Abstract
Pseudomonas aeruginosa is a gram-negative facultative opportunistic pathogen associated with severe infections in immunocompromised hosts and in patients with cystic fibrosis. P. aeruginosa strains show divergent pathogenicity in vivo and trigger apoptosis of and/or are internalized into human host cells. In the present study, we studied the molecular ordering of apoptosis signaling upon infection of human conjunctiva epithelial Chang cells with P. aeruginosa PAK as well as the role of bacterial pili in the response to the infection. Our results show that CD95 up-regulation is followed by early activation of caspase-8 and -3 and cleavage of the caspase-3 substrate poly(ADP-ribose) polymerase. The data also demonstrate release of apoptosis inducing factor into the cytosol of infected cells. Induction of mitochondrial alterations, i.e., mitochondrial depolarization and release of cytochrome c, as well as cleavage of caspase-9, -7, and -1 occurred only at later time points. In addition, our results demonstrate that pili are required for P. aeruginosa-induced apoptosis of human epithelial cells. While the two piliated P. aeruginosa strains, PAO-I and PAK, induced apoptosis of Chang cells within 3 h of infection, the pilus-deficient P. aeruginosa mutants PAKΔpilA and PAKΔpilAΔall were without effect. The pilus-deficient mutants failed to induce a significant up-regulation of CD95 on the cell surface and to trigger mitochondrial alterations or activation of caspase-8, -3, and -7. In addition, only the piliated wild-type strains induced caspase-1-mediated activation of interleukin-1β. Thus, pili are necessary for distinct infection-induced cellular responses of human epithelial cells.
Pseudomonas aeruginosa is a gram-negative facultative opportunistic pathogen which causes serious infections in hospitalized patients. After surgical resection or burn injury, localized infections can result in generalized and often fatal bacteremia. Patients with cystic fibrosis are chronically colonized with P. aeruginosa and very often develop life-threatening bronchiolar infections without bacteremia.
Infection of mammalian cells with P. aeruginosa has been shown to result in the internalization of P. aeruginosa, to trigger apoptotic, oncotic, or necrotic death of the infected cells, and finally, to induce the release of several pro-inflammatory mediators, in particular interleukin-1, IL-6, IL-8 and IL-10 (2, 10, 11, 13, 20-22, 30, 56, 67). Both cell-associated and extracellular products contribute to the pathogenicity of P. aeruginosa (41, 63, 66). Binding of P. aeruginosa to mammalian cells is mediated via bacterial lipopolysaccharide (LPS), alginate, flagella, and pili (16, 28, 53, 66). Type IV pili are long fibers that extend from the bacterial surface and are composed of a single structural protein, pilin. Pilin is encoded by the pilA gene on the pil operon (44). Pili mediate P. aeruginosa adherence to and invasion into mammalian cells (5, 8, 12, 15, 16, 49, 53). Furthermore, P. aeruginosa type IV pili are important for twitching motility and the proper operation of the type III secretion system and thus contribute to the virulence of P. aeruginosa (4, 8, 16, 26, 35, 36, 51). Consistent with those observations, pilus-deficient mutants were shown to display decreased adhesion to and invasion into mammalian cells (5) as well as decreased virulence in vitro and in animal models (7, 15, 63).
Although invasion and cytotoxicity of P. aeruginosa both depend on bacterial adherence, they constitute independent events that are mutually exclusive (14). The presence of a type III secretion system and the expression of several bacterial factors, in particular the exotoxins ExoS, ExoT, and ExoU, are required either for bacterial cytotoxicity or for the regulation of bacterial invasion (9, 18-20, 22, 23, 30). In mammalian conjunctiva epithelial Chang cells, P. aeruginosa-triggered apoptosis is dependent on a type III secretion-mediated up-regulation of CD95 and CD95 ligand on infected cells (25, 32). Signaling events induced by P. aeruginosa downstream of CD95 up-regulation included JNK activation and mitochondrial alterations. CD95 activation and apoptosis of lung epithelial cells were shown to be essential for host defense of mice against nasal infections with P. aeruginosa (25).
Generally, apoptosis is the result of a complex intracellular proteolysis mediated by a defined group of intracellular proteases known as caspases, leading to cleavage of important cellular substrates and thus to a more or less uniform execution of apoptosis (61). Upon triggering of death receptors such as CD95, procaspase-8 is recruited to a death-inducing signaling complex composed of the clustered receptors, the adapter molecule FADD, and procaspase-8 (6, 47, 48, 54). In this complex, procaspase-8 undergoes autoproteolytic processing and triggers the execution of apoptotic cell death through activation of caspase-3 (54). In contrast, death receptor-independent apoptosis pathways are mostly executed by a direct activation of the mitochondria with breakdown of the mitochondrial membrane potential and release of proapoptotic proteins (i.e., cytochrome c and apoptosis inducing factor [AIF]), leading to activation of caspase-9 as the initiator caspase (40, 43, 62). Similar to caspase-8, caspase-9 can activate all downstream effector caspases (17, 27, 70).
The aim of the present study was to characterize the molecular ordering of apoptosis pathways of human Chang epithelial cells upon infection with P. aeruginosa strains PAK and PAO-I and to evaluate the role of type IV pili in this process. We were able to define a signaling cascade with early activation of caspase-8 and -3 upstream of the mitochondria and late activation of caspase-9 and -7. In addition, we identified the release of AIF and caspase-1-mediated cleavage of IL-1β as novel molecular mechanisms involved in the cellular response to P. aeruginosa. Pilus-deficient mutants of P. aeruginosa, PAKΔpilA and PAKΔpilAΔall, failed to trigger apoptosis, to significantly up-regulate CD95, to activate caspases, and to induce mitochondrial alterations typical for apoptosis.
MATERIALS AND METHODS
Chemicals.
Dihydroethidine (DHE) and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanineiodide (JC1) were from Molecular Probes (Mobitec, Goettingen, Germany). The proton shuttle carbonyl cyanide m-chlorophenylhydrazone was purchased from Sigma and dissolved in dimethyl sulfoxide. Anti-CD95 (clone CH11) was from Upstate (Biomol, Hamburg, Germany), mouse anti-cytochrome c (clone 7H8 2C12) was from Pharmingen (Becton Dickinson, Heidelberg, Germany), rabbit anti-AIF (clone H-300), rabbit anti-caspase-1, rabbit anti-IL-1β, and alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) were from Santa Cruz Biotechnology (Heidelberg, Germany), and rabbit anti-cleaved caspase-7, rabbit anti-cleaved caspase-3, and rabbit anti-cleaved caspase-9 were from Cell Signaling Technology (New England BioLabs, Frankfurt, Germany). Mouse monoclonal anti-caspase-8 antibody was obtained from BioCheck (Münster, Germany) and used as previously described (3). All other chemicals were from Sigma-Aldrich, if not otherwise indicated.
Bacteria and cells.
The human conjunctiva epithelial cell line Chang was purchased from the American Type Culture Collection (Manassas, Va.). Chang cells were cultured in RPMI 1640 medium supplemented with 2 mM glutamine and 5% heat-inactivated fetal calf serum (FCS; Life Technologies, Karlsruhe, Germany). Cells were grown as monolayers in tissue culture flasks in a humidified atmosphere (5% CO2-95% air) at 37°C.
P. aeruginosa strain PAO-I was kindly provided by S. M. J. Fleiszig (University of California, Berkeley), P. aeruginosa strains PAK, PAKΔpilA (PAKΔpilArif1), and PAKΔpilAΔall (PAKΔpilAΔxcpP-Z) were generated by D. Nunn (34) and kindly provided by S. Lory (Department of Microbiology and Molecular Genetics, Harvard Medical School, Boston, Mass.) (Table 1). The type III secretion-negative mutant of P. aeruginosa PAK pcrD::Smr was kindly provided by J. Heesemann (Max von Pettenkofer-Institut, Munich, Germany) (31).
TABLE 1.
Bacterial strains used in the study
| P. aeruginosa strain | Relevant characteristics | Source (reference) |
|---|---|---|
| PAO-I | Laboratory strain, wild type | S. M. J. Fleiszig |
| PAK | Laboratory strain, wild type | S. Lory |
| PAKΔpilA | PAKΔpilArif1; prototrophic PAK strain, rifampin resistant, deletion of the pilA structural gene, export competent | S. Lory (34) |
| PAKΔpilAΔall | PAKΔpilAΔxcpP-Z; derivative of PAKΔpilArif1, deletion of the pilA structural gene and of xcpP-Z sequences, export deficient | S. Lory (34) |
| PAKpcrD::Smr | Type III secretion-deficient mutant | J. Heesemann (31) |
To confirm functional inactivity of pili in the mutant strains, we compared adhesion of the P. aeruginosa strains to and invasion into human conjunctiva Chang epithelial cells by gentamicin survival assays (24). Adhesion of the pilus-deficient mutants P. aeruginosa PAKΔpilA and PAKΔpilAΔall was nearly absent when compared with that of the piliated wild-type P. aeruginosa strains PAO-I and PAK. Similarly, only the piliated P. aeruginosa strains were internalized while the pilus-deficient PAK mutants failed to invade these cells (data not shown). However, even for the wild-type strains, internalization was an inefficient process that averaged one (PAK) to three (PAO-I) bacteria internalized into 80 Chang cells within 30 min.
Infection experiments.
Chang cells were seeded in RPMI 1640 medium supplemented with 2 mM l-glutamine and 5% FCS 24 h prior to infection at cell numbers yielding subconfluent cell layers for the infection experiments. Prior to infection, cells were washed twice in RPMI 1640 with 2 mM l-glutamine and maintained in the same medium during the infection. Bacteria were grown overnight at 37°C on tryptic soy agar plates, resuspended in tryptic soy broth (Difco Laboratories, Detroit, Mich.) to an optical density of 0.25, shaken at 130 rpm for 1 h at 37°C to reach the early logarithmic phase, pelleted by centrifugation, and resuspended in fresh tryptic soy broth. For infection periods longer than 90 min, medium was supplemented with 1% heat-inactivated FCS. Infection was started by inoculation of cells at a bacterium/host cell ratio of 1,000:1. Synchronous infection conditions and an enhanced bacterium-host cell interaction was achieved by a 2-min centrifugation (35 × g) step. The end of the centrifugation was defined as the start point in all experiments. Lower bacterium-to-host cell ratios (100:1 and 10:1) also resulted in apoptosis of the host cells but with slower kinetics. After the indicated infection time, cells were collected by using cell dissociation solution (Sigma-Aldrich) or accutase (PAA, Coelbe, Germany).
Quantification of apoptosis.
Cells were washed twice in 10 mM HEPES (pH 7.4), 140 mM NaCl, and 5 mM CaCl2, resuspended at a concentration of 2 × 105 cells/200 μl in the same buffer supplemented with fluorescein isothiocyanate (FITC)-annexin V (1:200 diluted; Roche Biochemicals, Mannheim, Germany), incubated for 15 min, and subjected to fluorescence-activated cell sorter (FACS) analysis with a FACSCalibur flow cytometer and CELLQuest software (Becton Dickinson).
Release of mitochondrial proteins.
Cells were incubated for 30 min on ice in 50 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)-KOH (pH 7.4), 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, 10 μM cytochalasin B, and 100 μM (each) aprotinin and leupeptin. After Dounce homogenization, samples were centrifuged at 15,000 × g for 15 min. The supernatants were added to 5× reducing sodium dodecyl sulfate (SDS) sample buffer (60 mM Tris-HCl [pH 6.8], 2.3% SDS, 10% glycerol, and 5% β-mercaptoethanol) and boiled for 5 min at 95°C, and aliquots of the cytosolic fraction corresponding to 20 μg of total protein were subjected to SDS-15% polyacrylamide gel electrophoresis. Proteins were transferred onto nitrocellulose membranes (Hybond; Amersham Pharmacia Biotech, Freiburg, Germany), the membranes were blocked for 1 h with 4% bovine serum albumin, and cytochrome c was detected by incubation with a monoclonal mouse anti-cytochrome c antibody (clone 7H8 2C12; Pharmingen) or a rabbit anti-AIF antibody followed by alkaline phosphatase-conjugated goat anti-mouse IgG and development with the Tropix-enhanced chemiluminescence detection system (PE Applied Biosystems, Bedford, Mass.). Equal protein loading was controlled by Ponceau red staining of the blot membranes.
Assessment of mitochondrial function.
For determination of mitochondrial membrane potential, cells were washed, resuspended in complete RPMI medium, and loaded with the cationic lipophilic fluorochrome JC1 (2.5 μg/ml) for 10 min at 37°C. Cells were washed twice with phosphate-buffered saline (PBS) and submitted to FACS analysis. The red fluorescence of JC1 aggregates corresponds to the mitochondrial membrane potential, whereas the green fluorescence of JC1 monomers is indicative of the mitochondrial mass.
The production of reactive oxygen species was analyzed by staining the cells with DHE. To this end, PBS-washed cells were incubated with DHE (5 μM) for 30 min at 37°C, washed, resuspended at 1 × 106/ml in PBS, and submitted to FACS analysis.
Forward and side scatter were used to establish size gates and to exclude bacteria and cellular debris from the analysis.
CD95 translocation.
Cells were harvested by using cell dissociation solution supplemented with 10 mM 1,10 phenanthroline (Sigma-Aldrich). Cells were washed twice with PBS containing 10 mM 1,10 phenanthroline and resuspended in 132 mM NaCl, 1 mM CaCl2, 0.7 mM MgCl2, 20 mM HEPES (pH 7.3), 5.4 mM KCl, 0.8 mM MgSO4, 0.2% sodium azide, 2% FCS, and 10 mM 1,10 phenanthroline. Cells were then incubated for 45 min on ice with mouse anti-human CD95 (clone CH11; Biomol), washed, and incubated for an additional 45 min on ice with an FITC-coupled goat anti-mouse IgG (Becton Dickinson). Finally, cells were washed and submitted to FACS analysis.
Caspase activation.
Cells were infected for the indicated time and lysed for 20 min on ice in TN1 buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Triton X-100) supplemented with 100 μg of aprotinin/ml, 100 μg of leupeptin/ml, 5 μg of pepstatin/ml, and 2 mM phenylmethylsulfonyl fluoride or, alternatively, lysed for 10 min on ice in 25 mM HEPES (pH 7.4), 0.2% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 125 mM NaCl, 10 mM (each) sodium fluoride and sodium pyrophosphate, and 100 μg of aprotinin/ml and 100 μg of leupeptin/ml. Lysates were centrifuged at 15,000 × g for 15 min. Proteins (20 μg/ml) were loaded and resolved by SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Hybond; Amersham Pharmacia Biotech). After blocking for 1 h with 5% nonfat dried milk in TBST (20 mM Tris-Cl [pH 7.5], 150 mM NaCl, 0.1% Tween 20), immunodetection was performed by overnight incubation at 4°C with the primary antibody followed by incubation with an alkaline phosphatase-conjugated secondary antibody (1 h at room temperature). The immunoblots were visualized by using the Tropix detection system. Equal protein loading was controlled by Ponceau red staining of membranes. In addition, an unspecific band detected by the antibody was used as a loading control.
Statistical evaluation.
Experiments were performed at least three times, and results were presented as means ± standard deviations, if not otherwise indicated. The significance of the results was analyzed by Student's t test.
RESULTS
Pili are required for P. aeruginosa-induced host cell apoptosis and activation of IL-1β.
To define the relevance of pili for the response of epithelial cells to an infection with P. aeruginosa, we compared induction of apoptosis in human Chang epithelial cells upon infection with wild-type P. aeruginosa strains PAO-I and PAK as well as two pilus-deficient mutants of PAK, PAKΔpilA and PAKΔpilAΔall.
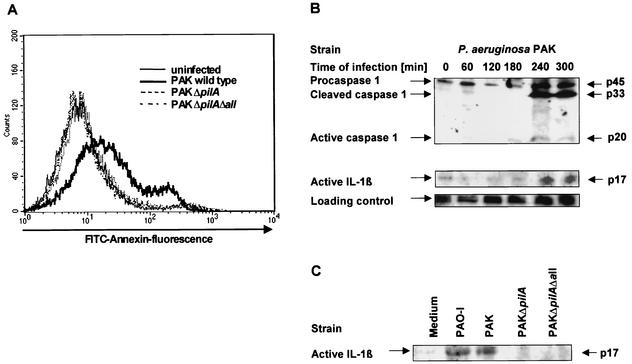
Only PAO-I and wild-type PAK triggered a breakdown of phosphatidylserine asymmetry as detected by an increase in FITC-annexin V binding within 3 h of infection while the pilus-deficient strains failed to induce significant apoptosis (Fig. 1A and Table 2). These results were further corroborated by the observation that morphological alterations of the cells indicative for apoptosis, i.e., cell rounding and membrane blebbing, were absent in cells infected with the pilus-deficient PAKΔpilA and PAKΔpilAΔall even after infection times of 6 h. Both events were readily detected after infection with wild-type strains (data not shown).
FIG. 1.
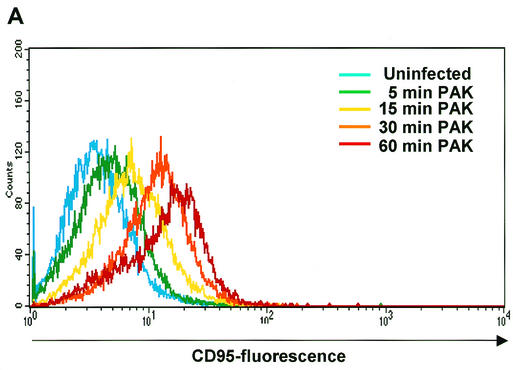
P. aeruginosa-induced apoptosis and activation of IL-1β require pilus-mediated adhesion of the bacteria to Chang epithelial cells. (A) Human Chang epithelial cells were infected for 3 h with P. aeruginosa PAK, PAKΔpilA and PAKΔpilAΔall. Cells were harvested, stained with FITC-annexin V, and submitted to FACS analysis. Data are representative of 4 independent experiments with similar results. (B) P. aeruginosa PAK induces a time-dependent activation of caspase-1 and cleavage of IL-1β. Caspase-1 and IL-1β cleavage was determined by Western blot analysis of cytosolic extracts (20 μg of protein/lane) of Chang cells infected for 0 to 5 h with P. aeruginosa PAK. Staining of an unspecific band is shown as a loading control. (C) The pilus-deficient mutants fail to activate IL-1β. Human Chang epithelial cells were infected for 4 h with P. aeruginosa PAK, PAKΔpilA, and PAKΔpilAΔall. IL-1β cleavage was then determined by Western blot analysis of cytosolic extracts. Shown are representatives of 3 similar experiments.
TABLE 2.
Pilus-deficient P. aeruginosa PAK mutants fail to induce significant apoptosis of human Chang conjunctiva epithelial cellsa
| P. aeruginosa strain | % Annexin-positive cellsb | % Cells with low Δψmc | % Cells with high ROSd |
|---|---|---|---|
| Without infection | 6.0 ± 3.0 | 2.5 ± 1.5 | 6.6 ± 3.3 |
| PAO-I | 35.1 ± 7.9e | 35.3 ± 8.4e | 41.5 ± 8.6e |
| PAK | 39.1 ± 11.0e | 37.0 ± 11.4e | 25.6 ± 5.6e |
| PAKΔpilA | 7.4 ± 4.7 | 4.1 ± 2.0 | 10.4 ± 5.1 |
| PAK ΔpilAΔall | 6.6 ± 3.5 | 4.4 ± 3.3 | 6.8 ± 2.2 |
Chang conjunctiva epithelial cells were infected with for 3 h with the P. aeruginosa strains as indicated. Data are means ± standard deviations of 3 experiments.
Apoptotic cell death was then determined by FACS analysis of FITC-annexin V-stained cells. Values represent percentages of FITC-annexin-positive cells.
Mitochondrial membrane potential was determined by FACS analysis of JC1-stained cells. Values represent percentages of cells with low Δψm.
Synthesis of ROS was determined by FACS analysis of DHE-stained cells. Values indicate percentages of ethidium-positive cells with high ROS production.
Significantly different from value for uninfected control cells (P < 0.05) as calculated by Student's t test.
Furthermore, we tested for a caspase-1-mediated activation of IL-1β in Chang cells upon infection with P. aeruginosa. Our results show that P. aeruginosa PAK and PAO-I trigger a time-dependent cleavage of caspase-1 within 4 to 5 h of infection and a concomitant cleavage of IL-1β (Fig. 1B and data not shown). In contrast to PAO-I and PAK, the pilus-deficient mutants PAKΔpilA and PAKΔpilAΔall failed to cleave IL-1β (Fig. 1C). The results indicate that only piliated P. aeruginosa triggers the caspase-1-mediated activation of IL-1β, and the pilus-deficient mutants were without effect.
Thus, pilus deficiency prevents several responses of host cells to an infection with P. aeruginosa, in particular, induction of apoptosis and activation of IL-1β in infected cells.
Molecular sequence of intracellular signaling during P. aeruginosa-induced apoptosis and requirement of bacterial pili for initiation of the signaling cascade.
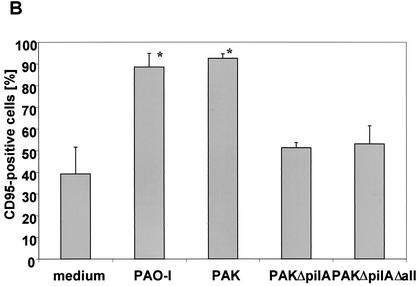
Next, we aimed to define the molecular sequence of P. aeruginosa-mediated apoptosis. Up-regulation of CD95 expression at the epithelial cell surface has been shown to constitute an early and critical event during infection with P. aeruginosa PAO-I (25, 32). We first examined the effect of the P. aeruginosa wild-type strain PAK on CD95 expression. P. aeruginosa PAK triggered a time-dependent up-regulation of CD95 on the surface of the infected cells that was already detectable after 5 to 10 min of infection and amounted to 46.8% (cells with an increase in fluorescence intensity) after 30 min of infection (Fig. 2A). We then tested whether the pilus-deficient P. aeruginosa strains were also able to induce CD95 up-regulation. Our results show that infection with the two piliated strains of P. aeruginosa, PAK and PAO-I, induced a rapid and marked increase in the exposure of CD95 on the surface of the infected cells while the pilus-deficient mutants almost completely failed to increase surface CD95 expression (Fig. 2B). This finding indicates that the up-regulation of CD95 on the cell surface is mediated by a pilus-dependent process.
FIG.2.
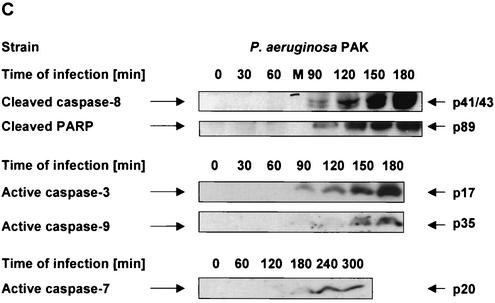
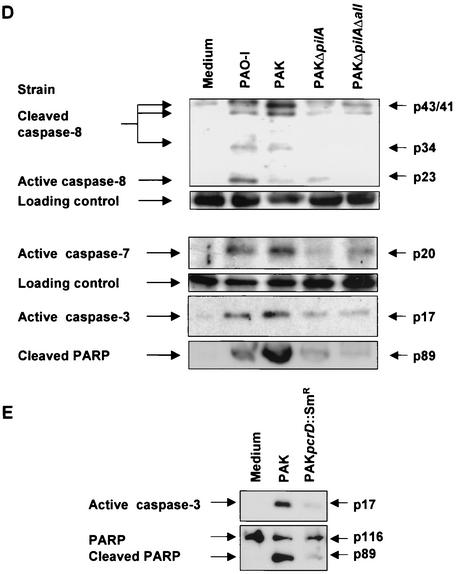
The P. aeruginosa-induced up-regulation of CD95 and the activation of caspases depends on the expression of bacterial pili and a functional type III secretion system. (A) P. aeruginosa PAK induces a time-dependent up-regulation of CD95 on the surface of infected Chang cells. Cells were infected for the indicated times with wild-type P. aeruginosa PAK. CD95 exposure on the cell surface was determined by FACS analysis of immunostained cells. (B) Infection of Chang epithelial cells with pilus-deficient PAK mutants fails to induce significant CD95 up-regulation on the cell surface when compared to wild-type PAK. Cells were left uninfected or infected for 90 min with P. aeruginosa PAK, PAKΔpilA, or PAKΔpilAΔall. CD95 exposure on the cell surface was determined by FACS analysis of immunostained cells. Data show means ± standard deviations (n = 3). ∗, significant difference from values for uninfected control cells (P < 0.05) as calculated by Student's t test. (C) P. aeruginosa PAK induces time-dependent activation of caspase-8, -9, -3, and -7 as well as cleavage of the caspase-3 substrate PARP. Caspase activation was determined by Western blot analysis of cytosolic extracts (20 μg of protein/ml/lane) of Chang cells infected for the indicated times with P. aeruginosa PAK. (D and E) While P. aeruginosa PAO-I and wild-type PAK induce activation of caspases as well as cleavage of the caspase-3 substrate PARP, the pilus-deficient mutant strains PAKΔpilA and PAKΔpilAΔall and the type III secretion-deficient mutant PAK pcrD::Smr fail to induce significant processing of caspases and PARP. Caspase activation was determined by Western blot analysis of cytosolic extracts (20 μg of protein/ml/lane) of cells infected for 3 h with P. aeruginosa PAK, PAKΔpilA, PAKΔpilAΔall, or PAK pcrD::Smr or left uninfected for the same time period. Staining of an unspecific band is shown as a loading control. Data show one representative of three independent experiments (A and C to E).
Furthermore, we aimed to identify the role of caspases in signaling of P. aeruginosa-induced apoptosis. Caspases have been shown to be central for both CD95 receptor- and stress-induced apoptosis. In death receptor-mediated pathways, caspase-8 constitutes the most apical caspase, whereas in mitochondrial pathways, caspase-9 functions as the initiator caspase and caspase-8 is only activated downstream of the mitochondria (3, 52, 60). To define the hierarchy of the caspase cascade during P. aeruginosa-induced apoptosis, we analyzed cleavage of caspase-8, -9, -3, and -7 as well as of the caspase-3 substrate poly(ADP-ribose) polymerase (PARP). Our results show that P. aeruginosa PAK induced an early activation of caspase-8 and -3 which was first detectable after 90 min of infection (Fig. 2C). In addition, cleavage of the caspase-3 substrate PARP followed the same time course. In contrast, activation of caspase-9 and -7 occurred only at later time points (150 to 180 min of infection), suggesting an activation of the mitochondrial apoptosis pathways downstream of caspase-8. Only the piliated P. aeruginosa PAO-I and PAK triggered activation of caspase-8, -3, and -7, whereas the pilus-deficient mutants failed to induce significant processing of caspase-8 and subsequent activation of effector caspase-3 and -7 as well as cleavage of the caspase substrate PARP (Fig. 2D).
Earlier investigations in our laboratory had shown that deficiency of the type III secretion system almost completely abolished P. aeruginosa-induced apoptosis (32). Therefore, we additionally analyzed caspase activation upon infection with the type III secretion-deficient P. aeruginosa mutant. Similar to the results obtained with the pilus-deficient mutants, PAK pcrD::Smr failed to induce significant activation of caspase-8 and cleavage of the caspase-3 substrate PARP (Fig. 2E).
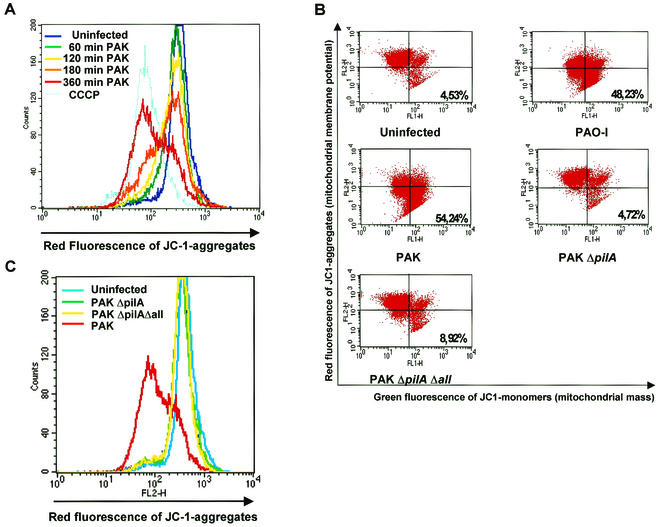
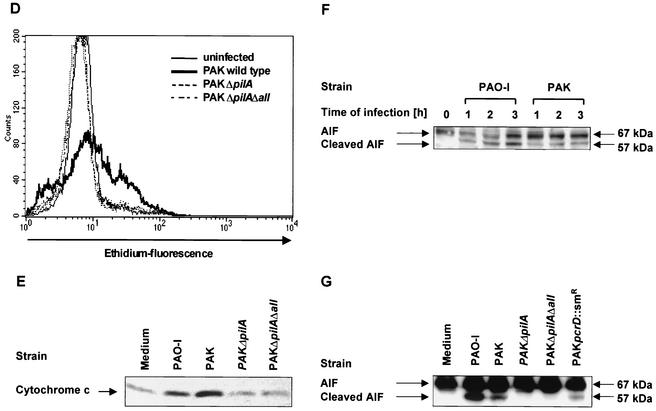
Mitochondria have been shown to be crucially involved in many forms of apoptosis. Apoptotic stimuli induce the release of proteins such as cytochrome c and/or AIF from the mitochondrial intermembrane space as well as hyperproduction of reactive oxygen species (ROS) and depolarization of the mitochondrial membrane potential (Δψm) (33, 62, 69). We have recently shown that P. aeruginosa PAO-I triggers mitochondrial changes, including the release of cytochrome c and mitochondrial membrane depolarization upon infection. To get further insight into the importance of mitochondria during P. aeruginosa-induced apoptosis, we first examined the time course of the mitochondrial membrane potential depolarization upon infection with P. aeruginosa PAK. Wild-type PAK induced time-dependent depolarization of the mitochondrial membrane potential within 3 to 6 h of infection (Fig. 3A). Mitochondrial membrane depolarization could only be observed after infection with the wild-type piliated strains while the pilus-deficient mutants failed to induce this response (Fig. 3B; Table 2). Even after 6 h of infection with the pilus-deficient strains, no alteration of the mitochondrial membrane potential could be detected (Fig. 3C), arguing against a delayed apoptotic response upon infection with the pilus-deficient strains. In addition, only P. aeruginosa PAO-I and PAK induced a marked increase in ROS production in infected Chang cells, which was absent in cells infected with the pilus-deficient mutants (Fig. 3D; Table 2).
FIG. 3.
The P. aeruginosa-induced mitochondrial alterations depend on the expression of bacterial pili and a functional type III secretion system. Infection of Chang epithelial cells with P. aeruginosa PAK induces a progressive mitochondrial depolarization, hyperproduction of ROS, and release of cytochrome c which are not observed after infection with pilus-deficient PAK mutants. (A) Chang conjunctiva epithelial cells were infected for the indicated times with wild-type P. aeruginosa PAK. The mitochondrial membrane potential was determined by FACS analysis of JC1-loaded cells. Complete depolarization with carbonyl cyanide m-chlorophenylhydrazone (CCCP) was included as a positive control. (B and C) Chang conjunctiva epithelial cells were left without infection or infected with the indicated P. aeruginosa strains for 3 h (B) or for 6 h (C). Cells were loaded with JC1 and subjected to FACS analysis. (B) Cells in the lower right quadrant represent cells with depolarized mitochondrial membrane potential (low Δψm). Numbers indicate percentages of the cells with low Δψm. (D) Chang conjunctiva epithelial cells were left without infection or infected for 3 h with the indicated P. aeruginosa strains. ROS production was determined by FACS analysis of cells loaded with DHE. Cytochrome c (E) and AIF (F and G) release from the mitochondria were determined by immunoblotting cytosol preparations from Chang cells infected for 3 h (E and G) or 1 to 3 h (F) with P. aeruginosa PAO-I, PAK, PAKΔpilA, PAKΔpilAΔall, or PAK pcrD::Smr as indicated and Chang cells which were left uninfected for the same time period. Data are representatives of 3 similar experiments (A to G).
Finally, we examined whether treatment of Chang cells with the pilus-deficient mutants induces release of cytochrome c, a protein localized in the mitochondrial intermembrane space. Cytochrome c release was observed 3 h after infection with P. aeruginosa PAO-I or PAK while the pilus-deficient mutants failed to induce significant cytochrome c release (Fig. 3E).
To identify further constituents of the signaling cascade, we tested whether infection of Chang cells resulted in mitochondrial release of AIF. AIF represents a mitochondrial flavoprotein which is sufficient to induce apoptosis-like morphology of isolated nuclei independently from Apaf1 and caspase-9 and has been implicated in caspase-independent mitochondrial pathways for the induction of programmed cell death (33, 62). The primary transcript of AIF constitutes a 67-kDa protein which is cleaved to a 57-kDa protein upon uptake into the mitochondria. While the 67-kDa AIF proform was detected in all cells, a time-dependent release of the 57-kDa mitochondrial AIF could be detected after infection with the two piliated wild-type strains of P. aeruginosa, PAO-I and PAK (Fig. 3F), while the pilus-deficient mutants failed to release processed AIF. In contrast, a small but detectable release of cleaved AIF could be detected in the cytosol of Chang cells upon infection with the type III secretion-deficient mutant PAK pcrD::Smr (Fig. 3G).
In summary, our data indicate that pili are required to initiate CD95 up-regulation, caspase activation, and mitochondrial alterations upon infection with P. aeruginosa.
DISCUSSION
Our results provide novel insights into the molecular ordering of P. aeruginosa PAK- and PAO-I-induced apoptosis. The rapid up-regulation of CD95 on the surface of the infected cells represented the signaling event that was first detected upon infection of human epithelial cells with wild-type piliated P. aeruginosa PAK. This is consistent with our earlier observations of apoptosis induction upon infection with P. aeruginosa PAO-I (32). Furthermore, we could demonstrate an infection-induced activation of caspase-8, -9, -3, and -7. Those caspases have been shown to be central for death receptor and mitochondrial apoptosis signaling pathways (3, 39, 46, 60, 64, 68). Our observations on the early processing of caspase-8 and -3 together with the delay in caspase-9 activation places CD95 up-regulation and activation of caspase-8 upstream of the mitochondria. Similar to earlier observations, this argues for a predominant role of death receptor signaling in P. aeruginosa-induced apoptosis (25). In contrast, during DNA damage- or stress-induced apoptosis that follow mitochondrial pathways, caspase-8 and -9 cleavage occur with a similar time course (52).
In addition, our results reveal novel molecular details of the interaction of P. aeruginosa strains with human host cells. In this regard, we could demonstrate, for the first time, a release of mature AIF from the mitochondria upon infection. AIF, which may be important for death-related nuclear changes (33, 62), has been suggested to mediate caspase-independent cell death (38, 58, 60). Thus, AIF release upon bacterial infection may ensure cell death in cells lacking adequate caspase activation. Furthermore, our data demonstrate that P. aeruginosa PAO-I and PAK induce caspase-1-mediated activation of IL-1β. This confirms earlier reports on the induction of proinflammatory IL-1β upon infection with P. aeruginosa (13, 67). Likewise, stimulation of endothelial cells and mononuclear phagocytes with LPS has been shown to trigger caspase-1-mediated cleavage of IL-1β (59). IL-1 has been identified as an important mediator of pulmonary inflammation induced by bacteria or bacterial products (37, 65), and increased levels of IL-1 contributed to the pathogenicity of P. aeruginosa in pneumonia and corneal injury (57, 67). Thus, similar to the caspase-1-mediated activation of IL-1β and IL-18, which has been shown to be essential for Shigella-induced inflammation (55), P. aeruginosa-induced cleavage of IL-1β may be related to the infection-induced inflammatory host cell response. In this regard, recent data reveal an involvement of the CD95-CD95 ligand system in the onset of cytokine production during gram-negative bacterial pneumonia (45). Since infection-induced activation of IL-1β required the presence of bacterial pili, these bacterial adhesins seem to be also necessary for inflammatory host cell responses to an infection with P. aeruginosa.
Consistent with earlier observations on a requirement of pili for adherence and cytotoxicity of P. aeruginosa (5, 7, 8, 23, 35, 50), our data reveal that pili are necessary for infection-induced apoptosis. The centrifugation step used in the infection protocol to increase bacterium-host cell interactions was not sufficient to elicit a full apoptotic response. On the other hand, earlier findings from our laboratory revealed that a functional type III secretion system is also required to elicit a full apoptotic response upon infection with P. aeruginosa (32). However, neither pilus deficiency nor deficiency of the type III system completely abolished infection-induced apoptotic signaling. In particular, a minor up-regulation of the CD95 receptor on the surface of the infected cells, a limited activation of caspases and the mitochondria, and the release of some mature AIF could still be detected. Those changes may result from residual type III secretion of the pilus-deficient mutants or some pilus-mediated protein secretion of the type III secretion-deficient mutant (8). In this regard, P. aeruginosa type IV pili have been shown to assist in type III secretion-system-dependent delivery of cytotoxins (7, 42). In particular, PilA, the major structural subunit of type IV pili, PilT, and PilU seem to be required for efficient protein secretion (7, 23, 35). Furthermore, in a recent report, P. aeruginosa LPS was shown to induce an up-regulation of Trail/APO 2 ligand in monocytes and macrophages (29). Thus, multiple factors, including type III-dependent toxins, pili, and LPS, may be involved in the induction of apoptosis upon infection with P. aeruginosa.
In summary, we provide insight into the molecular ordering of human epithelial cell apoptosis initiated by P. aeruginosa infection, placing activation of the caspase cascade upstream of the mitochondria. We conclude that induction of epithelial cell apoptosis by P. aeruginosa requires bacterial expression of a full repertoire of virulence factors including adhesins, a functional type III secretion system, expression of type III secreted cytotoxins, and an appropriate activation of signaling cascades within the epithelial cells. The outcome of an infection with P. aeruginosa may depend on the balance of bacterial invasion, the induction of apoptosis, and the inflammatory host cell response.
Acknowledgments
We gratefully acknowledge E. Gulbins for carefully reviewing the manuscript and E. Faber for technical assistance.
This work was supported by a grant of the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Fö. 01KS9602) and the Interdisziplinäres Zentrum für Klinische Forschung der Universität Tübingen (IZKF) (grant no. S.05.00023.2/IIB9 to V.J.).
Editor: V. J. DiRita
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apodaca, G., M. Bomsel, R. Lindstedt, J. Engel, D. Frank, K. E. Mostov, and J. Wiener-Kronish. 1995. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect. Immun. 63:1541-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belka, C., J. Rudner, S. Wesselborg, A. Stepczynska, P. Marini, A. Lepple-Wienhues, H. Faltin, M. Bamberg, W. Budach, and K. Schulze-Osthoff. 2000. Differential role of caspase-8 and BID activation during radiation- and CD95-induced apoptosis. Oncogene 19:1181-1190. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, D. E. 1980. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can. J. Microbiol 26:146-154. [DOI] [PubMed] [Google Scholar]
- 5.Chi, E., T. Mehl, D. Nunn, and S. Lory. 1991. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect. Immun. 59:822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinnaiyan, A. M., C. G. Tepper, M. F. Seldin, K. O'Rourke, F. C. Kischkel, S. Hellbardt, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J. Biol. Chem. 271:4961-4965. [DOI] [PubMed] [Google Scholar]
- 7.Comolli, J. C., A. R. Hauser, L. Waite, C. B. Whitchurch, J. S. Mattick, and J. N. Engel. 1999. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect. Immun. 67:3625-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comolli, J. C., L. L. Waite, K. E. Mostov, and J. N. Engel. 1999. Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect. Immun. 67:3207-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowell, B. A., D. Y. Chen, D. W. Frank, A. J. Vallis, and S. M. Fleiszig. 2000. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect. Immun. 68:403-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dacheux, D., B. Toussaint, M. Richard, G. Brochier, J. Croize, and I. Attree. 2000. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect. Immun. 68:2916-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMango, E., A. J. Ratner, R. Bryan, S. Tabibi, and A. Prince. 1998. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Investig. 101:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doig, P., T. Todd, P. A. Sastry, K. K. Lee, R. S. Hodges, W. Paranchych, and R. T. Irvin. 1988. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect. Immun. 56:1641-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epelman, S., T. F. Bruno, G. G. Neely, D. E. Woods, and C. H. Mody. 2000. Pseudomonas aeruginosa exoenzyme S induces transcriptional expression of proinflammatory cytokines and chemokines. Infect. Immun. 68:4811-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, D. J., D. W. Frank, V. Finck-Barbancon, C. Wu, and S. M. Fleiszig. 1998. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect. Immun. 66:1453-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farinha, M. A., B. D. Conway, L. M. Glasier, N. W. Ellert, R. T. Irvin, R. Sherburne, and W. Paranchych. 1994. Alteration of the pilin adhesin of Pseudomonas aeruginosa PAO results in normal pilus biogenesis but a loss of adherence to human pneumocyte cells and decreased virulence in mice. Infect. Immun. 62:4118-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari, D., A. Stepczynska, M. Los, S. Wesselborg, and K. Schulze-Osthoff. 1998. Differential regulation and ATP requirement for caspase-8 and caspase-3 activation during CD95- and anticancer drug-induced apoptosis. J. Exp. Med. 188:979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finck-Barbancon, V., and D. W. Frank. 2001. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 183:4330-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol 25:547-557. [DOI] [PubMed] [Google Scholar]
- 20.Fleiszig, S. M., J. P. Wiener-Kronish, H. Miyazaki, V. Vallas, K. E. Mostov, D. Kanada, T. Sawa, T. S. Yen, and D. W. Frank. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleiszig, S. M., T. S. Zaidi, and G. B. Pier. 1995. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect. Immun. 63:4072-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frithz-Lindsten, E., Y. Du, R. Rosqvist, and A. Forsberg. 1997. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol 25:1125-1139. [DOI] [PubMed] [Google Scholar]
- 23.Garrity-Ryan, L., B. Kazmierczak, R. Kowal, J. Comolli, A. Hauser, and J. N. Engel. 2000. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68:7100-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grassme, H., E. Gulbins, B. Brenner, K. Ferlinz, K. Sandhoff, K. Harzer, F. Lang, and T. F. Meyer. 1997. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 91:605-615. [DOI] [PubMed] [Google Scholar]
- 25.Grassme, H., S. Kirschnek, J. Riethmueller, A. Riehle, G. von Kurthy, F. Lang, M. Weller, and E. Gulbins. 2000. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science 290:527-530. [DOI] [PubMed] [Google Scholar]
- 26.Graupner, S., V. Frey, R. Hashemi, M. G. Lorenz, G. Brandes, and W. Wackernagel. 2000. Type IV pilus genes pilA and pilC of Pseudomonas stutzeri are required for natural genetic transformation, and pilA can be replaced by corresponding genes from nontransformable species. J. Bacteriol. 182:2184-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 28.Gupta, S. K., R. S. Berk, S. Masinick, and L. D. Hazlett. 1994. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect. Immun. 62:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halaas, O., R. Vik, A. Ashkenazi, and T. Espevik. 2000. Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scand. J. Immunol 51:244-250. [DOI] [PubMed] [Google Scholar]
- 30.Hauser, A. R., and J. N. Engel. 1999. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect. Immun. 67:5530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornef, M. W., A. Roggenkamp, A. M. Geiger, M. Hogardt, C. A. Jacobi, and J. Heesemann. 2000. Triggering the ExoS regulon of Pseudomonas aeruginosa: a GFP-reporter analysis of exoenzyme (Exo) S, ExoT and ExoU synthesis. Microb. Pathog. 29:329-343. [DOI] [PubMed] [Google Scholar]
- 32.Jendrossek, V., H. Grassme, I. Mueller, F. Lang, and E. Gulbins. 2001. Pseudomonas aeruginosa-induced apoptosis involves mitochondria and stress-activated protein kinases. Infect. Immun. 69:2675-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joza, N., S. A. Susin, E. Daugas, W. L. Stanford, S. K. Cho, C. Y. Li, T. Sasaki, A. J. Elia, H. Y. Cheng, L. Ravagnan, K. F. Ferri, N. Zamzami, A. Wakeham, R. Hakem, H. Yoshida, Y. Y. Kong, T. W. Mak, J. C. Zuniga-Pflucker, G. Kroemer, and J. M. Penninger. 2001. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549-554. [DOI] [PubMed] [Google Scholar]
- 34.Kagami, Y., M. Ratliff, M. Surber, A. Martinez, and D. N. Nunn. 1998. Type II protein secretion by Pseudomonas aeruginosa: genetic suppression of a conditional mutation in the pilin-like component XcpT by the cytoplasmic component XcpR. Mol. Microbiol. 27:221-233. [DOI] [PubMed] [Google Scholar]
- 35.Kang, P. J., A. R. Hauser, G. Apodaca, S. M. Fleiszig, J. Wiener-Kronish, K. Mostov, and J. N. Engel. 1997. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol. Microbiol. 24:1249-1262. [DOI] [PubMed] [Google Scholar]
- 36.Kohler, T., L. K. Curty, F. Barja, C. van Delden, and J. C. Pechere. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leff, J. A., J. W. Baer, M. E. Bodman, J. M. Kirkman, P. F. Shanley, L. M. Patton, C. J. Beehler, J. M. McCord, and J. E. Repine. 1994. Interleukin-1-induced lung neutrophil accumulation and oxygen metabolite-mediated lung leak in rats. Am. J. Physiol. 266:L2-L8. [DOI] [PubMed] [Google Scholar]
- 38.Leist, M., and M. Jaattela. 2001. Four deaths and a funeral: from caspases to alternative mechanisms. Nat. Rev. Mol. Cell. Biol. 2:589-598. [DOI] [PubMed] [Google Scholar]
- 39.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 40.Li, P., D. Nijhawan, I. Budihardjo, S. M. Srinivasula, M. Ahmad, E. S. Alnemri, and X. Wang. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479-489. [DOI] [PubMed] [Google Scholar]
- 41.Lomholt, J. A., K. Poulsen, and M. Kilian. 2001. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect. Immun. 69:6284-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu, H. M., S. T. Motley, and S. Lory. 1997. Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol. Microbiol. 25:247-259. [DOI] [PubMed] [Google Scholar]
- 43.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 44.Mattick, J. S., C. B. Whitchurch, and R. A. Alm. 1996. The molecular genetics of type-4 fimbriae in Pseudomonas aeruginosa-a review. Gene 179:147-155. [DOI] [PubMed] [Google Scholar]
- 45.Matute-Bello, G., C. W. Frevert, W. C. Liles, M. Nakamura, J. T. Ruzinski, K. Ballman, V. A. Wong, C. Vathanaprida, and T. R. Martin. 2001. Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect. Immun. 69:5768-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIlroy, D., H. Sakahira, R. V. Talanian, and S. Nagata. 1999. Involvement of caspase 3-activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuli. Oncogene 18:4401-4408. [DOI] [PubMed] [Google Scholar]
- 47.Medema, J. P., C. Scaffidi, F. C. Kischkel, A. Shevchenko, M. Mann, P. H. Krammer, and M. E. Peter. 1997. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16:2794-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muzio, M., A. M. Chinnaiyan, F. C. Kischkel, K. O'Rourke, A. Shevchenko, J. Ni, C. Scaffidi, J. D. Bretz, M. Zhang, R. Gentz, M. Mann, P. H. Krammer, M. E. Peter, and V. M. Dixit. 1996. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 85:817-827. [DOI] [PubMed] [Google Scholar]
- 49.Plotkowski, M. C., A. M. Saliba, S. H. Pereira, M. P. Cervante, and O. Bajolet-Laudinat. 1994. Pseudomonas aeruginosa selective adherence to and entry into human endothelial cells. Infect. Immun. 62:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajan, S., G. Cacalano, R. Bryan, A. J. Ratner, C. U. Sontich, A. van Heerckeren, P. Davis, and A. Prince. 2000. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells: analysis of the effects of cystic fibrosis transmembrane conductance regulator dysfunction and bacterial virulence factors. Am. J. Respir. Cell Mol. Biol. 23:304-312. [DOI] [PubMed] [Google Scholar]
- 51.Roncero, C., A. Darzins, and M. J. Casadaban. 1990. Pseudomonas aeruginosa transposable bacteriophages D3112 and B3 require pili and surface growth for adsorption. J. Bacteriol. 172:1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudner, J., A. Lepple-Wienhues, W. Budach, J. Berschauer, B. Friedrich, S. Wesselborg, K. Schulze-Osthoff, and C. Belka. 2001. Wild-type, mitochondrial and ER-restricted Bcl-2 inhibit DNA damage-induced apoptosis but do not affect death receptor-induced apoptosis. J. Cell Sci. 114:4161-4172. [DOI] [PubMed] [Google Scholar]
- 53.Saiman, L., K. Ishimoto, S. Lory, and A. Prince. 1990. The effect of piliation and exoproduct expression on the adherence of Pseudomonas aeruginosa to respiratory epithelial monolayers. J. Infect. Dis. 161:541-548. [DOI] [PubMed] [Google Scholar]
- 54.Salvesen, G. S., and V. M. Dixit. 1999. Caspase activation: the induced-proximity model. Proc. Natl. Acad. Sci. USA 96:10964-10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 56.Sawa, T., D. B. Corry, M. A. Gropper, M. Ohara, K. Kurahashi, and J. P. Wiener-Kronish. 1997. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J. Immunol. 159:2858-2866. [PubMed] [Google Scholar]
- 57.Schultz, M. J., A. W. Rijneveld, S. Florquin, C. K. Edwards, C. A. Dinarello, and T. van der Poll. 2002. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol 282:L285-L290. [DOI] [PubMed] [Google Scholar]
- 58.Schulze-Osthoff, K., A. C. Bakker, B. Vanhaesebroeck, R. Beyaert, W. A. Jacob, and W. Fiers. 1992. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 267:5317-5323. [PubMed] [Google Scholar]
- 59.Schumann, R. R., C. Belka, D. Reuter, N. Lamping, C. J. Kirschning, J. R. Weber, and D. Pfeil. 1998. Lipopolysaccharide activates caspase-1 (interleukin-1-converting enzyme) in cultured monocytic and endothelial cells. Blood 91:577-584. [PubMed] [Google Scholar]
- 60.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 61.Stroh, C., and K. Schulze-Osthoff. 1998. Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 5:997-1000. [DOI] [PubMed] [Google Scholar]
- 62.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, B. E. Snow, G. M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, N. Larochette, D. R. Goodlett, R. Aebersold, D. P. Siderovski, J. M. Penninger, and G. Kroemer. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441-446. [DOI] [PubMed] [Google Scholar]
- 63.Tang, H. B., E. DiMango, R. Bryan, M. Gambello, B. H. Iglewski, J. B. Goldberg, and A. Prince. 1996. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect. Immun. 64:37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 65.Ulich, T. R., L. R. Watson, S. M. Yin, K. Z. Guo, P. Wang, H. Thang, and J. del Castillo. 1991. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am. J. Pathol. 138:1485-1496. [PMC free article] [PubMed] [Google Scholar]
- 66.Vasil, M. L. 1986. Pseudomonas aeruginosa: biology, mechanisms of virulence, epidemiology. J. Pediatr. 108:800-805. [DOI] [PubMed] [Google Scholar]
- 67.Xue, M. L., M. D. Willcox, A. Lloyd, D. Wakefield, and A. Thakur. 2001. Regulatory role of IL-1beta in the expression of IL-6 and IL-8 in human corneal epithelial cells during Pseudomonas aeruginosa colonization. Clin. Exp. Ophthalmol. 29:171-174. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguchi, N., I. R. Kieba, J. Korostoff, P. S. Howard, B. J. Shenker, and E. T. Lally. 2001. Maintenance of oxidative phosphorylation protects cells from Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Cell. Microbiol. 3:811-823. [DOI] [PubMed] [Google Scholar]
- 69.Zamzami, N., P. Marchetti, M. Castedo, D. Decaudin, A. Macho, T. Hirsch, S. A. Susin, P. X. Petit, B. Mignotte, and G. Kroemer. 1995. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 182:367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou, H., Y. Li, X. Liu, and X. Wang. 1999. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 274:11549-11556. [DOI] [PubMed] [Google Scholar]