Abstract
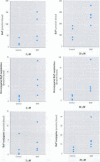
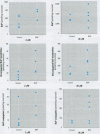
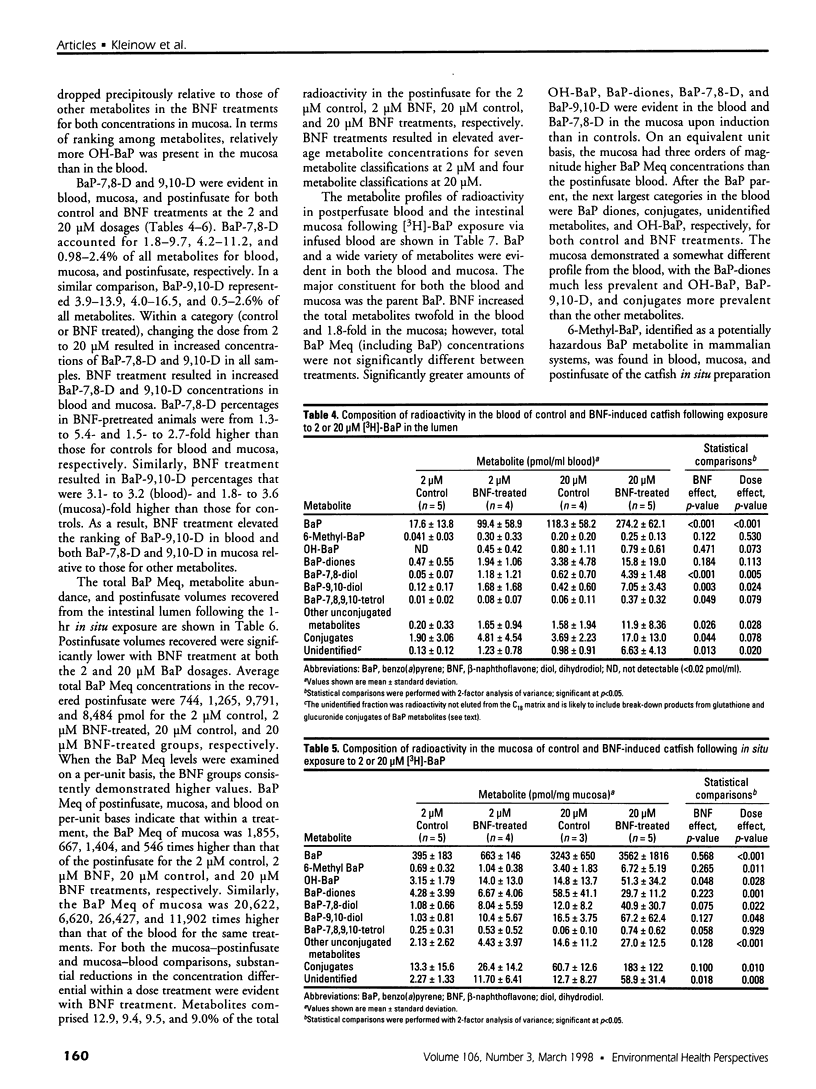
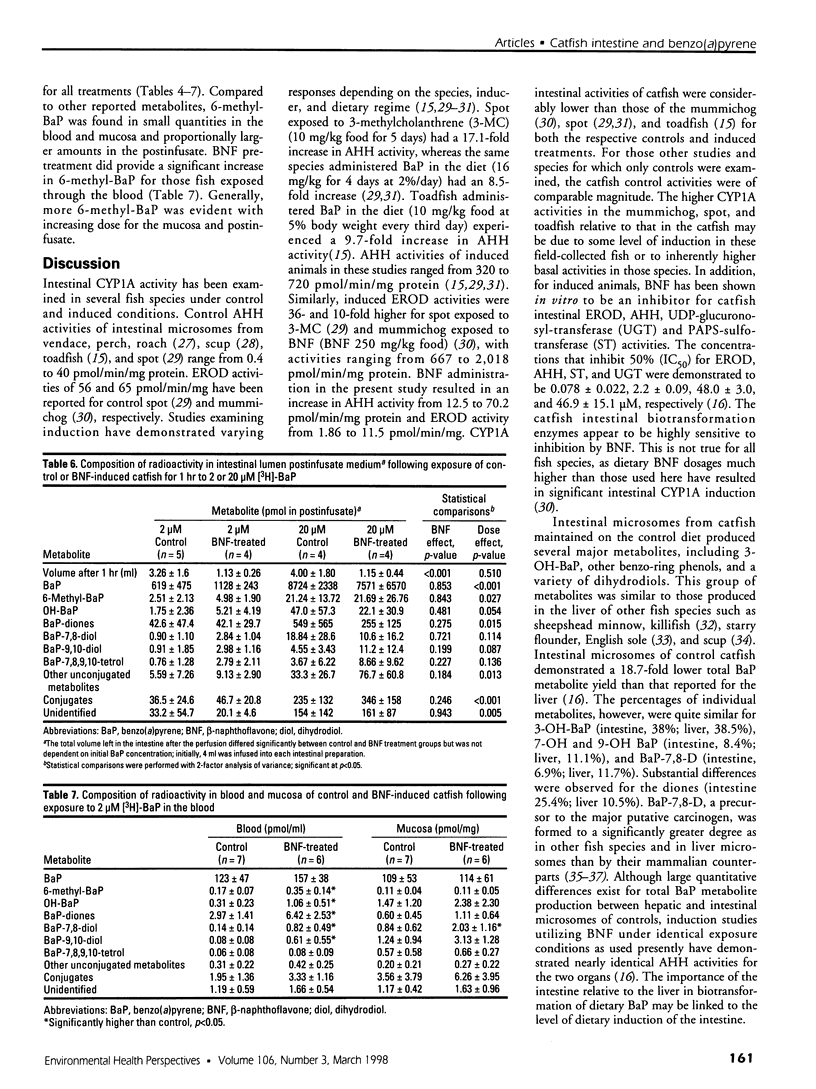
In the aquatic environment, diet is an important route of exposure for the common contaminant and procarcinogen benzo(a)pyrene (BaP). Dietary organisms vary in their BaP content and in contaminated areas often contain other xenobiotics including cytochrome P4501A inducers. This study examined the effect of dose and previous dietary exposure to the inducer ss-naphthoflavone (BNF) upon the intestinal metabolism of BaP and the systemic bioavailability of BaP-derived products in catfish. BaP was administered at 2 and 20 microM into in situ-isolated perfused intestines of control and BNF-pretreated catfish. The intestine formed an array of metabolites in all treatments including potentially hazardous metabolites such as BaP-7,8 and 9,10 dihydrodiols and 6-methyl-BaP. BNF treatment disproportionally increased the contribution of BaP-7,8 and 9,10 dihydrodiols relative to the contributions of other metabolites. A greater percentage of metabolites was evident as conjugates in 2 microM controls, whereas a greater percentage of unconjugated metabolites was evident for 20 microM controls and BNF treatments of both dosages. BNF pretreatment and the higher 20 microM BaP dosage resulted in greater bioavailability, with 2.6-5.5-fold and 3.0-6. 3-fold increases in systemically available BaP products, respectively. Metabolites represented 10.2-23.1% of the increased bioavailability with BNF treatment, suggesting that mechanisms, in addition to induced metabolism, may be operative. These results indicate that intestinal bioavailability, level of biotransformation, and the metabolic profile of BaP-derived products entering the blood from the intestine may be altered by dose and dietary BNF pretreatment.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Pesonen M., Johansson C. Differential induction of cytochrome P-450-dependent monooxygenase, epoxide hydrolase, glutathione transferase and UDP glucuronosyl transferase activities in the liver of the rainbow trout by beta-naphthoflavone or Clophen A50. Biochem Pharmacol. 1985 Sep 15;34(18):3309–3314. doi: 10.1016/0006-2952(85)90351-x. [DOI] [PubMed] [Google Scholar]
- Bock K. W., von Clausbruch U. C., Winne D. Absorption and metabolism of naphthalene and benzo(a)pyrene in the rat jejunum in situ. Med Biol. 1979 Oct;57(5):262–264. [PubMed] [Google Scholar]
- Burke M. D., Mayer R. T. Ethoxyresorufin: direct fluorimetric assay of a microsomal O-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab Dispos. 1974 Nov-Dec;2(6):583–588. [PubMed] [Google Scholar]
- Cavalieri E. L., Devanesan P. D., Rogan E. G. Radical cations in the horseradish peroxidase and prostaglandin H synthase mediated metabolism and binding of benzo[a]pyrene to deoxyribonucleic acid. Biochem Pharmacol. 1988 Jun 1;37(11):2183–2187. doi: 10.1016/0006-2952(88)90579-5. [DOI] [PubMed] [Google Scholar]
- Eling T. E., Thompson D. C., Foureman G. L., Curtis J. F., Hughes M. F. Prostaglandin H synthase and xenobiotic oxidation. Annu Rev Pharmacol Toxicol. 1990;30:1–45. doi: 10.1146/annurev.pa.30.040190.000245. [DOI] [PubMed] [Google Scholar]
- Flesher J. W., Myers S. R., Stansbury K. H. The site of substitution of the methyl group in the bioalkylation of benzo[a]pyrene. Carcinogenesis. 1990 Mar;11(3):493–496. doi: 10.1093/carcin/11.3.493. [DOI] [PubMed] [Google Scholar]
- Goldin B. R. Intestinal microflora: metabolism of drugs and carcinogens. Ann Med. 1990 Feb;22(1):43–48. doi: 10.3109/07853899009147240. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Korzekwa K. R. Cytochromes P450 expression systems. Annu Rev Pharmacol Toxicol. 1995;35:369–390. doi: 10.1146/annurev.pa.35.040195.002101. [DOI] [PubMed] [Google Scholar]
- Guiney P. D., Smolowitz R. M., Peterson R. E., Stegeman J. J. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in vascular endothelium with toxicity in early life stages of lake trout. Toxicol Appl Pharmacol. 1997 Apr;143(2):256–273. doi: 10.1006/taap.1996.8051. [DOI] [PubMed] [Google Scholar]
- Haasch M. L., Quardokus E. M., Sutherland L. A., Goodrich M. S., Lech J. J. Hepatic CYP1A1 induction in rainbow trout by continuous flowthrough exposure to beta-naphthoflavone. Fundam Appl Toxicol. 1993 Jan;20(1):72–82. doi: 10.1006/faat.1993.1009. [DOI] [PubMed] [Google Scholar]
- Hattemer-Frey H. A., Travis C. C. Benzo-a-pyrene: environmental partitioning and human exposure. Toxicol Ind Health. 1991 May;7(3):141–157. doi: 10.1177/074823379100700303. [DOI] [PubMed] [Google Scholar]
- Hawkins W. E., Walker W. W., Overstreet R. M., Lytle T. F., Lytle J. S. Dose-related carcinogenic effects of water-borne benzo[a]pyrene on livers of two small fish species. Ecotoxicol Environ Saf. 1988 Dec;16(3):219–231. doi: 10.1016/0147-6513(88)90052-8. [DOI] [PubMed] [Google Scholar]
- Holder G., Yagi H., Dansette P., Jerina D. M., Levin W., Lu A. Y., Conney A. H. Effects of inducers and epoxide hydrase on the metabolism of benzo(a)pyrene by liver microsomes and a reconstituted system: analysis by high pressure liquid chromatography. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4356–4360. doi: 10.1073/pnas.71.11.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M. O., Altman A. H., Li C. L., Schell J. D., Jr Biotransformation, hepatopancreas DNA binding and pharmacokinetics of benzo[a]pyrene after oral and parenteral administration to the American lobster, Homarus americanus. Chem Biol Interact. 1995 Mar 30;95(1-2):141–160. doi: 10.1016/0009-2797(94)03355-2. [DOI] [PubMed] [Google Scholar]
- James M. O., Altman A. H., Morris K., Kleinow K. M., Tong Z. Dietary modulation of phase 1 and phase 2 activities with benzo(a)pyrene and related compounds in the intestine but not the liver of the channel catfish, Ictalurus punctatus. Drug Metab Dispos. 1997 Mar;25(3):346–354. [PubMed] [Google Scholar]
- James M. O., Bend J. R. Polycyclic aromatic hydrocarbon induction of cytochrome P-450-dependent mixed-function oxidases in marine fish. Toxicol Appl Pharmacol. 1980 Jun 15;54(1):117–133. doi: 10.1016/0041-008x(80)90012-5. [DOI] [PubMed] [Google Scholar]
- James M. O., Little P. J. Modification of benzo(a)pyrene metabolism in hepatic microsomes from untreated and induced rats by imidazole derivatives which inhibit monooxygenase activity and enhance epoxide hydrolase activity. Drug Metab Dispos. 1983 Jul-Aug;11(4):350–354. [PubMed] [Google Scholar]
- James M. O., Schell J. D., Boyle S. M., Altman A. H., Cromer E. A. Southern flounder hepatic and intestinal metabolism and DNA binding of benzo[a]pyrene (BaP) metabolites following dietary administration of low doses of BaP, BaP-7,8-dihydrodiol or a BaP metabolite mixture. Chem Biol Interact. 1991;79(3):305–321. doi: 10.1016/0009-2797(91)90111-j. [DOI] [PubMed] [Google Scholar]
- Kinoshita N., Shears B., Gelboin H. V. K-region and non-K-region metabolism of benzo(a)pyrene by rat liver microsomes. Cancer Res. 1973 Aug;33(8):1937–1944. [PubMed] [Google Scholar]
- Kirby G. M., Bend J. R., Smith I. R., Hayes M. A. The role of glutathione S-transferases in the hepatic metabolism of benzo[a]pyrene in white suckers (Catostomus commersoni) from polluted and reference sites in the Great Lakes. Comp Biochem Physiol C. 1990;95(1):25–30. doi: 10.1016/0742-8413(90)90077-m. [DOI] [PubMed] [Google Scholar]
- Kleeman J. M., Olson J. R., Peterson R. E. Species differences in 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity and biotransformation in fish. Fundam Appl Toxicol. 1988 Feb;10(2):206–213. doi: 10.1016/0272-0590(88)90304-1. [DOI] [PubMed] [Google Scholar]
- Kleinow K. M., Haasch M. L., Williams D. E., Lech J. J. A comparison of hepatic P450 induction in rat and trout (Oncorhynchus mykiss): delineation of the site of resistance of fish to phenobarbital-type inducers. Comp Biochem Physiol C. 1990;96(2):259–270. doi: 10.1016/0742-8413(90)90006-u. [DOI] [PubMed] [Google Scholar]
- Kloepper-Sams P. J., Stegeman J. J. The temporal relationships between P450E protein content, catalytic activity, and mRNA levels in the teleost Fundulus heteroclitus following treatment with beta-naphthoflavone. Arch Biochem Biophys. 1989 Feb 1;268(2):525–535. doi: 10.1016/0003-9861(89)90319-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Komatsu S., Nishi T., Hara H., Hayashi K. ATP-dependent transport for glucuronides in canalicular plasma membrane vesicles. Biochem Biophys Res Commun. 1991 Apr 30;176(2):622–626. doi: 10.1016/s0006-291x(05)80229-3. [DOI] [PubMed] [Google Scholar]
- Lindström-Seppä P., Koivusaari U., Hänninen O. Extrahepatic xenobiotic metabolism in North-European Freshwater fish. Comp Biochem Physiol C. 1981;69(2):259–263. doi: 10.1016/0306-4492(81)90137-4. [DOI] [PubMed] [Google Scholar]
- Lindwall G., Boyer T. D. Excretion of glutathione conjugates by primary cultured rat hepatocytes. J Biol Chem. 1987 Apr 15;262(11):5151–5158. [PubMed] [Google Scholar]
- Little P. J., James M. O., Pritchard J. B., Bend J. R. Benzo(a)pyrene metabolism in hepatic microsomes from feral and 3-methycholanthrene-treated southern flounder, Paralichthys lethostigma. J Environ Pathol Toxicol Oncol. 1984 Jul;5(4-5):309–320. [PubMed] [Google Scholar]
- Maccubbin A. E., Black P., Trzeciak L., Black J. J. Evidence for polynuclear aromatic hydrocarbons in the diet of bottom-feeding fish. Bull Environ Contam Toxicol. 1985 Jun;34(6):876–882. doi: 10.1007/BF01609820. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Genetic differences in microsomal electron transport: the Ah locus. Methods Enzymol. 1978;52:226–240. doi: 10.1016/s0076-6879(78)52026-0. [DOI] [PubMed] [Google Scholar]
- Nemoto N., Gelboin H. V. Enzymatic conjugation of benzo (a) pyrene oxides, phenols and dihydrodiols with UDP-glucuronic acid. Biochem Pharmacol. 1976 May 15;25(10):1221–1226. doi: 10.1016/0006-2952(76)90373-7. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Mallett A. K., Wise A. The effect of diet on the mammalian gut flora and its metabolic activities. Crit Rev Toxicol. 1985;16(1):31–103. doi: 10.3109/10408448509041324. [DOI] [PubMed] [Google Scholar]
- Salhab A. S., James M. O., Wang S. L., Shiverick K. T. Positional metabolism of benzo(a)pyrene in rat placenta and maternal liver. Comparison of induction effects. Drug Metab Dispos. 1986 Jul-Aug;14(4):471–476. [PubMed] [Google Scholar]
- Shiverick K. T., Swanson C., Salhab A. S., James M. O. Differential induction of ethoxyresorufin-O-deethylase in tissues of the rat placenta. J Pharmacol Exp Ther. 1986 Sep;238(3):1108–1113. [PubMed] [Google Scholar]
- Stansbury K. H., Flesher J. W., Gupta R. C. Mechanism of aralkyl-DNA adduct formation from benzo[a]pyrene in vivo. Chem Res Toxicol. 1994 Mar-Apr;7(2):254–259. doi: 10.1021/tx00038a019. [DOI] [PubMed] [Google Scholar]
- Stegeman J. J., Binder R. L., Orren A. Hepatic and extrahepatic microsomal electron transport components and mixed-function oxygenases in the marine fish Stenotomus versicolor. Biochem Pharmacol. 1979 Dec 1;28(23):3431–3439. doi: 10.1016/0006-2952(79)90083-2. [DOI] [PubMed] [Google Scholar]
- Stegeman J. J., Miller M. R., Hinton D. E. Cytochrome P450IA1 induction and localization in endothelium of vertebrate (teleost) heart. Mol Pharmacol. 1989 Nov;36(5):723–729. [PubMed] [Google Scholar]
- Surh Y. J., Miller J. A. Roles of electrophilic sulfuric acid ester metabolites in mutagenesis and carcinogenesis by some polynuclear aromatic hydrocarbons. Chem Biol Interact. 1994 Jun;92(1-3):351–362. doi: 10.1016/0009-2797(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Miranda C. L., Henderson M. C., Buhler D. R., Williams D. E., Bailey G. S. Inhibition of in vitro aflatoxin B1-DNA binding in rainbow trout by CYP1A inhibitors: alpha-naphthoflavone, beta-naphthoflavone and trout CYP1A1 peptide antibody. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995 Mar;110(3):273–280. doi: 10.1016/0742-8413(95)00005-9. [DOI] [PubMed] [Google Scholar]
- Van Veld P. A., Patton J. S., Lee R. F. Effect of preexposure to dietary benzo[a]pyrene (BP) on the first-pass metabolism of BP by the intestine of toadfish (Opsanus tau): in vivo studies using portal vein-catheterized fish. Toxicol Appl Pharmacol. 1988 Feb;92(2):255–265. doi: 10.1016/0041-008x(88)90385-7. [DOI] [PubMed] [Google Scholar]
- Van Veld P. A., Vetter R. D., Lee R. F., Patton J. S. Dietary fat inhibits the intestinal metabolism of the carcinogen benzo[a]pyrene in fish. J Lipid Res. 1987 Jul;28(7):810–817. [PubMed] [Google Scholar]
- Varanasi U., Nishimoto M., Reichert W. L., Le Eberhart B. T. Comparative metabolism of benzo(a)pyrene and covalent binding to hepatic DNA in English sole, starry flounder, and rat. Cancer Res. 1986 Aug;46(8):3817–3824. [PubMed] [Google Scholar]
- Vetter R. D., Carey M. C., Patton J. S. Coassimilation of dietary fat and benzo(a)pyrene in the small intestine: an absorption model using the killifish. J Lipid Res. 1985 Apr;26(4):428–434. [PubMed] [Google Scholar]
- Yeh G. C., Lopaczynska J., Poore C. M., Phang J. M. A new functional role for P-glycoprotein: efflux pump for benzo(alpha)pyrene in human breast cancer MCF-7 cells. Cancer Res. 1992 Dec 1;52(23):6692–6695. [PubMed] [Google Scholar]
- van Veld P. A., Stegeman J. J., Woodin B. R., Patton J. S., Lee R. F. Induction of monooxygenase activity in the intestine of spot (Leiostomus xanthurus), a marine teleost, by dietary polycyclic aromatic hydrocarbons. Drug Metab Dispos. 1988 Sep-Oct;16(5):659–665. [PubMed] [Google Scholar]