Abstract
DtxR-type metal ion-dependent repressors, present in many bacterial pathogens, may regulate expression of virulence genes such as that encoding diphtheria toxin. SirR, a DtxR homologue initially identified in Staphylococcus epidermidis, governs the expression of the adjacent sitABC operon encoding a putative metal ion ABC transporter system. We identified a sirR homologue, mntR, in Staphylococcus aureus and demonstrated by gel shift assay that the corynebacterial repressor DtxR binds to the S. aureus mntABC operator in the presence of Fe2+ or Mn2+. Since a mutant DtxR, DtxR(E175K), functions as an iron-independent hyperrepressor in certain settings, we constructed a heterodiploid S. aureus strain expressing dtxR(E175K) from the native mntR promoter. Transcription of the S. aureus mntABC operon was repressed in the presence of Fe2+ or Mn2+ in wild-type and heterodiploid S. aureus strains. Under metal ion-limiting conditions, mntABC transcription was reduced but not abolished in S. aureus isolates expressing dtxR(E175K) compared with an isogenic control, suggesting that DtxR(E175K) binds the S. aureus MntR box in vivo. Under all conditions tested, mntABC transcription in the dtxR(E175K)-expressing strain was reduced relative to the isogenic control, indicating that DtxR(E175K) function was constitutively active. In the mouse skin abscess model, dtxR(E175K)-expressing S. aureus recombinants showed significantly reduced CFU levels compared with the isogenic wild-type control. We conclude that the S. aureus MntR box is recognized by corynebacterial DtxR proteins and thus belongs to the DtxR family of metal-dependent operator sites. Moreover, constitutive repression by DtxR(E175K) reduces the virulence of S. aureus in the mouse skin abscess model.
Staphylococcus aureus is a major human pathogen that causes a variety of diseases ranging from minor skin ailments to life-threatening deep infections such as endocarditis, meningitis, arthritis, and toxic shock syndrome (16, 30, 40). Despite the introduction of new antibiotics, the morbidity and mortality from serious S. aureus infections remain high and drug resistance is emerging as a new threat (5). The identification and characterization of specific virulence genes continue to be a high priority, as these genes may prove to be important future drug targets.
The diphtheria toxin repressor, DtxR, from Corynebacterium diphtheriae, has been shown to be an iron-dependent repressor that controls the expression of a series of iron-sensitive genes, including the diphtheria toxin gene, the gene for heme oxygenase, and genes involved in siderophore production (1, 24, 27, 33). In addition to C. diphtheriae, DtxR homologues have been found in Streptomyces spp. (8), Brevibacterium lactofermentum (19), Mycobacterium spp. (6), Streptococcus mutans (13), Treponema pallidum (9), Staphylococcus epidermidis (11), and S. aureus (12). There is evidence to suggest that these DtxR homologues are important in regulating genes encoding metal ion transport systems related to virulence and oxidative stress defense. S. epidermidis SirR, S. mutans SloR, and T. pallidum TroR are known to bind a specific operator region that regulates an ATP-binding cassette (ABC) transporter system. The streptococcal DtxR homologue, SloR, has been found to regulate the sloABC operon, the latter being members of the lipoprotein receptor antigen I (LraI) family of ABC transporter systems. Interestingly, a mutation in the S. mutans sloC gene results in a loss of virulence in the rat model of endocarditis (13). Another LraI locus in Streptococcus parasanguis, known as fimA, has also been implicated in virulence in an endocarditis model (3).
Sun et al. isolated and characterized a series of dtxR mutants created by PCR mutagenesis and found that a single amino acid substitution of lysine for glutamic acid at position 175 [mutant DtxR(E175K)] conferred an iron-insensitive, hyperrepressor phenotype (31). Wild-type DtxR of C. diphtheriae requires Fe2+ ions to transcriptionally regulate the tox gene (33). Since DtxR in C. diphtheriae and its closest phylogenetic homologue, IdeR in Mycobacterium tuberculosis (28), are likely to be global regulators that control the expression of iron-responsive genes involved in iron acquisition and virulence, heterodiploids expressing DtxR(E175K) would be expected to have a dominant negative phenotype and to be constitutively unable to upregulate iron-repressed genes. Along these lines, Manabe et al. reported that an M. tuberculosis heterodiploid expressing DtxR(E175K) was attenuated compared with a wild-type strain in a mouse model of tuberculosis (17). In vitro, by gel shift assay, the DtxR protein was demonstrated to bind to putative IdeR-regulated genes. Likewise, IdeR binds to the C. diphtheriae tox promoter (28).
Horsburgh et al., using primers designed from the S. epidermidis sirR gene, isolated and sequenced the S. aureus homologue, which they termed mntR (12). In this study, using in vitro gel retardation assays, we demonstrate that DtxR(C102D), a biologically active iron-dependent repressor, binds to the S. aureus MntR box in the 5′ untranslated region of the mntABC operon of S. aureus. Furthermore, using reverse transcription (RT)-PCR and molecular beacons, we show that transcription of mntABC is repressed under conditions containing Fe2+ or Mn2+ ions. S. aureus expressing the metal ion-independent repressor DtxR(E175K) inhibited mntABC expression under conditions with and without iron. In addition, this strain was attenuated in a mouse skin abscess model compared with the parent strain containing an empty plasmid.
MATERIALS AND METHODS
Strains and plasmids.
S. aureus RN4220 and RN6390 were gifts from Richard Novick. S. aureus was cultured at 37°C in tryptic soy broth or tryptic soy agar. Escherichia coli (DH5α) was cultured at 37°C in Luria-Bertani broth or on Luria-Bertani agar. Staphylococcal siderophore detection media (SSD) (2 mM KH2PO4, 7.9 mM NaCl, 17.2 mM NH4Cl, 2% [vol/vol] 1.5 M Tris-HCl [pH 8.8] solution, 20 mM glucose, 0.6% [wt/vol] Casamino Acids [Difco], 39 μM tryptophan, 32 μM nicotinic acid, and 6 μM thiamine-HCl) were used as the minimal media for both iron-depleted and iron-rich media (15). The iron-depleted medium was prepared by treating the minimal medium with 10 g of Chelex 100 (Sigma) per liter and stirring for 1 h at room temperature to remove divalent and trivalent metal ions. The iron-rich medium was SSD medium containing ferric ammonium acetate at a final concentration of 50 μg/ml. Antibiotics were added at the following concentrations: ampicillin, 100 μg/ml; tetracycline, 5 μg/ml.
Construction of the dtxR(E175K) shuttle vector plasmid.
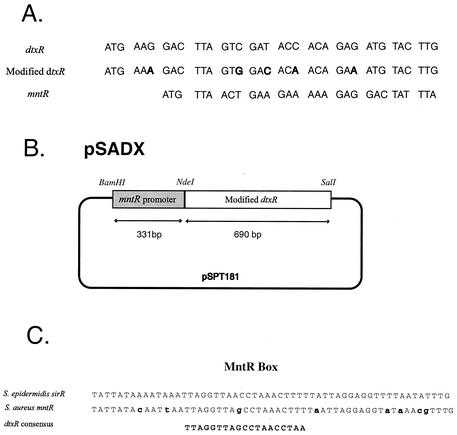
The mntR gene of S. aureus was identified in The Institute of Genomic Research (TIGR, Rockville, Md.) database as a homologue of the S. epidermidis sirR gene (11). The first 10 codons of dtxR(E175K) from C. diphtheriae were altered to reflect S. aureus codon usage (Fig. 1A). The modified DNA fragment of dtxR(E175K) was amplified by PCR from pNBV1/SAD (17) with primers SADX F-1 (5′-CATATGAAAGACTTAGTGGACACAACAGAAATGTACTTGCGTACTATC) and SADXR-2 (5′-GTCGACTTAGAGTTCTTCGATACGAATAGT) (Fig. 1). The PCR products were cloned into pCRII (Invitrogen Corp., Carlsbad, Calif.), and the resulting plasmid was designated pSDTXR/PCRII. Two hundred base pairs of upstream sequence from mntR including the putative promoter was amplified with MntRPro F-1 (5′-GGATCCTTGCAGTTGTTGTTGTATAGG) and MntRPro R-2 (5′-CATATGACTTTCACCTCACATACATTG) and then cloned into pCRII, digested by BamHI and NdeI, and ligated into the same sites in the pSDTXR/PCRII. The resulting plasmid was designated pSADX1. The plasmids were extracted by using the Qiagen (Chatsworth, Calif.) plasmid purification system. After a restriction digest by BamHI and SalI, the fragment encoding the putative mntR promoter and modified dtxR(E175K) were cloned into the pSPT181 multi-copy shuttle plasmid and designated pSADX. This construct was then transformed into the restriction-deficient RN4220 recipient by electroporation (22) and transduced at 37°C into RN6390 with phage 80α by the method described by Novick et al. (18). MA2004 is the heterodiploid S. aureus strain expressing dtxR(E175K) from the mntR promoter in recombinant pSADX, while MA2181 is the control S. aureus RN6390 containing only the parent shuttle vector pSPT181.
FIG. 1.
(A) Sequences of the initial 10 or 12 codons of dtxR, modified dtxR (modified to S. aureus codon preferences), and mntR. (B) Schematic representation of the pSADX shuttle vector plasmid containing the mntR promoter and the modified dtxR. (C) The MntR box. The alignment of the S. aureus MntR box with the S. epidermidis SirR box (11) and the consensus DtxR box (14) is shown.
Cell extract preparation and Western blotting.
The S. aureus MA2004 heterodiploid and MA2181 control strains were cultured in tryptic soy broth containing 5 μg of tetracycline/ml for 18 h. After pelleting, the cells were resuspended in 1.0 ml of TEG buffer (25 mM Tris-HCl, 5 mM EGTA [pH 8.0]), and cell extracts were prepared from lysostaphin-treated cells by using an adaptation of the method originally described by Schindler and Schuhardt (23). One microgram of whole-cell protein extract was separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Hybond; Amersham Pharmacia, Piscataway, N.J.) by the semidry technique (Transblot SD; Bio-Rad, Hercules, Calif.). The blot was blocked with 5% nonfat milk in phosphate-buffered saline with 0.1% Tween 20 for 1 h and incubated at 4°C with anti-DtxR polyclonal antibodies (35). After being washed, the membrane was incubated with horseradish peroxidase-conjugated antirabbit antibody diluted in phosphate-buffered saline with 0.1% Tween 20 at room temperature for 2 h. After being washed, the Supersignal chemiluminescent substrate (Pierce, Rockford, Ill.) was used for autoradiograph development.
DNA gel shift assay.
The gel electrophoresis mobility shift assay (EMSA) with purified DtxR protein was performed as previously described (33). Radiolabeled mntABC promoter/operator region DNA containing the MntR box was generated by PCR by using 100 ng of 32P-end-labeled primer mixed with 150 ng of unlabeled primer and template DNA from gel-purified 100-bp cold fragments containing the MntR box PCR, amplified by using primers 5′-CTCTTTTTCTTCAGTTAACATACT and 5′-GCCGCGTACTGGTATCGATAAGGA. Binding reactions were carried out with 10 mM Tris-HCl (pH 7.4)-5 mM MgCl2-50 mM KCl-1 mM dithiothreitol-5% glycerol-50 μg of calf thymus DNA/ml-5 μg of bovine serum albumin. Freshly prepared FeSO4 or MnSO4 was added at 125 μM. For the divalent metal ion-free sample, the divalent metal iron chelator EDTA at a concentration of 0.1 mM was added to the reaction solution. Binding reactions were equilibrated for 30 min. Samples were immediately submitted to electrophoresis at 200 V on a 5% nondenaturing polyacrylamide gel in 0.5× Tris-borate EDTA buffer. For the MntR EMSA, purified MntR recognized a 106-bp α-32P-labeled PCR product containing the 5′promoter/operator (P/O) region of the S. aureus mntABC P/O. The end-labeled fragments (20 fmol) were incubated for 15 min at room temperature with either purified MntR (100 ng) or His-tagged MntR (100 ng). To determine the metal-dependent nature of MntR binding to the mntABC, P/O samples were incubated prior to resolution by native gel electrophoresis in the presence of EDTA (0.5 mM).
RT and real-time PCR.
RNA was extracted from S. aureus grown for 24 to 36 h under iron-rich or iron-deleted conditions in minimal medium containing 50 μM MgCl2 with an RNeasy RNA kit (Qiagen, Inc.). Before cell lysis, the S. aureus was incubated in Tris-EDTA buffer with 50 μg of lysostaphin (Sigma, St. Louis, Mo.)/ml for 10 min at 37°C followed by silica bead beating to destroy the cell walls. The contaminating DNA in 10 μl of total RNA was digested by 2 μl of DNase I (Ambion). First-strand cDNA was synthesized from 1 μg of total RNA by incubation with mntABC reverse primer at 65°C for 15 min, followed by standing on ice for 5 min. RNA was incubated in reverse transcriptase buffer, 5 mM concentrations of deoxynucleoside triphosphates, and 16 U of murine leukemia virus reverse transcriptase (Roche) at 37°C for 1 h. Aliquots of first-strand cDNA were used in the PCR.
Molecular beacons were designed based on the specific 190-bp sequence of mntABC of S. aureus by using an in silico algorithm. A DNA folding program to estimate the stability of the hairpin stem (now available at http://www.cbr.nrc.ca/services/dnafold_e.php) was used. Each 50-μl reaction mixture contained a 0.2 μM concentration of the primer set, 100 μM concentrations of deoxynucleoside triphosphates, 1 U of Platinum Taq DNA polymerase (Life Technology), 3 mM MgCl2, 500 mM KCl, 10 mM Tris-HCl (pH 8.4), and 2.5 μl of reverse-transcribed sample. The PCRs were performed on the iCycler (Bio-Rad) with the following cycle conditions: preincubation at 65°C for 10 min followed by denaturation at 94°C for 2 min, and then 32 cycles of denaturation at 94°C for 30 s, annealing at 57°C for 1 min, and extension at 72°C for 30 s, followed by a final extension at 72°C for 10 min. Fluorescence was monitored during the annealing step.
Mouse skin abscess model.
Hairless but immunocompetent SKH1 male mice, 6 weeks of age, were anesthetized and injected subcutaneously with the MA2181 control and the MA2004 heterodiploid strains of S. aureus. There were five mice in each group. After 7 days bacterial numbers in each lesion were measured by CFU counts by using a previously described method (2, 4). Significant differences between groups were assessed by nonparametric statistics. Animals were treated according to the American Association for Accreditation of Laboratory Animal Care guidelines under a study protocol approved by the appropriate institutional review board.
RESULTS
Identification of the S. aureus mntR gene.
Using the 19-bp palindromic consensus sequence for DtxR identified in C. diphtheriae (5′-TTAGGTTAGCCTAACCTAA-3′), we found a similar sequence that corresponded with 16 bp of the 19-bp DtxR-box consensus sequence in contig 6186 from the then-unfinished S. aureus genome sequence data provided by TIGR (http://www.tigr.org) (14). Interestingly, this sequence is identical to the SirR box upstream of the S. epidermidis sitABC gene (11) (Fig. 1C). Moreover, a BLAST search of the downstream region revealed three open reading frames with strong matches to the sitABC operon of S. epidermidis. The three encoded proteins, now designated MntA, MntB, and MntC, have 83, 95, and 83% similarity and 67, 83, and 72% identity, respectively, with S. epidermidis SitA, SitB, and SitC. The S. aureus operon has the same orientation and organization as previously reported for the S. epidermidis sitABC operon (13).
DtxR(C102D) binds to the MntR box in the promoter region of mntABC in S. aureus.
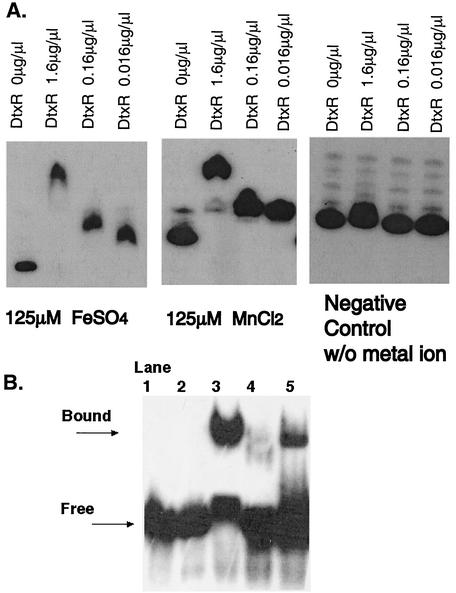
To examine whether the DtxR-related proteins are capable of binding to the putative MntR box, a DNA gel shift binding assay was done. DtxR(C102D), in which a cysteine is changed to an aspartate at position 102, was used because of greater stability and ease of purification. Both DtxR and DtxR(C102D) function as iron-dependent repressors. Although the regulatory activity of DtxR(C102D) is not as sensitive to the concentration of iron as is that of DtxR, it is able to suppress the expression of the tox gene (34). A 200-bp 32P-labeled MntR box upstream of the mntABC operon was amplified by PCR and incubated with DtxR(C102D) in the presence or absence of Fe2+ or Mn2+. The MntR box shifted in a metal ion-dependent manner with both Mn2+ and Fe2+ (Fig. 2A). Furthermore, the addition of EDTA, a divalent metal ion chelator, to the running buffer abolished binding even in the presence of metal ions (Fig. 2B and data not shown).
FIG. 2.
DtxR and MntR recognize the putative MntR box, and binding is metal ion dependent. (A) EMSA showed that increasing concentrations of DtxR in the presence of a 125 μM concentration of either FeSO4 (left panel) or MnCl2 (middle panel) bound to and shifted the MntR box in a dose-dependent manner. In the absence of metal ion (right panel), no shift was seen when DtxR was added to the MntR box. (B) EMSA with MntR demonstrated that divalent cations are required for binding and shifting of the MntR box. Lane 1, S. aureus mntABC promoter/operator (P/O); lane 2, mntABC P/O (in excess) with His-tagged MntR and 0.5 mM EDTA; lane 3, mntABC P/O with His-tagged MntR; lane 4, mntABC P/O with untagged MntR and 0.5 mM EDTA; lane 5, mntABC P/O with untagged MntR.
Expression of the constitutively active DtxR(E175K) hyperrepressor in S. aureus.
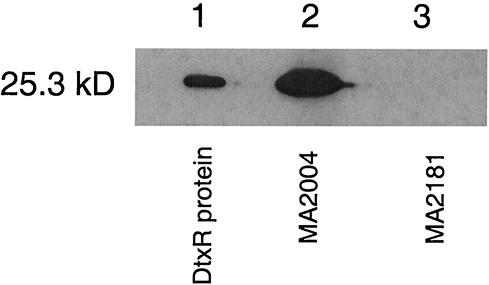
In order to express the C. diphtheriae dtxR(E175K) gene efficiently in S. aureus, we altered the codon usage of the first 10 codons of the dtxR(E175K) gene to S. aureus codon preferences and fused the modified gene to a 331-bp DNA fragment containing the native S. aureus mntR promoter and 5′ untranslated regulatory sequences. The resulting shuttle vector, pSADX, derived from pSPT181 (Fig. 1A and B), was transformed into the parent S. aureus strain RN6390 to produce the MA2004 heterodiploid strain. We confirmed that this strain expressed the 25.3-kDa protein by using anti-DtxR polyclonal antibodies in Western blotting (Fig. 3, lane 2). Purified DtxR run on the same gel was also detected by using this antibody (Fig. 3, lane 1), whereas the parent strain containing only an empty plasmid, i.e., the MA2181 control, showed no immunoreactivity by Western blotting (Fig. 3, lane 3).
FIG. 3.
Expression of the 25.3-kDa DtxR protein by the S. aureus MA2004 heterodiploid. The DtxR protein produced from the mntR promoter in the heterodiploid (lane 2) and native (lane 1) DtxR is recognized by an anti-DtxR antibody by Western blot analysis. The parent S. aureus strain containing an empty plasmid does not make a detectable cross-reacting protein, such as MntR, PerR, Zur, or Fur, the endogenous Fe-dependent regulators in S. aureus (lane 3).
Transcription of mntABC is divalent metal ion dependent by RT-PCR.
Previous studies have shown that the expression of S. aureus mntABC is MntR dependent and similarly that the expression of S. epidermidis sitABC is SirR dependent (11, 12). Hence, to evaluate the repressor function of DtxR(E175K) in the S. aureus heterodiploid strain MA2004, we chose to monitor mntABC transcriptional levels. RT-PCR using molecular beacons was carried out to quantify transcription of the mntABC operon. A 190-bp segment in mntB was selected by a computer algorithm as the best molecular beacon target sequence for mntABC. RNA samples from the S. aureus MA2181 control (RN6390 transformed with pSPT181) and from the MA2004 heterodiploid (RN6390 transformed with pSADX) were grown for 24 h in 125 μM FeSO4, 125 μM MnCl2, or Fe2+- and Mn2+-restricted medium. As may be seen in Table 1, the empty vector control strain MA2181 revealed a 78-fold derepression of mntABC transcription upon shifting from Mn2+-replete conditions to divalent metal ion-free conditions (0.5 ± 0.15 to 38.8 ± 4.14 pmol [means ± standard deviations]). Similarly, iron withdrawal led to a 491-fold derepression of mntABC transcription (0.079 ± 0.017 to 38.8 ± 4.14 pmol). Thus, in the control strains mntABC transcription appears to be dependent on either Mn2+ or Fe2+, as was observed by Horsburgh et al. (12). Addition of the constitutively active dtxR(E175K) gene in the heterodiploid strain MA2004 produced a reduction in the amount of derepression by iron or manganese withdrawal but did not fully achieve constitutively active repression. Withdrawal of Mn2+ produced a 24-fold derepression in contrast to the 78-fold derepression with the control strains (0.12 ± 0.008 to 2.91 ± 0.9 pmol). Likewise, withdrawal of iron led to a significant increase, from undetectable levels of transcription to 2.91 pmol. Comparing the amount of additional repression achieved by expression of the constitutively active dtxR(E175K) gene in the heterodiploid strain MA2004 in the presence of Mn2+ or Fe2+, there was a 4.2- or >10.4-fold increase in repression mediated by the presence of the constitutively active repressor, respectively. Under Fe2+-and Mn2+-free conditions, the repression mediated by the presence of DtxR(E175K) increased 13.3-fold (Table 1). Table 1 shows that under iron-replete conditions, the wild-type control strain MA2181 showed some degree of mntABC transcription (0.079 ± 0.017 pmol) while the heterodiploid MA2004 containing the constitutively active dtxR(E175K) gene repressed transcription of mntABC to <0.0076 pmol, which was below the limit of detection of our highly sensitive RT-PCR molecular beacon assay.
TABLE 1.
Quantitative mntABC transcriptional levels in S. aureus strains exposed to different divalent metal ion concentrations
| Metal ion (concn) | Mean ± SD (pmol) of mntABC transcriptional levels in:
|
Ratio | |
|---|---|---|---|
| MA2181 empty vector control (S. aureus pSPT181) | MA2004 heterodiploid [S. aureus pSPT181- PmntR::dtxR(E175K)] | ||
| Fe2+- and Mn2+-free | 38.8 ± 4.14 | 2.91 ± 0.9 | 13.3:1 |
| Fe2+ (125 μM) | 0.079 ± 0.017 | <0.0076a | >10.4:1 |
| Mn2+ (125 μM) | 0.5 ± 0.15 | 0.12 ± 0.008 | 4.2:1 |
Undetectable; the lower limit of detection was 0.0076 pmol.
Virulence assessment of the DtxR(E175K)-containing S. aureus strain by use of the mouse skin abscess model.
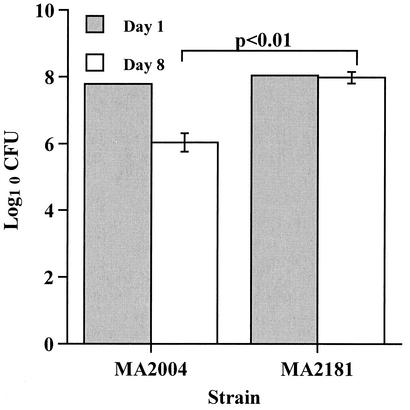
To evaluate whether MntR-mediated divalent cation-independent gene regulation plays a role in the virulence of S. aureus, we evaluated the pathogenicity of our empty vector control strain MA2181 and the heterodiploid MA2004 strain containing the constitutively active dtxR(E175K) gene. We postulated that the heterodiploid strain MA2004 might show a virulence defect, because our earlier data indicated that the presence of the dtxR(E175K) gene altered the ability of S. aureus to derepress mntR-dependent gene expression following a shift into Fe2+- and Mn2+-free media. A comparison of virulence between the MA2004 heterodiploid and the MA2181 control strain was accomplished by using the murine skin abscess model of infection. The inocula of MA2004 and MA2181 were 1.1 × 108 CFU and 0.6 × 108 CFU, respectively. Seven days after infection, a statistically significant difference between the mean CFU of the MA2181 control strain and the mean CFU of the MA2004 heterodiploid was recovered from each lesion (1.04 × 108 ± 0.46 × 108 CFU of MA2181 and 1.41 × 106 ± 0.96 × 106 CFU of MA2004) (Fig. 4).
FIG. 4.
The S. aureus MA2004 heterodiploid expressing DtxR is significantly less virulent in a mouse skin abscess model than is a strain carrying the empty MA2181 plasmid, suggesting that the MutR-mediated gene regulation is essential for virulence in this model.
DISCUSSION
In 1936 Pappenheimer and Johnson demonstrated that the addition of low concentrations of iron to the growth medium resulted in inhibition of toxin production by C. diphtheriae (20). Uchida et al. demonstrated that the diphtheria toxin structural gene, tox, was carried by corynebacteriophage β and that the addition of iron results in the immediate repression of toxin synthesis (38). Boyd et al. and Schmitt and Holmes described the molecular cloning of an iron-dependent regulatory element, dtxR (diphtheria toxin repressor), from genomic libraries of nontoxigenic C. diphtheriae (1, 27). DtxR can specifically bind to the palindromic sequence in the tox operator in the presence of iron and thereby repress the transcription of the tox gene. At low iron concentrations, DtxR is believed to undergo a conformational change which reduces its affinity for the tox operator and leads to derepression of tox transcription (37).
Recently, a single missense mutation was identified by PCR mutagenesis using a positive genetic selection system to clone dtxR alleles with hyperrepressor phenotypes. One of the mutant proteins encoded by this family of mutant alleles, DtxR(E175K), was found to bind to the tox operator site and function as an iron-independent repressor (31). At least seven promoters are already known to be negatively regulated by DtxR (14, 24-26, 29, 32) in C. diphtheriae. These promoters are the iron-regulated promoters (IRPs), designated IRP1 through IRP5, as well as the promoters for tox and heme oxygenase (hmuO). DtxR acts as a global regulatory protein to control the expression of diphtheria toxin, to regulate the high-affinity iron uptake system, and to govern expression of other iron sensitive genes.
Iron, in vivo, normally remains bound to host proteins such as ferritin, transferrin, lactoferrin, hemoglobin, and iron sulfur proteins; only a minor fraction (10 to 18%) is available in the free form (7, 36). Because free iron is scarce in mammalian hosts, it has been postulated that a shift into an iron-restrictive environment prompts not only the transcription of genes for high-affinity iron acquisition but also the transcription of virulence genes which enhance microbial survival within the host in pathogenic bacteria. The construction of heterodiploid bacteria in which one DtxR allele is a hyperrepressor would be anticipated to interfere with the normal induction of such genes at low iron concentrations, resulting in an attenuation of virulence in pathogenic bacteria such as S. aureus. This concept was supported by the work of Manabe et al., in which an M. tuberculosis DtxR(E175K)-expressing heterodiploid strain was attenuated compared to the wild-type control in a mouse model. These results suggested that the iron-dependent repressor IdeR may control genes essential for virulence in M. tuberculosis (17).
S. epidermidis has a sitABC operon, which works as an ATP binding cassette transporter, and a staphylococcal iron regulator repressor, SirR, with homology to DtxR, the gene for which is immediately upstream of the sitABC operon and is divergently transcribed as a 645-bp open reading frame. Since SirR can bind to the sitABC operator site (the SirR box) in the presence of ferrous or manganese ions, it appears to be an iron-dependent repressor of sitABC (11). S. aureus has recently been shown to have a homologous locus known as mntR and mntABC (12). The MntA, MntB, and MntC proteins share 83, 95, and 83% similarity and 67, 83, and 72% identity with S. epidermidis SitA, SitB, and SitC, respectively.
S. aureus MntA has a consensus ATP binding motif which includes a P loop (GPNGAGKA) (21). MntC has a consensus prelipoprotein signal peptide cleavage sequence of staphylococcal lipoprotein (ILAACG) at position 14 (10). Upstream of mntABC, there is strong homology with the repressor binding site, an 18- to 19-bp putative MntR box sequence in S. aureus closely related to the SirR box consensus in S. epidermidis and the DtxR consensus sequence in C. diphtheriae. Using allelic replacement to inactivate mntR, mntA, and mntH, Horsburgh et al. showed that the mutants have defective growth in the absence of metal and an increased susceptibility to intracellularly generated superoxide radicals due to a diminished uptake of manganese. They clearly demonstrated that MntR acts as a negative and positive regulator of mntABC and mntH, respectively (12).
In this study DtxR(C102D) was substituted for wild-type DtxR and used for EMSA in order to assess whether the DtxR-like proteins can bind to the S. aureus MntR box. Analysis of point mutations at residue 102 showed that only Asp can substitute for Cys to generate an active DtxR protein (34). However, the iron-dependent regulatory activity of DtxR(C102D) is not as strong as that of wild-type DtxR. Our data demonstrate that the DtxR(C102D) does indeed bind to the S. aureus MntR box in the presence of Fe2+ or Mn2+ ions, and the affinity seems to be related to the concentration of these metal ions. These data indicate that DtxR-related proteins, as well as MntR, may act as a repressor and control the expression of mntABC.
Real-time RT-PCR was carried out to identify the transcriptional levels of an mntABC mRNA under iron-free, high-ferric ion, or high-manganese ion conditions. To quantify the reverse transcripts, we employed real-time PCR using molecular beacons (39). Under manganese- and iron-free conditions, the MA2181 control strain expressed 38.8 ± 4.14 pmol of mntABC whereas the MA2004 heterodiploid expressed 2.91 ± 0.9 pmol of mntABC. These results confirmed the activity of DtxR(E175K) as an iron-insensitive hyperrepressor in S. aureus. In the presence of 125 μM ferrous sulfate, the level of expression of mntABC in the control strain MA2181 was 0.079 ± 0.017 pmol and the level in the MA2004 heterodiploid was below the level of detection. In the presence of 125 μM manganese ion, transcription in the MA2181 control strain was 0.5 ± 0.15 pmol and in the MA2004 heterodiploid strains it was 0.12 ± 0.008 pmol. These data show that mntABC transcription was repressed in both the wild-type and heterodiploid backgrounds and that the repression was stronger in the heterodiploid. DtxR(E175K) is deduced to be a repressor of the tox gene in iron-free conditions but in this study failed to fully repress the mntABC gene. This partial activity of DtxR(E175K) in S. aureus suggests that differences between the DtxR consensus binding sequence of C. diphtheriae and that of the S. aureus MntR box may influence the affinity of the foreign DtxR protein for DNA binding in S. aureus. These data further demonstrate that S. aureus mntABC transcription is Mn2+ dependent, suggesting that mntR is responsive to either Mn2+ or Fe2+ levels, as fully described by Horsburgh et al. (12).
For the virulence study, we used the subcutaneous abscess assay on hairless mice. On day 1, S. aureus was inoculated subcutaneously; the lesions were extracted on day 8, and colonies were counted. Bacterial survival by CFU counts of the MA2004 heterodiploid was statistically significantly less than that of the MA2181 control strain. In terms of the abscess size, MA2004 tended to cause smaller lesions than MA2181. However, the appearance of the lesions caused by the different strains was almost the same. Future experiments using different doses of heterodiploids could more clearly resolve these differences. This result indicates that DtxR(E175K) can attenuate the virulence of S. aureus. Also, mntABC or other genes regulated by MntR or DtxR may be related to virulence, since the expression of DtxR(E175K) reduced the virulence of S. aureus. Alternatively, the mutation in the repressors may affect growth or survival in the metal-limited environment found in mammalian hosts.
An earlier study in which S. aureus mntR was disrupted showed that there was no attenuation in the skin abscess model (12). The mntR mutant strain showed high-level expression of MntR-dependent genes irrespective of metal ion concentration. In contrast, our study, in which a constitutively active repressor DtxR(E175K) was added, revealed that mntABC transcription was significantly reduced at all metal ion concentrations, and especially under Fe2+-replete conditions. Thus, an inability to derepress MntR-dependent genes during infection (when free metal ion concentrations are believed to be low) attenuates S. aureus virulence, while constitutive derepression, as in the case of mntR mutation, does not. The findings also suggest that activators which interfere with metal ion release and derepression in repressors such as MntR may have novel antibacterial effects.
Acknowledgments
This work was supported by Public Health Service grants K08 AI 01689-01, AI36973, AI37856, and AI43846 from the National Institutes of Health. R. Harrison and J. Murphy were supported by a grant from NIAID 1R43AI49617-1, and M. Ando received partial support from Advanced Microbial Solutions Corp.
We are grateful for the assistance of Naomi Gauchet in preparing the manuscript.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Boyd, J., M. N. Oza, and J. R. Murphy. 1990. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc. Natl. Acad. Sci. USA 87:5968-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunce, C., L. Wheeler, G. Reed, J. Musser, and N. Barg. 1992. Murine model of cutaneous infection with gram-positive cocci. Infect. Immun. 60:2636-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnette-Curley, D., V. Wells, H. Viscount, C. L. Munro, J. C. Fenno, P. Fives-Taylor, and F. L. Macrina. 1995. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect. Immun. 63:4669-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, P. F., S. J. Foster, E. Ingham, and M. O. Clements. 1998. The Staphylococcus aureus alternative sigma factor sigmaB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J. Bacteriol. 180:6082-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, M. L. 1992. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 257:1050-1055. [DOI] [PubMed] [Google Scholar]
- 6.Dussurget, O., M. Rodriguez, and I. Smith. 1996. An ideR mutant of Mycobacterium smegmatis has derepressed siderophore production and an altered oxidative-stress response. Mol. Microbiol. 22:535-544. [DOI] [PubMed] [Google Scholar]
- 7.Guerinot, M. L. 1994. Microbial iron transport. Annu. Rev. Microbiol. 48:743-772. [DOI] [PubMed] [Google Scholar]
- 8.Gunter-Seeboth, K., and T. Schupp. 1995. Cloning and sequence analysis of the Corynebacterium diphtheriae dtxR homologue from Streptomyces lividans and S. pilosus encoding a putative iron repressor protein. Gene 166:117-119. [DOI] [PubMed] [Google Scholar]
- 9.Hardham, J. M., L. V. Stamm, S. F. Porcella, J. G. Frye, N. Y. Barnes, J. K. Howell, S. L. Mueller, J. D. Radolf, G. M. Weinstock, and S. J. Norris. 1997. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transport system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene 197:47-64. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, S., and H. C. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 11.Hill, P. J., A. Cockayne, P. Landers, J. A. Morrissey, C. M. Sims, and P. Williams. 1998. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect. Immun. 66:4123-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horsburgh, M. J., S. J. Wharton, A. G. Cox, E. Ingham, S. Peacock, and S. J. Foster. 2002. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol. Microbiol. 44:1269-1286. [DOI] [PubMed] [Google Scholar]
- 13.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and role in virulence. Infect. Immun. 68:4441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, J. H., T. Wang, K. Ault, J. Liu, M. P. Schmitt, and R. K. Holmes. 1997. Identification and characterization of three new promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. Infect. Immun. 65:4273-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsay, J. A., and T. V. Riley. 1994. Staphylococcal iron requirements, siderophore production, and iron-regulated protein expression. Infect. Immun. 62:2309-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 17.Manabe, Y. C., B. J. Saviola, L. Sun, J. R. Murphy, and W. R. Bishai. 1999. Attenuation of virulence in Mycobacterium tuberculosis expressing a constitutively active iron repressor. Proc. Natl. Acad. Sci. USA 96:12844-12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novick, R. P., I. Edelman, and S. Lofdahl. 1986. Small Staphylococcus aureus plasmids are transduced as linear multimers that are formed and resolved by replicative processes. J. Mol. Biol. 192:209-220. [DOI] [PubMed] [Google Scholar]
- 19.Oguiza, J. A., X. Tao, A. T. Marcos, J. F. Martin, and J. R. Murphy. 1995. Molecular cloning, DNA sequence analysis, and characterization of the Corynebacterium diphtheriae dtxR homolog from Brevibacterium lactofermentum. J. Bacteriol. 177:465-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappenheimer, A. M., Jr. 1977. Diphtheria toxin. Annu. Rev. Biochem. 46:69-94. [DOI] [PubMed] [Google Scholar]
- 21.Saraste, M., P. R. Sibbald, and A. Wittinghofer. 1990. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 15:430-434. [DOI] [PubMed] [Google Scholar]
- 22.Schenk, S., and R. A. Laddaga. 1992. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol. Lett. 73:133-138. [DOI] [PubMed] [Google Scholar]
- 23.Schindler, C. A., and V. T. Schuhardt. 1964. Lysostaphin: a new bacteriolytic agent for the staphylococcus. Proc. Natl. Acad. Sci. USA 51:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt, M. P. 1997. Transcription of the Corynebacterium diphtheriae hmuO gene is regulated by iron and heme. Infect. Immun. 65:4634-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt, M. P. 1997. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J. Bacteriol. 179:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt, M. P., and R. K. Holmes. 1994. Cloning, sequence, and footprint analysis of two promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. J. Bacteriol. 176:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt, M. P., and R. K. Holmes. 1991. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect. Immun. 59:1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt, M. P., M. Predich, L. Doukhan, I. Smith, and R. K. Holmes. 1995. Characterization of an iron-dependent regulatory protein (IdeR) of Mycobacterium tuberculosis as a functional homolog of the diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae. Infect. Immun. 63:4284-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitt, M. P., B. G. Talley, and R. K. Holmes. 1997. Characterization of lipoprotein IRP1 from Corynebacterium diphtheriae, which is regulated by the diphtheria toxin repressor (DtxR) and iron. Infect. Immun. 65:5364-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheagren, J. N. 1984. Staphylococcus aureus. The persistent pathogen (first of two parts). N. Engl. J. Med. 310:1368-1373. [DOI] [PubMed] [Google Scholar]
- 31.Sun, L., J. vanderSpek, and J. R. Murphy. 1998. Isolation and characterization of iron-independent positive dominant mutants of the diphtheria toxin repressor DtxR. Proc. Natl. Acad. Sci. USA 95:14985-14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tai, S. P., A. E. Krafft, P. Nootheti, and R. K. Holmes. 1990. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb. Pathog. 9:267-273. [DOI] [PubMed] [Google Scholar]
- 33.Tao, X., J. Boyd, and J. R. Murphy. 1992. Specific binding of the diphtheria tox regulatory element DtxR to the tox operator requires divalent heavy metal ions and a 9-base-pair interrupted palindromic sequence. Proc. Natl. Acad. Sci. USA 89:5897-5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao, X., and J. R. Murphy. 1993. Cysteine-102 is positioned in the metal binding activation site of the Corynebacterium diphtheriae regulatory element DtxR. Proc. Natl. Acad. Sci. USA 90:8524-8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao, X., H. Y. Zeng, and J. R. Murphy. 1995. Transition metal ion activation of DNA binding by the diphtheria tox repressor requires the formation of stable homodimers. Proc. Natl. Acad. Sci. USA 92:6803-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trivier, D., and R. J. Courcol. 1996. Iron depletion and virulence in Staphylococcus aureus. FEMS Microbiol. Lett. 141:117-127. [DOI] [PubMed] [Google Scholar]
- 37.Twigg, P. D., G. Parthasarathy, L. Guerrero, T. M. Logan, and D. L. D. Caspar. 2001. Disordered to ordered folding in the regulation of diphtheria toxin repressor activity. Proc. Natl. Acad. Sci. USA 98:11259-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uchida, T., D. M. Gill, and A. M. Pappenheimer, Jr. 1971. Mutation in the structural gene for diphtheria toxin carried by temperate phage. Nat. New Biol. 233:8-11. [DOI] [PubMed] [Google Scholar]
- 39.van Schie, R. C., S. A. Marras, J. M. Conroy, N. J. Nowak, J. J. Catanese, and P. J. de Jong. 2000. Semiautomated clone verification by real-time PCR using molecular beacons. BioTechniques 29:1296-1306. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson, B. J. 1997. Biology, p. 1-38. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone, New York, N.Y.