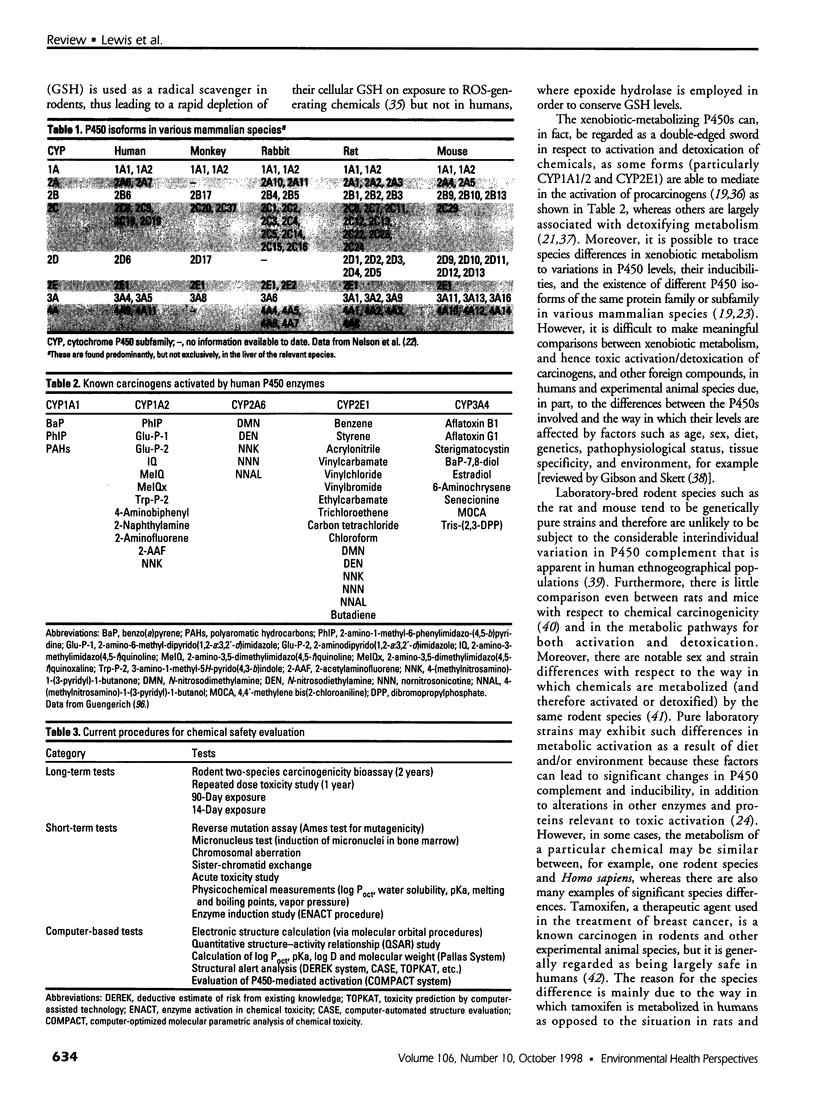
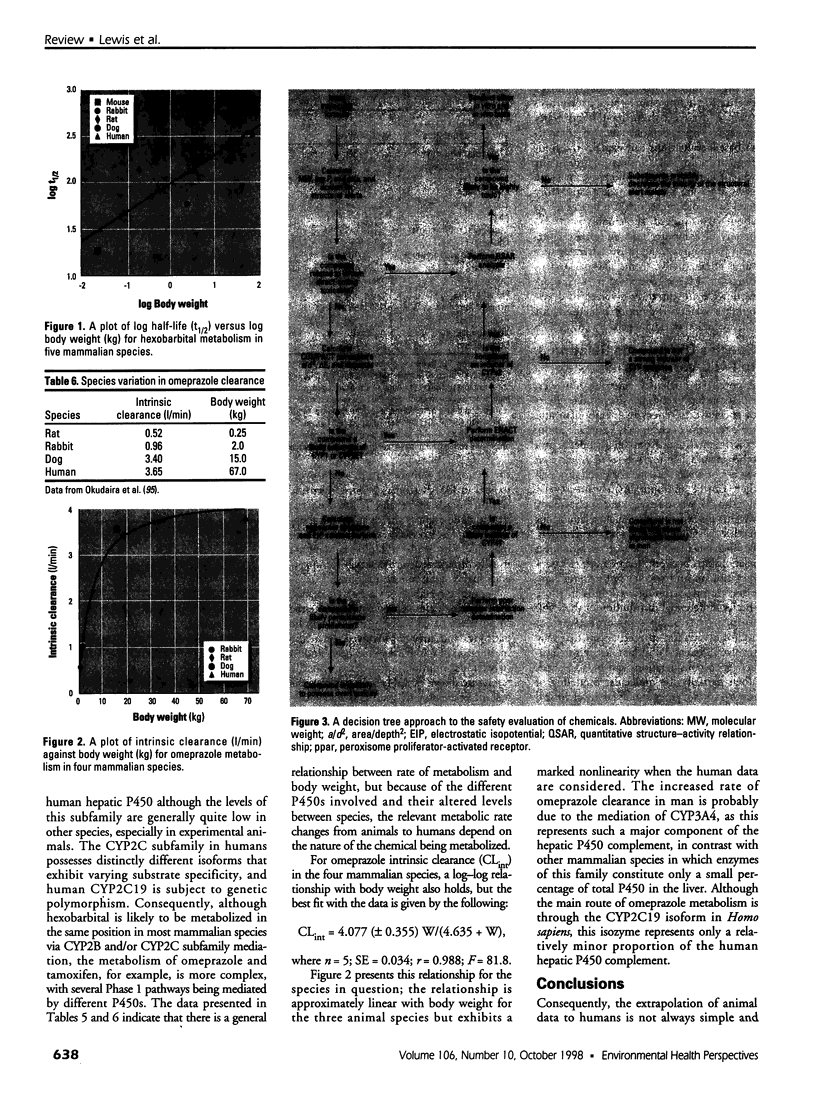
Abstract
The importance of cytochrome P450 isoforms to species differences in the metabolism of foreign compounds and activation of procarcinogens has been identified. The possible range of P450 isozymes in significant variations in toxicity exhibited by experimental rodent species may have a relevance to chemical risk assessment, especially as human P450s are likely to show changes in the way they metabolize xenobiotics. Consequently, in the safety evaluation of chemicals, we should be cautious in extrapolating results from experimental animal models to humans. This paper focuses on examples in which species differences in P450s lead to significant alterations in carcinogenic response, and includes a discussion of the current procedures for toxicity screening, with an emphasis on short-term tests.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S. Too many rodent carcinogens: mitogenesis increases mutagenesis. Science. 1990 Aug 31;249(4972):970–971. doi: 10.1126/science.2136249. [DOI] [PubMed] [Google Scholar]
- Andersson T., Miners J. O., Veronese M. E., Tassaneeyakul W., Tassaneeyakul W., Meyer U. A., Birkett D. J. Identification of human liver cytochrome P450 isoforms mediating omeprazole metabolism. Br J Clin Pharmacol. 1993 Dec;36(6):521–530. doi: 10.1111/j.1365-2125.1993.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby J. International Commission for Protection Against Environmental Mutagens and Carcinogens. Two million rodent carcinogens? The role of SAR and QSAR in their detection. Mutat Res. 1994 Feb 1;305(1):3–12. doi: 10.1016/0027-5107(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Ashby J., Paton D. The influence of chemical structure on the extent and sites of carcinogenesis for 522 rodent carcinogens and 55 different human carcinogen exposures. Mutat Res. 1993 Mar;286(1):3–74. doi: 10.1016/0027-5107(93)90003-x. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Prediction of rodent carcinogenicity for 44 chemicals: results. Mutagenesis. 1994 Jan;9(1):7–15. doi: 10.1093/mutage/9.1.7. [DOI] [PubMed] [Google Scholar]
- Benigni R., Giuliani A. Quantitative structure-activity relationship (QSAR) studies in genetic toxicology: mathematical models and the "biological activity" term of the relationship. Mutat Res. 1994 Apr 15;306(2):181–186. doi: 10.1016/0027-5107(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Benigni R., Richard A. M. QSARS of mutagens and carcinogens: two case studies illustrating problems in the construction of models for noncongeneric chemicals. Mutat Res. 1996 Nov 4;371(1-2):29–46. doi: 10.1016/s0165-1218(96)90092-0. [DOI] [PubMed] [Google Scholar]
- Bogen K. T. Improved prediction of carcinogenic potencies from mutagenic potencies for chemicals positive in rodents and the Ames test. Environ Mol Mutagen. 1995;25(1):37–49. doi: 10.1002/em.2850250107. [DOI] [PubMed] [Google Scholar]
- Boobis A. R., Sesardic D., Murray B. P., Edwards R. J., Singleton A. M., Rich K. J., Murray S., de la Torre R., Segura J., Pelkonen O. Species variation in the response of the cytochrome P-450-dependent monooxygenase system to inducers and inhibitors. Xenobiotica. 1990 Nov;20(11):1139–1161. doi: 10.3109/00498259009046835. [DOI] [PubMed] [Google Scholar]
- Cohen S. M. Human relevance of animal carcinogenicity studies. Regul Toxicol Pharmacol. 1995 Feb;21(1):75–86. doi: 10.1006/rtph.1995.1012. [DOI] [PubMed] [Google Scholar]
- Debnath A. K., Shusterman A. J., Lopez de Compadre R. L., Hansch C. International Commission for Protection Against Environmental Mutagens and Carcinogens. The importance of the hydrophobic interaction in the mutagenicity of organic compounds. Mutat Res. 1994 Feb 1;305(1):63–72. doi: 10.1016/0027-5107(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Di Carlo F. J. Carcinogenesis bioassay data: correlation by species and sex. Drug Metab Rev. 1984;15(3):409–413. doi: 10.3109/03602538409029968. [DOI] [PubMed] [Google Scholar]
- Ennever F. K., Noonan T. J., Rosenkranz H. S. The predictivity of animal bioassays and short-term genotoxicity tests for carcinogenicity and non-carcinogenicity to humans. Mutagenesis. 1987 Mar;2(2):73–78. doi: 10.1093/mutage/2.2.73. [DOI] [PubMed] [Google Scholar]
- Enslein K., Borgstedt H. H. A QSAR model for the estimation of carcinogenicity: example application to an azo-dye. Toxicol Lett. 1989 Dec;49(2-3):107–121. doi: 10.1016/0378-4274(89)90027-1. [DOI] [PubMed] [Google Scholar]
- Fung V. A., Huff J., Weisburger E. K., Hoel D. G. Predictive strategies for selecting 379 NCI/NTP chemicals evaluated for carcinogenic potential: scientific and public health impact. Fundam Appl Toxicol. 1993 May;20(4):413–436. doi: 10.1006/faat.1993.1053. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Slone T. H., Manley N. B., Bernstein L. Target organs in chronic bioassays of 533 chemical carcinogens. Environ Health Perspect. 1991 Jun;93:233–246. doi: 10.1289/ehp.9193233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. A., de Morais S. M. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics. 1994 Dec;4(6):285–299. doi: 10.1097/00008571-199412000-00001. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Gelboin H. V. Role of human cytochromes P450 in the metabolic activation of chemical carcinogens and toxins. Drug Metab Rev. 1994;26(1-2):165–183. doi: 10.3109/03602539409029789. [DOI] [PubMed] [Google Scholar]
- Gori G. B. Adjudicating cancer causation: scientific, political, and legal conflicts. Regul Toxicol Pharmacol. 1991 Jun;13(3):309–325. doi: 10.1016/0273-2300(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Gori G. B. Are animal tests relevant in cancer risk assessment? A persistent issue becomes uncomfortable. Regul Toxicol Pharmacol. 1991 Jun;13(3):225–227. doi: 10.1016/0273-2300(91)90064-3. [DOI] [PubMed] [Google Scholar]
- Gori G. B. Cancer risk assessment: the science that is not. Regul Toxicol Pharmacol. 1992 Aug;16(1):10–20. doi: 10.1016/0273-2300(92)90019-6. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Comparisons of catalytic selectivity of cytochrome P450 subfamily enzymes from different species. Chem Biol Interact. 1997 Oct 24;106(3):161–182. doi: 10.1016/s0009-2797(97)00068-9. [DOI] [PubMed] [Google Scholar]
- Hansch C., Zhang L. Quantitative structure-activity relationships of cytochrome P-450. Drug Metab Rev. 1993;25(1-2):1–48. doi: 10.3109/03602539308993972. [DOI] [PubMed] [Google Scholar]
- Ioannides C., Parke D. V. Induction of cytochrome P4501 as an indicator of potential chemical carcinogenesis. Drug Metab Rev. 1993;25(4):485–501. doi: 10.3109/03602539308993983. [DOI] [PubMed] [Google Scholar]
- Ioannides C., Parke D. V. The cytochrome P450 I gene family of microsomal hemoproteins and their role in the metabolic activation of chemicals. Drug Metab Rev. 1990;22(1):1–85. doi: 10.3109/03602539008991444. [DOI] [PubMed] [Google Scholar]
- Lee Y., Buchanan B. G., Klopman G., Dimayuga M., Rosenkranz H. S. The potential of organ specific toxicity for predicting rodent carcinogenicity. Mutat Res. 1996 Oct 28;358(1):37–62. doi: 10.1016/0027-5107(96)00110-8. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Bird M. G., Parke D. V. Molecular modelling of CYP2E1 enzymes from rat, mouse and man: an explanation for species differences in butadiene metabolism and potential carcinogenicity, and rationalization of CYP2E substrate specificity. Toxicology. 1997 Mar 28;118(2-3):93–113. doi: 10.1016/s0300-483x(96)03583-4. [DOI] [PubMed] [Google Scholar]
- Lewis D. F. Comparison between rodent carcinogenicity test results of 44 chemicals and a number of predictive systems. Regul Toxicol Pharmacol. 1994 Dec;20(3 Pt 1):215–222. doi: 10.1006/rtph.1994.1073. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Lake B. G. Molecular modelling of members of the P4502A subfamily: application to studies of enzyme specificity. Xenobiotica. 1995 Jun;25(6):585–598. doi: 10.3109/00498259509061877. [DOI] [PubMed] [Google Scholar]
- Lewis D. F., Langley G. R. A validation study of the COMPACT and HazardExpert techniques with 40 chemicals. Mutat Res. 1996 Aug 12;369(3-4):157–174. doi: 10.1016/s0165-1218(96)90023-3. [DOI] [PubMed] [Google Scholar]
- Lijinsky W. Life-span and cancer: the induction time of tumors in diverse animal species treated with nitrosodiethylamine. Carcinogenesis. 1993 Nov;14(11):2373–2375. doi: 10.1093/carcin/14.11.2373. [DOI] [PubMed] [Google Scholar]
- Lipnick R. L. Outliers: their origin and use in the classification of molecular mechanisms of toxicity. Sci Total Environ. 1991 Dec;109-110:131–153. doi: 10.1016/0048-9697(91)90175-e. [DOI] [PubMed] [Google Scholar]
- Lorenz J., Glatt H. R., Fleischmann R., Ferlinz R., Oesch F. Drug metabolism in man and its relationship to that in three rodent species: monooxygenase, epoxide hydrolase, and glutathione S-transferase activities in subcellular fractions of lung and liver. Biochem Med. 1984 Aug;32(1):43–56. doi: 10.1016/0006-2944(84)90007-3. [DOI] [PubMed] [Google Scholar]
- Magdó I., Ferenczy G. G., Bencz Z. Prediction of carcinogenicity from molecular structure; modification and reinvestigation of the method. Cancer Lett. 1994 Jun 30;81(2):201–207. doi: 10.1016/0304-3835(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Manjgaladze M., Chen S., Frame L. T., Seng J. E., Duffy P. H., Feuers R. J., Hart R. W., Leakey J. E. Effects of caloric restriction on rodent drug and carcinogen metabolizing enzymes: implications for mutagenesis and cancer. Mutat Res. 1993 Dec;295(4-6):201–222. doi: 10.1016/0921-8734(93)90021-t. [DOI] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Martin A. P., Palumbi S. R. Body size, metabolic rate, generation time, and the molecular clock. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4087–4091. doi: 10.1073/pnas.90.9.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell E. E. Historical review of the rodent bioassay and future directions. Regul Toxicol Pharmacol. 1995 Feb;21(1):38–86. doi: 10.1006/rtph.1995.1007. [DOI] [PubMed] [Google Scholar]
- McGregor D. B., Pangrekar J., Rosenkranz H. S., Klopman G. A reexamination of the low prevalence of carcinogens in an early carcinogen screen. Regul Toxicol Pharmacol. 1994 Feb;19(1):97–105. doi: 10.1006/rtph.1994.1008. [DOI] [PubMed] [Google Scholar]
- Mersch-Sundermann V., Rosenkranz H. S., Klopman G. Structural basis of the genotoxicity of polycyclic aromatic hydrocarbons. Mutagenesis. 1992 May;7(3):211–218. doi: 10.1093/mutage/7.3.211. [DOI] [PubMed] [Google Scholar]
- Nedelcheva V., Gut I. P450 in the rat and man: methods of investigation, substrate specificities and relevance to cancer. Xenobiotica. 1994 Dec;24(12):1151–1175. doi: 10.3109/00498259409038673. [DOI] [PubMed] [Google Scholar]
- Negishi M., Iwasaki M., Juvonen R. O., Sueyoshi T., Darden T. A., Pedersen L. G. Structural flexibility and functional versatility of cytochrome P450 and rapid evolution. Mutat Res. 1996 Feb 19;350(1):43–50. doi: 10.1016/0027-5107(95)00089-5. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Koymans L., Kamataki T., Stegeman J. J., Feyereisen R., Waxman D. J., Waterman M. R., Gotoh O., Coon M. J., Estabrook R. W. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996 Feb;6(1):1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- Orr W. C., Sohal R. S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994 Feb 25;263(5150):1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Parke D. V. The cytochromes P450 and mechanisms of chemical carcinogenesis. Environ Health Perspect. 1994 Oct;102(10):852–853. doi: 10.1289/ehp.94102852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poso A., von Wright A., Gynther J. An empirical and theoretical study on mechanisms of mutagenic activity of hydrazine compounds. Mutat Res. 1995 Nov;332(1-2):63–71. doi: 10.1016/0027-5107(95)00155-2. [DOI] [PubMed] [Google Scholar]
- Richard A. M. International Commission for Protection Against Environmental Mutagens and Carcinogens. Application of SAR methods to non-congeneric data bases associated with carcinogenicity and mutagenicity: issues and approaches. Mutat Res. 1994 Feb 1;305(1):73–97. doi: 10.1016/0027-5107(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Sachse C., Brockmöller J., Bauer S., Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997 Feb;60(2):284–295. [PMC free article] [PubMed] [Google Scholar]
- Sanderson D. M., Earnshaw C. G. Computer prediction of possible toxic action from chemical structure; the DEREK system. Hum Exp Toxicol. 1991 Jul;10(4):261–273. doi: 10.1177/096032719101000405. [DOI] [PubMed] [Google Scholar]
- Sarver J. G., White D., Erhardt P., Bachmann K. Estimating xenobiotic half-lives in humans from rat data: influence of log P. Environ Health Perspect. 1997 Nov;105(11):1204–1209. doi: 10.1289/ehp.971051204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng J. E., Gandy J., Turturro A., Lipman R., Bronson R. T., Parkinson A., Johnson W., Hart R. W., Leakey J. E. Effects of caloric restriction on expression of testicular cytochrome P450 enzymes associated with the metabolic activation of carcinogens. Arch Biochem Biophys. 1996 Nov 1;335(1):42–52. doi: 10.1006/abbi.1996.0480. [DOI] [PubMed] [Google Scholar]
- Smith D. A. Species differences in metabolism and pharmacokinetics: are we close to an understanding? Drug Metab Rev. 1991;23(3-4):355–373. doi: 10.3109/03602539109029764. [DOI] [PubMed] [Google Scholar]
- Stoloff L. An analysis of the 1987 list of IARC-identified human carcinogens and the correlated animal studies. Regul Toxicol Pharmacol. 1992 Feb;15(1):10–13. doi: 10.1016/0273-2300(92)90079-o. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Spalding J., Stasiewicz S., Ashby J. Prediction of the outcome of rodent carcinogenicity bioassays currently being conducted on 44 chemicals by the National Toxicology Program. Mutagenesis. 1990 Jan;5(1):3–14. doi: 10.1093/mutage/5.1.3. [DOI] [PubMed] [Google Scholar]
- Wang S., Milne G. W. Applications of computers to toxicological research. Chem Res Toxicol. 1993 Nov-Dec;6(6):748–753. doi: 10.1021/tx00036a002. [DOI] [PubMed] [Google Scholar]
- Wiseman H., Kaur H., Halliwell B. DNA damage and cancer: measurement and mechanism. Cancer Lett. 1995 Jun 29;93(1):113–120. doi: 10.1016/0304-3835(95)03792-U. [DOI] [PubMed] [Google Scholar]
- Wiseman H., Lewis D. F. The metabolism of tamoxifen by human cytochromes P450 is rationalized by molecular modelling of the enzyme-substrate interactions: potential importance to its proposed anti-carcinogenic/carcinogenic actions. Carcinogenesis. 1996 Jun;17(6):1357–1360. doi: 10.1093/carcin/17.6.1357. [DOI] [PubMed] [Google Scholar]