Abstract
Interest in the toxicological aspects of oxidative stress has grown in recent years, and research has become increasingly focused on the mechanistic aspects of oxidative damage and cellular responses in biological systems. Toxic consequences of oxidative stress at the subcellular level include lipid peroxidation and oxidative damage to DNA and proteins. These effects are often used as end points in the study of oxidative stress. Typically, mammalian species have been used as models to study oxidative stress and to elucidate the mechanisms underlying cellular damage and response, largely because of the interest in human health issues surrounding oxidative stress. However, it is becoming apparent that oxidative stress also affects aquatic organisms exposed to environmental pollutants. Research in fish has demonstrated that mammalian and piscine systems exhibit similar toxicological and adaptive responses to oxidative stress. This suggests that piscine models, in addition to traditional mammalian models, may be useful for further understanding the mechanisms underlying the oxidative stress response.
Full text
PDF


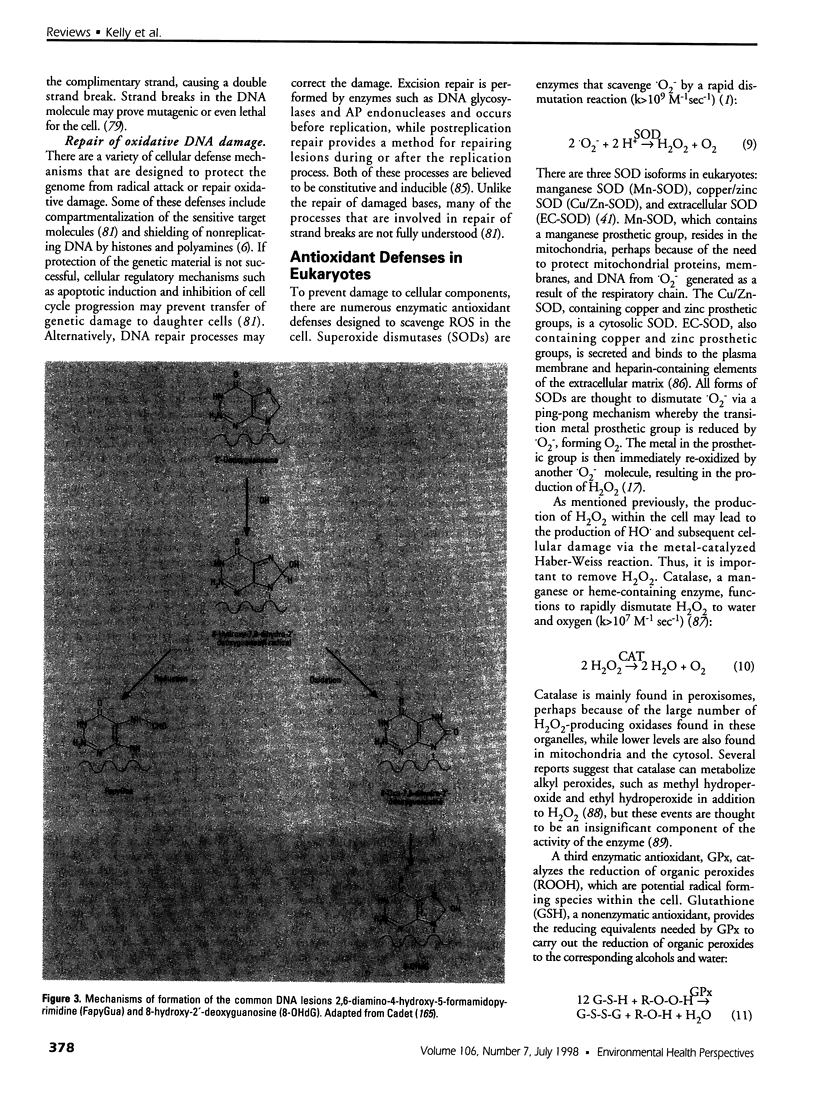






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Altman S. A., Zastawny T. H., Randers-Eichhorn L., Cacciuttolo M. A., Akman S. A., Dizdaroglu M., Rao G. Formation of DNA-protein cross-links in cultured mammalian cells upon treatment with iron ions. Free Radic Biol Med. 1995 Dec;19(6):897–902. doi: 10.1016/0891-5849(95)00095-f. [DOI] [PubMed] [Google Scholar]
- Babich H., Palace M. R., Stern A. Oxidative stress in fish cells: in vitro studies. Arch Environ Contam Toxicol. 1993 Feb;24(2):173–178. doi: 10.1007/BF01141344. [DOI] [PubMed] [Google Scholar]
- Bachur N. R., Gordon S. L., Gee M. V. A general mechanism for microsomal activation of quinone anticancer agents to free radicals. Cancer Res. 1978 Jun;38(6):1745–1750. [PubMed] [Google Scholar]
- Bailey G. S., Williams D. E., Hendricks J. D. Fish models for environmental carcinogenesis: the rainbow trout. Environ Health Perspect. 1996 Mar;104 (Suppl 1):5–21. doi: 10.1289/ehp.96104s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey G., Hendricks J., Dashwood R. Anticarcinogenesis in fish. Mutat Res. 1992 Jun;267(2):243–250. doi: 10.1016/0027-5107(92)90068-d. [DOI] [PubMed] [Google Scholar]
- Baud L., Hagege J., Sraer J., Rondeau E., Perez J., Ardaillou R. Reactive oxygen production by cultured rat glomerular mesangial cells during phagocytosis is associated with stimulation of lipoxygenase activity. J Exp Med. 1983 Dec 1;158(6):1836–1852. doi: 10.1084/jem.158.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlett B. S., Stadtman E. R. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997 Aug 15;272(33):20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Bjørneboe A., Bjørneboe G. E., Drevon C. A. Absorption, transport and distribution of vitamin E. J Nutr. 1990 Mar;120(3):233–242. doi: 10.1093/jn/120.3.233. [DOI] [PubMed] [Google Scholar]
- Bruhwyler J., Chleide E., Liégeois J. F., Carreer F. Nitric oxide: a new messenger in the brain. Neurosci Biobehav Rev. 1993 Winter;17(4):373–384. doi: 10.1016/s0149-7634(05)80114-9. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984 May 11;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Joyce A., Ingold K. U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983 Feb 15;221(1):281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Caron de Fromentel C., Pakdel F., Chapus A., Baney C., May P., Soussi T. Rainbow trout p53: cDNA cloning and biochemical characterization. Gene. 1992 Mar 15;112(2):241–245. doi: 10.1016/0378-1119(92)90383-z. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Aust A. E. Photochemical reduction of ferric iron by chelators results in DNA strand breaks. Arch Biochem Biophys. 1993 Feb 1;300(2):544–550. doi: 10.1006/abbi.1993.1075. [DOI] [PubMed] [Google Scholar]
- Cohen G. M., d'Arcy Doherty M. Free radical mediated cell toxicity by redox cycling chemicals. Br J Cancer Suppl. 1987 Jun;8:46–52. [PMC free article] [PubMed] [Google Scholar]
- Comporti M. Lipid peroxidation and cellular damage in toxic liver injury. Lab Invest. 1985 Dec;53(6):599–623. [PubMed] [Google Scholar]
- Costa M., Zhitkovich A., Gargas M., Paustenbach D., Finley B., Kuykendall J., Billings R., Carlson T. J., Wetterhahn K., Xu J. Interlaboratory validation of a new assay for DNA-protein crosslinks. Mutat Res. 1996 Jul 10;369(1-2):13–21. doi: 10.1016/s0165-1218(96)90043-9. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Oxidative damage to DNA in mammalian chromatin. Mutat Res. 1992 Sep;275(3-6):331–342. doi: 10.1016/0921-8734(92)90036-o. [DOI] [PubMed] [Google Scholar]
- Dooley T. P. Recent advances in cutaneous melanoma oncogenesis research. Oncol Res. 1994;6(1):1–9. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases: regularities and irregularities. Harvey Lect. 1983 1984;79:51–75. [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Fuhrman B., Oiknine J., Aviram M. Iron induces lipid peroxidation in cultured macrophages, increases their ability to oxidatively modify LDL, and affects their secretory properties. Atherosclerosis. 1994 Nov;111(1):65–78. doi: 10.1016/0021-9150(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Furono K., Suetsuga T., Sugihara N. Effects of metal ions on lipid peroxidation in cultured rat hepatocytes loaded with alpha-linolenic acid. J Toxicol Environ Health. 1996 Jun 7;48(2):121–129. doi: 10.1080/009841096161375. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Aspects to consider when detecting and measuring lipid peroxidation. Free Radic Res Commun. 1986;1(3):173–184. doi: 10.3109/10715768609083149. [DOI] [PubMed] [Google Scholar]
- Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9(1):1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Hasspieler B. M., Behar J. V., Di Giulio R. T. Glutathione-dependent defense in channel catfish (Ictalurus punctatus) and brown bullhead (Ameriurus nebulosus). Ecotoxicol Environ Saf. 1994 Jun;28(1):82–90. doi: 10.1006/eesa.1994.1036. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Irani K., Xia Y., Zweier J. L., Sollott S. J., Der C. J., Fearon E. R., Sundaresan M., Finkel T., Goldschmidt-Clermont P. J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997 Mar 14;275(5306):1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Iyengar A., Müller F., Maclean N. Regulation and expression of transgenes in fish -- a review. Transgenic Res. 1996 May;5(3):147–166. doi: 10.1007/BF01969704. [DOI] [PubMed] [Google Scholar]
- Janero D. R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Kantengwa S., Polla B. S. Phagocytosis of Staphylococcus aureus induces a selective stress response in human monocytes-macrophages (M phi): modulation by M phi differentiation and by iron. Infect Immun. 1993 Apr;61(4):1281–1287. doi: 10.1128/iai.61.4.1281-1287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappus H. Overview of enzyme systems involved in bio-reduction of drugs and in redox cycling. Biochem Pharmacol. 1986 Jan 1;35(1):1–6. doi: 10.1016/0006-2952(86)90544-7. [DOI] [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Binding of human extracellular-superoxide dismutase C to cultured cell lines and to blood cells. Lab Invest. 1989 May;60(5):659–666. [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- King J. K., Egner P. A., Kensler T. W. Generation of DNA base modification following treatment of cultured murine keratinocytes with benzoyl peroxide. Carcinogenesis. 1996 Feb;17(2):317–320. doi: 10.1093/carcin/17.2.317. [DOI] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Inhibition and reactivation of Mn-catalase. Implications for valence changes at the active site manganese. J Biol Chem. 1983 Nov 25;258(22):13646–13648. [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982 May 25;257(10):5751–5754. [PubMed] [Google Scholar]
- Korbashi P., Kohen R., Katzhendler J., Chevion M. Iron mediates paraquat toxicity in Escherichia coli. J Biol Chem. 1986 Sep 25;261(27):12472–12476. [PubMed] [Google Scholar]
- Kosugi H., Kato T., Kikugawa K. Formation of yellow, orange, and red pigments in the reaction of alk-2-enals with 2-thiobarbituric acid. Anal Biochem. 1987 Sep;165(2):456–464. doi: 10.1016/0003-2697(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Krause M. K., Rhodes L. D., Van Beneden R. J. Cloning of the p53 tumor suppressor gene from the Japanese medaka (Oryzias latipes) and evaluation of mutational hotspots in MNNG-exposed fish. Gene. 1997 Apr 11;189(1):101–106. doi: 10.1016/s0378-1119(96)00841-4. [DOI] [PubMed] [Google Scholar]
- Krinsky N. I. Mechanism of action of biological antioxidants. Proc Soc Exp Biol Med. 1992 Jun;200(2):248–254. doi: 10.3181/00379727-200-43429. [DOI] [PubMed] [Google Scholar]
- Kuo C. H., Uetsuki T., Kim C. H., Tanaka H., Li B. S., Taira E., Higuchi H., Okamoto H., Yoshikawa K., Miki N. Determination of a necdin cis-acting element required for neuron specific expression by using zebra fish. Biochem Biophys Res Commun. 1995 Jun 15;211(2):438–446. doi: 10.1006/bbrc.1995.1833. [DOI] [PubMed] [Google Scholar]
- Landi L., Cabrini L., Fiorentini D., Stefanelli C., Pedulli G. F. The antioxidant activity of ubiquinol-3 in homogeneous solution and in liposomes. Chem Phys Lipids. 1992 Apr;61(2):121–130. doi: 10.1016/0009-3084(92)90004-9. [DOI] [PubMed] [Google Scholar]
- Lang J., Vigo-Pelfrey C., Martin F. Liposomes composed of partially hydrogenated egg phosphatidylcholines: fatty acid composition, thermal phase behavior and oxidative stability. Chem Phys Lipids. 1990 Mar;53(1):91–101. doi: 10.1016/0009-3084(90)90137-g. [DOI] [PubMed] [Google Scholar]
- Largillière C., Mélancon S. B. Free malondialdehyde determination in human plasma by high-performance liquid chromatography. Anal Biochem. 1988 Apr;170(1):123–126. doi: 10.1016/0003-2697(88)90098-x. [DOI] [PubMed] [Google Scholar]
- Leanderson P., Tagesson C. Iron bound to the lipophilic iron chelator, 8-hydroxyquinoline, causes DNA strand breakage in cultured lung cells. Carcinogenesis. 1996 Mar;17(3):545–550. doi: 10.1093/carcin/17.3.545. [DOI] [PubMed] [Google Scholar]
- Leroyer V., Werner L., Shaughnessy S., Goddard G. J., Orr F. W. Chemiluminescence and oxygen radical generation by Walker carcinosarcoma cells following chemotactic stimulation. Cancer Res. 1987 Sep 15;47(18):4771–4775. [PubMed] [Google Scholar]
- Lucesoli F., Fraga C. G. Oxidative damage to lipids and DNA concurrent with decrease of antioxidants in rat testes after acute iron intoxication. Arch Biochem Biophys. 1995 Jan 10;316(1):567–571. doi: 10.1006/abbi.1995.1076. [DOI] [PubMed] [Google Scholar]
- Malins D. C., Gunselman S. J. Fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry reveal a remarkable degree of structural damage in the DNA of wild fish exposed to toxic chemicals. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):13038–13041. doi: 10.1073/pnas.91.26.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malins D. C., Ostrander G. K., Haimanot R., Williams P. A novel DNA lesion in neoplastic livers of feral fish: 2,6-diamino-4-hydroxy-5-formamidopyrimidine. Carcinogenesis. 1990 Jun;11(6):1045–1047. doi: 10.1093/carcin/11.6.1045. [DOI] [PubMed] [Google Scholar]
- Mangold K., Chang Y. J., Mathews C., Marien K., Hendricks J., Bailey G. Expression of ras genes in rainbow trout liver. Mol Carcinog. 1991;4(2):97–102. doi: 10.1002/mc.2940040204. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem. 1992 Apr 5;267(10):6696–6701. [PubMed] [Google Scholar]
- Marshall P. J., Warso M. A., Lands W. E. Selective microdetermination of lipid hydroperoxides. Anal Biochem. 1985 Feb 15;145(1):192–199. doi: 10.1016/0003-2697(85)90347-1. [DOI] [PubMed] [Google Scholar]
- Mashino T., Fridovich I. Superoxide radical initiates the autoxidation of dihydroxyacetone. Arch Biochem Biophys. 1987 May 1;254(2):547–551. doi: 10.1016/0003-9861(87)90136-6. [DOI] [PubMed] [Google Scholar]
- Mather-Mihaich E., Di Giulio R. T. Oxidant, mixed-function oxidase and peroxisomal responses in channel catfish exposed to a bleached kraft mill effluent. Arch Environ Contam Toxicol. 1991 Apr;20(3):391–397. doi: 10.1007/BF01064409. [DOI] [PubMed] [Google Scholar]
- Matkovics B., Novák R., Hanh H. D., Szabó L., Varga I., Zaleśna A comparative study of some more important experimental animal peroxide metabolism enzymes. Comp Biochem Physiol B. 1977;56(1):31–34. doi: 10.1016/0305-0491(77)90218-8. [DOI] [PubMed] [Google Scholar]
- Mazeaud F., Maral J., Michelson A. M. Distribution of superoxide dismutase and glutathione peroxidase in the carp: erythrocyte manganese SOD. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1161–1168. doi: 10.1016/0006-291x(79)90239-0. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M. Superoxide radical: controversies, contradictions, and paradoxes. Proc Soc Exp Biol Med. 1995 Jun;209(2):112–117. doi: 10.3181/00379727-209-43885c. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Mitchell J., Jiang H., Berry L., Meyrick B. Effect of antioxidants on lipopolysaccharide-stimulated induction of mangano superoxide dismutase mRNA in bovine pulmonary artery endothelial cells. J Cell Physiol. 1996 Nov;169(2):333–340. doi: 10.1002/(SICI)1097-4652(199611)169:2<333::AID-JCP12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975 Aug 1;56(1):1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun. 1975 Mar 17;63(2):463–468. doi: 10.1016/0006-291x(75)90710-x. [DOI] [PubMed] [Google Scholar]
- Nishimoto M., Roubal W. T., Stein J. E., Varanasi U. Oxidative DNA damage in tissues of English sole (Parophrys vetulus) exposed to nitrofurantoin. Chem Biol Interact. 1991;80(3):317–326. doi: 10.1016/0009-2797(91)90091-k. [DOI] [PubMed] [Google Scholar]
- Nohl H., Jordan W., Hegner D. Identification of free hydroxyl radicals in respiring rat heart mitochondria by spin trapping with the nitrone DMPO. FEBS Lett. 1981 Jan 26;123(2):241–244. doi: 10.1016/0014-5793(81)80297-9. [DOI] [PubMed] [Google Scholar]
- O'Brien P. J. Intracellular mechanisms for the decomposition of a lipid peroxide. I. Decomposition of a lipid peroxide by metal ions, heme compounds, and nucleophiles. Can J Biochem. 1969 May;47(5):485–492. doi: 10.1139/o69-076. [DOI] [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloneperoxidase of the leukocyte of normal blood. 3. The reaction of ferric myeloperoxidase with superoxide anion. Biochim Biophys Acta. 1972 Oct 12;284(2):355–359. doi: 10.1016/0005-2744(72)90130-1. [DOI] [PubMed] [Google Scholar]
- Okada S., Minamiyama Y., Hamazaki S., Toyokuni S., Sotomatsu A. Glutathione cycle dependency of ferric nitrilotriacetate-induced lipid peroxidation in mouse proximal renal tubules. Arch Biochem Biophys. 1993 Feb 15;301(1):138–142. doi: 10.1006/abbi.1993.1125. [DOI] [PubMed] [Google Scholar]
- Otto D. M., Moon T. W. 3,3',4,4'-tetrachlorobiphenyl effects on antioxidant enzymes and glutathione status in different tissues of rainbow trout. Pharmacol Toxicol. 1995 Oct;77(4):281–287. doi: 10.1111/j.1600-0773.1995.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Otto D. M., Moon T. W. Phase I and II enzymes and antioxidant responses in different tissues of brown bullheads from relatively polluted and non-polluted systems. Arch Environ Contam Toxicol. 1996 Jul;31(1):141–147. doi: 10.1007/BF00203918. [DOI] [PubMed] [Google Scholar]
- Parihar M. S., Dubey A. K. Lipid peroxidation and ascorbic acid status in respiratory organs of male and female freshwater catfish Heteropneustes fossilis exposed to temperature increase. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995 Nov;112(3):309–313. doi: 10.1016/0742-8413(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Park E. M., Thomas J. A. S-thiolation of creatine kinase and glycogen phosphorylase b initiated by partially reduced oxygen species. Biochim Biophys Acta. 1988 Feb 17;964(2):151–160. doi: 10.1016/0304-4165(88)90161-4. [DOI] [PubMed] [Google Scholar]
- Pedrajas J. R., Peinado J., López-Barea J. Oxidative stress in fish exposed to model xenobiotics. Oxidatively modified forms of Cu,Zn-superoxide dismutase as potential biomarkers. Chem Biol Interact. 1995 Dec 22;98(3):267–282. doi: 10.1016/0009-2797(95)03651-2. [DOI] [PubMed] [Google Scholar]
- Pietarinen-Runtti P., Raivio K. O., Linnainmaa K., Ekman A., Saksela M., Kinnula V. L. Differential effects of tumor necrosis factor and asbestos fibers on manganese superoxide dismutase induction and oxidant-induced cytotoxicity in human mesothelial cells. Cell Biol Toxicol. 1996 Jun;12(3):167–175. doi: 10.1007/BF00148170. [DOI] [PubMed] [Google Scholar]
- Porter N. A., Caldwell S. E., Mills K. A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995 Apr;30(4):277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Quinlan T., Spivack S., Mossman B. T. Regulation of antioxidant enzymes in lung after oxidant injury. Environ Health Perspect. 1994 Jun;102 (Suppl 2):79–87. doi: 10.1289/ehp.9410279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinheckel T., Wiswedel I., Noack H., Augustin W. Electrophoretic evidence for the impairment of complexes of the respiratory chain during iron/ascorbate induced peroxidation in isolated rat liver mitochondria. Biochim Biophys Acta. 1995 Oct 4;1239(1):45–50. doi: 10.1016/0005-2736(95)00142-p. [DOI] [PubMed] [Google Scholar]
- Remacle J., Raes M., Toussaint O., Renard P., Rao G. Low levels of reactive oxygen species as modulators of cell function. Mutat Res. 1995 Feb;316(3):103–122. doi: 10.1016/0921-8734(95)90004-7. [DOI] [PubMed] [Google Scholar]
- SESHADRI SASTRY P., JAYARAMAN J., RAMASARMA T. Distribution of coenzyme Q in rat liver cell fractions. Nature. 1961 Feb 18;189:577–577. doi: 10.1038/189577a0. [DOI] [PubMed] [Google Scholar]
- Salgo M. G., Corongiu F. P., Sevanian A. Enhanced interfacial catalysis and hydrolytic specificity of phospholipase A2 toward peroxidized phosphatidylcholine vesicles. Arch Biochem Biophys. 1993 Jul;304(1):123–132. doi: 10.1006/abbi.1993.1330. [DOI] [PubMed] [Google Scholar]
- Scarpa M., Stevanato R., Viglino P., Rigo A. Superoxide ion as active intermediate in the autoxidation of ascorbate by molecular oxygen. Effect of superoxide dismutase. J Biol Chem. 1983 Jun 10;258(11):6695–6697. [PubMed] [Google Scholar]
- Schweich M. D., Lison D., Lauwerys R. The role of vitamin E in the susceptibility of rat lung and liver microsomes to iron-stimulated peroxidation. Environ Res. 1995 Jul;70(1):62–69. doi: 10.1006/enrs.1995.1047. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Grist E., Thompson K., Woodhead A. D. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Woodhead A. D., Grist E. Animal model for ultraviolet radiation-induced melanoma: platyfish-swordtail hybrid. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8922–8926. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanian A., Kim E. Phospholipase A2 dependent release of fatty acids from peroxidized membranes. J Free Radic Biol Med. 1985;1(4):263–271. doi: 10.1016/0748-5514(85)90130-8. [DOI] [PubMed] [Google Scholar]
- Sevanian A., Muakkassah-Kelly S. F., Montestruque S. The influence of phospholipase A2 and glutathione peroxidase on the elimination of membrane lipid peroxides. Arch Biochem Biophys. 1983 Jun;223(2):441–452. doi: 10.1016/0003-9861(83)90608-2. [DOI] [PubMed] [Google Scholar]
- Sevanian A., Stein R. A., Mead J. F. Metabolism of epoxidized phosphatidylcholine by phospholipase A2 and epoxide hydrolase. Lipids. 1981 Nov;16(11):781–789. doi: 10.1007/BF02535029. [DOI] [PubMed] [Google Scholar]
- Sharma M. K., Buettner G. R. Interaction of vitamin C and vitamin E during free radical stress in plasma: an ESR study. Free Radic Biol Med. 1993 Jun;14(6):649–653. doi: 10.1016/0891-5849(93)90146-l. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kondo K., Hayaishi O. Role of prostaglandin endoperoxides in the serum thiobarbituric acid reaction. Arch Biochem Biophys. 1981 Feb;206(2):271–276. doi: 10.1016/0003-9861(81)90091-6. [DOI] [PubMed] [Google Scholar]
- Spolarics Z. Endotoxin stimulates gene expression of ROS-eliminating pathways in rat hepatic endothelial and Kupffer cells. Am J Physiol. 1996 Apr;270(4 Pt 1):G660–G666. doi: 10.1152/ajpgi.1996.270.4.G660. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Berlett B. S. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997 May;10(5):485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- Tappel M. E., Chaudiere J., Tappel A. L. Glutathione peroxidase activities of animal tissues. Comp Biochem Physiol B. 1982;73(4):945–949. doi: 10.1016/0305-0491(82)90341-8. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Toyokuni S., Sagripanti J. L. Increased 8-hydroxydeoxyguanosine in kidney and liver of rats continuously exposed to copper. Toxicol Appl Pharmacol. 1994 May;126(1):91–97. doi: 10.1006/taap.1994.1094. [DOI] [PubMed] [Google Scholar]
- Ursini F., Maiorino M., Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim Biophys Acta. 1985 Mar 29;839(1):62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Ursini F., Maiorino M., Valente M., Ferri L., Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982 Feb 15;710(2):197–211. doi: 10.1016/0005-2760(82)90150-3. [DOI] [PubMed] [Google Scholar]
- Waldeck A. R., Stocker R. Radical-initiated lipid peroxidation in low density lipoproteins: insights obtained from kinetic modeling. Chem Res Toxicol. 1996 Sep;9(6):954–964. doi: 10.1021/tx960057s. [DOI] [PubMed] [Google Scholar]
- Washburn P. C., Di Giulio R. T. Nitrofurantoin-stimulated superoxide production by channel catfish (Ictalurus punctatus) hepatic microsomal and soluble fractions. Toxicol Appl Pharmacol. 1988 Sep 30;95(3):363–377. doi: 10.1016/0041-008x(88)90355-9. [DOI] [PubMed] [Google Scholar]
- Whittaker P., Wamer W. G., Chanderbhan R. F., Dunkel V. C. Effects of alpha-tocopherol and beta-carotene on hepatic lipid peroxidation and blood lipids in rats with dietary iron overload. Nutr Cancer. 1996;25(2):119–128. doi: 10.1080/01635589609514434. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Carpenter H. M., Buhler D. R., Kelly J. D., Dutchuk M. Alterations in lipid peroxidation, antioxidant enzymes, and carcinogen metabolism in liver microsomes of vitamin E-deficient trout and rat. Toxicol Appl Pharmacol. 1992 Sep;116(1):78–84. doi: 10.1016/0041-008x(92)90147-k. [DOI] [PubMed] [Google Scholar]
- Winston G. W. Oxidants and antioxidants in aquatic animals. Comp Biochem Physiol C. 1991;100(1-2):173–176. doi: 10.1016/0742-8413(91)90148-m. [DOI] [PubMed] [Google Scholar]
- Xia Y., Dawson V. L., Dawson T. M., Snyder S. H., Zweier J. L. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]