar]
- 9.van Lohuizen M, Tijms M, Voncken JW, Schumacher A, Magnuson T, Wientjens E. Interaction of mouse polycomb-group (Pc-G) proteins Enx1 and Enx2 with Eed: indication for separate Pc-G complexes. Mol Cell Biol. 1998;18:3572–3579. doi: 10.1128/mcb.18.6.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto N, Brock HW, Nomura M, Kyba M, Hodgson J, Fujita Y, Takihara Y, Shimada K, Higashinakagawa T. RAE28, BMI1, and M33 are members of heterogeneous multimeric mammalian Polycomb group complexes. Biochem Biophys Res Commun. 1998;245:356–365. doi: 10.1006/bbrc.1998.8438. [DOI] [PubMed] [Google Scholar]
- 11.Satijn DP, Otte AP. RING1 interacts with multiple Polycombgroup proteins and displays tumorigenic activity. Mol Cell Biol. 1999;19:57–68. doi: 10.1128/mcb.19.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardos JI, Saurin AJ, Tissot C, Duprez E, Freemont PS. HPC3 is a new human polycomb orthologue that interacts and associates with RING1 and Bmi1 and has transcriptional repression properties. J Biol Chem. 2000;275:28785–28792. doi: 10.1074/jbc.M001835200. [DOI] [PubMed] [Google Scholar]
- 13.Ng J, Hart CM, Morgan K, Simon JA. A Drosophila ESC-E(Z) protein complex is distinct from other polycomb group complexes and contains covalently modified ESC. Mol Cell Biol. 2000;20:3069–3078. doi: 10.1128/mcb.20.9.3069-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satijn DP, Hamer KM, den Blaauwen J, Otte AP. The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos. Mol Cell Biol. 2001;21:1360–1369. doi: 10.1128/MCB.21.4.1360-1369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tie F, Furuyama T, Prasad-Sinha J, Jane E, Harte PJ. The Drosophila Polycomb Group proteins ESC and E(Z) are present in a complex containing the histone-binding protein p55 and the histone deacetylase RPD3. Development. 2001;128:275–286. doi: 10.1242/dev.128.2.275. [DOI] [PubMed] [Google Scholar]
- 16.Furuyama T, Tie F, Harte PJ. Polycomb group proteins ESC and E(Z) are present in multiple distinct complexes that undergo dynamic changes during development. Genesis. 2003;35:114–124. doi: 10.1002/gene.10173. [DOI] [PubMed] [Google Scholar]
- 17.van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nat Genet. 1999;23:474–478. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- 18.Sewalt RG, Lachner M, Vargas M, Hamer KM, den Blaauwen JL, Hendrix T, Melcher M, Schweizer D, Jenuwein T, Otte AP. Selective interactions between vertebrate polycomb homologs and the SUV39H1 histone lysine methyltransferase suggest that histone H3-K9 methylation contributes to chromosomal targeting of polycomb group proteins. Mol Cell Biol. 2002;22:5539–5553. doi: 10.1128/MCB.22.15.5539-5553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal polycomb sites. Cell. 2002;111:185–196. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 20.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 21.Caldas C, Aparicio S. Cell memory and cancer—the story of the trithorax and Polycomb group genes. Cancer Metastasis Rev. 1999;18:313–329. doi: 10.1023/a:1006333610078. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs JJ, van Lohuizen M. Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta. 2002;1602:151–161. doi: 10.1016/s0304-419x(02)00052-5. [DOI] [PubMed] [Google Scholar]
- 23.van Lohuizen M. Functional analysis of mouse Polycomb group genes. Cell Mol Life Sci. 1998;54:71–79. doi: 10.1007/s000180050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raaphorst FM, Otte AP, Meijer CJ. Polycomb-group genes as regulators of mammalian lymphopoiesis. Trends Immunol. 2001;22:682–690. doi: 10.1016/s1471-4906(01)02082-8. [DOI] [PubMed] [Google Scholar]
- 25.Lessard J, Sauvageau G. Polycomb group genes as epigenetic regulators of normal and leukemic hemopoiesis. Exp Hematol. 2003;31:567–585. doi: 10.1016/s0301-472x(03)00081-x. [DOI] [PubMed] [Google Scholar]
- 26.Alkema MJ, Jacobs H, van Lohuizen M, Berns A. Perturbation of B and T cell development and predisposition to lymphomagenesis in Emu Bmi1 transgenic mice require the Bmi1 RING finger. Oncogene. 1997;15:899–910. doi: 10.1038/sj.onc.1201262. [DOI] [PubMed] [Google Scholar]
- 27.Satijn DP, Olson DJ, van der Vlag J, Hamer KM, Lambrechts C, Masselink H, Gunster MJ, Sewalt RG, van Driel R, Otte AP. Interference with the expression of a novel human polycomb protein, hPc2, results in cellular transformation and apoptosis. Mol Cell Biol. 1997;17:6076–6086. doi: 10.1128/mcb.17.10.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13:2678–2690. doi: 10.1101/gad.13.20.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 30.Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, van Lohuizen M, Band V, Campisi J, Dimri GP. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raaphorst FM, van Kemenade FJ, Blokzijl T, Fieret E, Hamer KM, Satijn DP, Otte AP, Meijer CJ. Coexpression of BMI-1 and EZH2 polycomb group genes in Reed-Sternberg cells of Hodgkin's disease. Am J Pathol. 2000;157:709–715. doi: 10.1016/S0002-9440(10)64583-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Kemenade FJ, Raaphorst FM, Blokzijl T, Fieret E, Hamer KM, Satijn DP, Otte AP, Meijer CJ. Coexpression of BMI-1 and EZH2 polycomb-group proteins is associated with cycling cells and degree of malignancy in B-cell non-Hodgkin lymphoma. Blood. 2001;97:3896–3901. doi: 10.1182/blood.v97.12.3896. [DOI] [PubMed] [Google Scholar]
- 34.Visser HP, Gunster MJ, Kluin-Nelemans HC, Manders EM, Raaphorst FM, Meijer CJ, Willemze R, Otte AP. The Polycomb group protein EZH2 is upregulated in proliferating, cultured human mantle cell lymphoma. Br J Haematol. 2001;112:950–958. doi: 10.1046/j.1365-2141.2001.02641.x. [DOI] [PubMed] [Google Scholar]
- 35.Raaphorst FM, Vermeer M, Fieret E, Blokzijl T, Dukers D, Sewalt RG, Otte AP, Willemze R, Meijer CJ. Site-specific expression of polycomb-group genes encoding the HPC-HPH/PRC1 complex in clinically defined primary nodal and cutaneous large B-cell lymphomas. Am J Pathol. 2004;164:533–542. doi: 10.1016/S0002-9440(10)63143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dukers DF, Van Galen JC, Giroth C, Jansen P, Sewalt RG, Otte AP, Kluin-Nelemans HC, Meijer CJ, Raaphorst FM. Unique polycomb gene expression pattern in Hodgkin's lymphoma and Hodgkin's lymphoma-derived cell lines. Am J Pathol. 2004;164:873–881. doi: 10.1016/S0002-9440(10)63175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vonlanthen S, Heighway J, Altermatt HJ, Gugger M, Kappeler A, Borner MM, Lohuizen M, Betticher DC. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer. 2001;84:1372–1376. doi: 10.1054/bjoc.2001.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimri GP, Martinez JL, Jacobs JJ, Keblusek P, Itahana K, van Lohuizen M, Campisi J, Wazer DE, Band V. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002;62:4736–4745. [PubMed] [Google Scholar]
- 39.Matsuo F, Yano K, Saito H, Morotomi K, Kato M, Yoshimoto M, Kasumi F, Akiyama F, Sakamoto G, Miki Y. Mutation analysis of the mel-18 gene that shows decreased expression in human breast cancer cell lines. Breast Cancer. 2002;9:33–38. doi: 10.1007/BF02967544. [DOI] [PubMed] [Google Scholar]
- 40.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci USA. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raaphorst FM, Meijer CJLM, Fieret E, Blokzijl T, Mommers E, Buerger H, Packeisen J, Sewalt RGAB, Otte AP, van Diest PJ. Poorly differentiated breast carcinoma is associated with increased expression of the human Polycomb group gene EZH2. Neoplasia. 2003;5:481–488. doi: 10.1016/s1476-5586(03)80032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 43.LaTulippe E, Satagopan J, Smith A, Scher H, Scardino P, Reuter V, Gerald WL. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res. 2002;62:4499–4506. [PubMed] [Google Scholar]
- 44.Kim JH, Yoon SY, Kim CN, Joo JH, Moon SK, Choe IS, Choe YK, Kim JW. The Bmi-1 oncoprotein is overexpressed in human colorectal cancer and correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett. 2004;203:217–224. doi: 10.1016/j.canlet.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 45.Witsuba II, Gazdar AF. Characteristic genetic alterations in lung cancer. Methods Mol Med. 2003;74:3–28. doi: 10.1385/1-59259-323-2:03. [DOI] [PubMed] [Google Scholar]
- 46.Sutedja TG, Venmans BJ, Smit EF, Postmus PE. Fluorescence bronchoscopy for early detection of lung cancer: a clinical perspective. Lung Cancer. 2001;34:157–168. doi: 10.1016/s0169-5002(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 47.Ferreux E, Lont AP, Horenblas S, Gallee MP, Raaphorst FM, Von Knebel DM, Meijer CJ, Snijders PJ. Evidence for at least three alternative mechanisms targeting the p16INK4A/cyclin D/Rb pathway in penile carcinoma, one of which is mediated by high-risk human papillomavirus. J Pathol. 2003;201:109–118. doi: 10.1002/path.1394. [DOI] [PubMed] [Google Scholar]
- 48.Raaphorst FM, Otte AP, van Kemenade FJ, Blokzijl T, Fieret E, Hamer KM, Satijn DP, Meijer CJ. Distinct bmi-1 and ezh2 expression patterns in thymocytes and mature T cells suggest a role for polycomb genes in human T cell differentiation. J Immunol. 2001;166:5925–5934. doi: 10.4049/jimmunol.166.10.5925. [DOI] [PubMed] [Google Scholar]
- 49.Van Galen JC, Dukers DF, Giroth C, Sewalt RG, Otte AP, Meijer CJ, Raaphorst FM. Distinct expression patterns of polycomb oncoproteins and their binding partners during the germinal center reaction. Eur J Immunol. 2004;34:1870–1881. doi: 10.1002/eji.200424985. [DOI] [PubMed] [Google Scholar]
- 50.Raaphorst FM, van Kemenade FJ, Fieret E, Hamer KM, Satijn DP, Otte AP, Meijer CJ. Cutting edge: polycomb gene expression patterns reflect distinct B cell differentiation stages in human germinal centers. J Immunol. 2000;164:1–4. doi: 10.4049/jimmunol.164.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Raaphorst FM, Meijer CJ, Fieret E, Blokzijl T, Mommers E, Buerger H, Packeisen J, Sewalt RA, Otte AP, van Diest PJ. Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia. 2003;5:481–488. doi: 10.1016/s1476-5586(03)80032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamasaki L. Role of the RB tumor suppressor in cancer. Cancer Treat Res. 2003;115:209–239. doi: 10.1007/0-306-48158-8_9. [DOI] [PubMed] [Google Scholar]