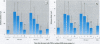Abstract
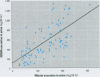
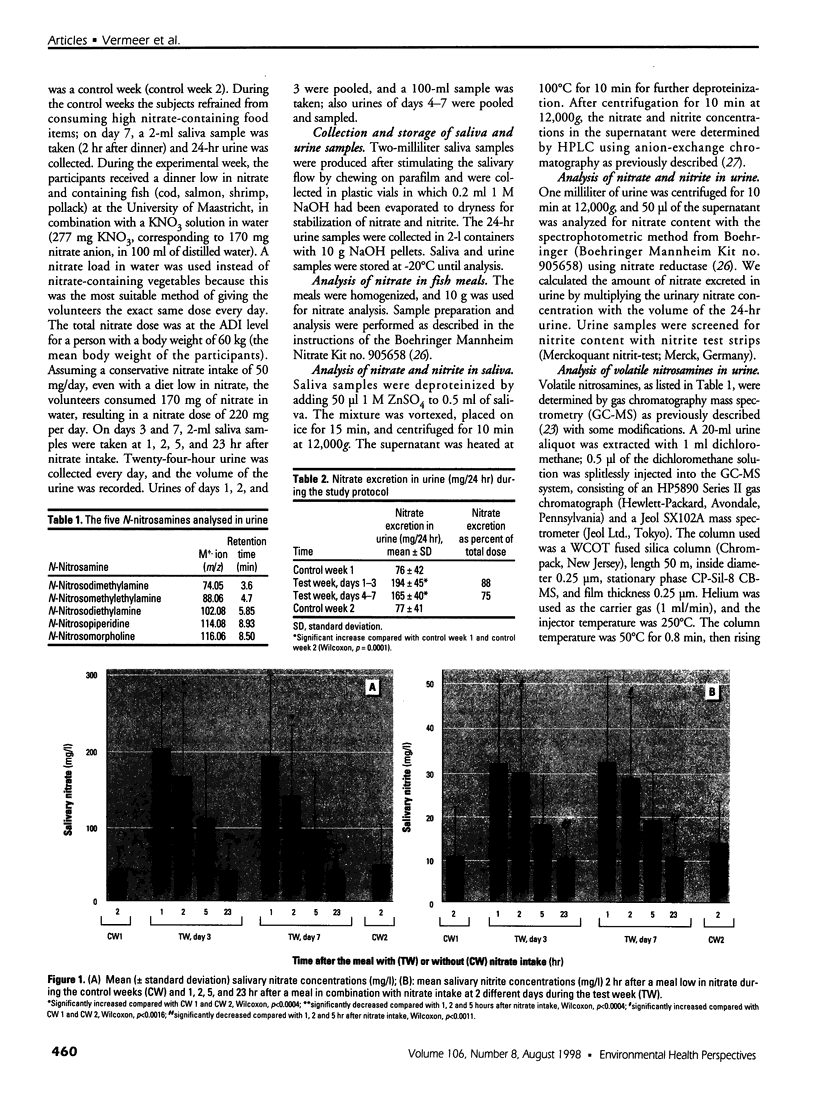
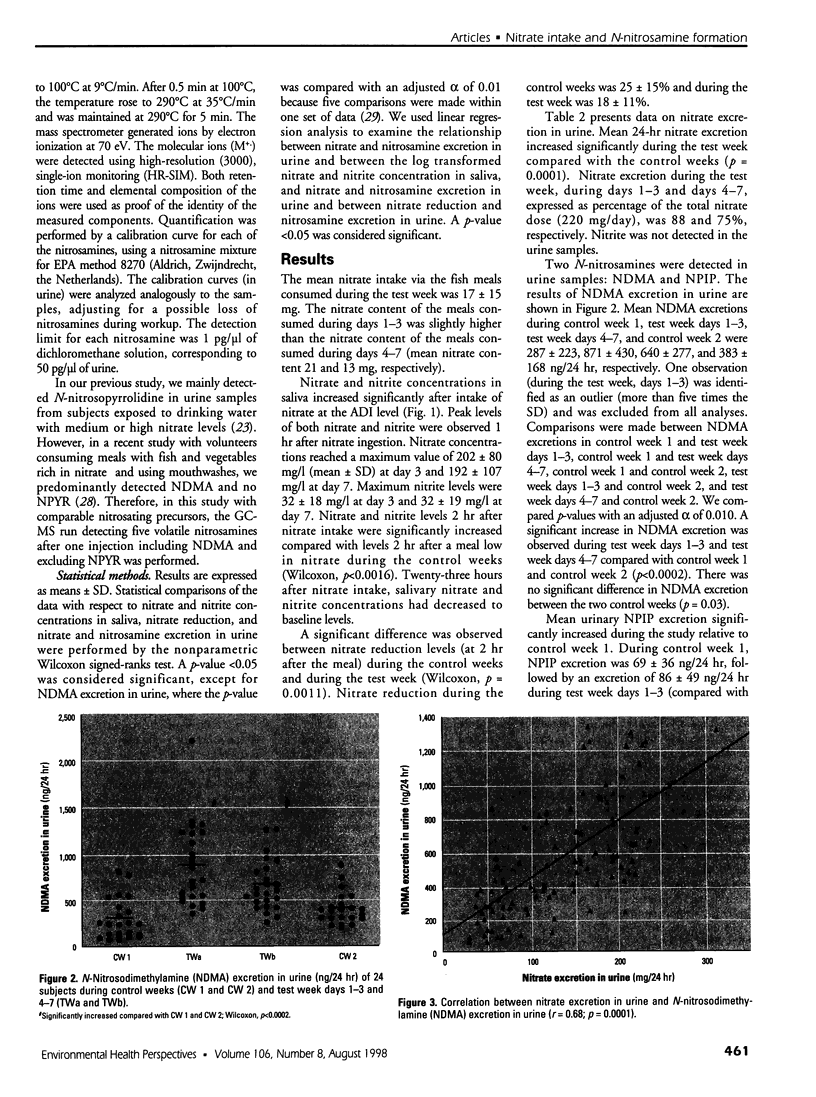
Formation of nitrite from ingested nitrate can result in several adverse health effects and implies a genotoxic risk as a consequence of endogenous formation of carcinogenic N-nitroso compounds. We studied the formation of volatile N-nitrosamines after intake of nitrate at the acceptable daily intake (ADI) level in combination with a fish meal rich in amines as nitrosatable precursors. Twenty-five volunteers consumed this meal during 7 consecutive days; a diet low in nitrate was consumed during 1 week before and 1 week after the test week. Nitrate intake at the ADI level resulted in a significant rise in mean salivary nitrate and nitrite concentrations. Mean urinary nitrate excretion increased from 76 mg/24 hr in the first control week to 194 and 165 mg/24 hr in the test week, followed by a decline to 77 mg/24 hr in the second control week. The urine samples were analyzed for volatile N-nitrosamines, and both N-nitrosodimethylamine (NDMA) and N-nitrosopiperidine (NPIP) were detected in the samples. Mean urinary NDMA excretion significantly increased from 287 ng/24 hr in the control week to 871 and 640 ng/24 hr in the test week and declined to 383 ng/24 hr in the second control week. Excretion of NPIP was not directly related to the nitrate intake and composition of the diet. Nitrate excretion and NDMA excretion were significantly correlated, as well as salivary nitrate and nitrite concentration and NDMA excretion. We conclude that nitrate intake at the ADI level in combination with a fish meal containing nitrosatable precursors increases NDMA excretion in urine and thus demonstrates increased formation of carcinogenic N-nitrosamines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayrton A. D., Smith J. N., Ioannides C. Bioactivation of N-nitrosopiperidine to mutagens: role of hepatic cytochrome P-450 proteins and contribution of cytosolic fraction. Carcinogenesis. 1987 Nov;8(11):1691–1695. doi: 10.1093/carcin/8.11.1691. [DOI] [PubMed] [Google Scholar]
- Bartholomew B., Hill M. J. The pharmacology of dietary nitrate and the origin of urinary nitrate. Food Chem Toxicol. 1984 Oct;22(10):789–795. doi: 10.1016/0278-6915(84)90116-9. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Ohshima H., Shuker D. E., Pignatelli B., Calmels S. Exposure of humans to endogenous N-nitroso compounds: implications in cancer etiology. Mutat Res. 1990 May;238(3):255–267. doi: 10.1016/0165-1110(90)90017-6. [DOI] [PubMed] [Google Scholar]
- Boeing H. Epidemiological research in stomach cancer: progress over the last ten years. J Cancer Res Clin Oncol. 1991;117(2):133–143. doi: 10.1007/BF01613137. [DOI] [PubMed] [Google Scholar]
- Bruning-Fann C. S., Kaneene J. B. The effects of nitrate, nitrite and N-nitroso compounds on human health: a review. Vet Hum Toxicol. 1993 Dec;35(6):521–538. [PubMed] [Google Scholar]
- Caygill C. P. Epidemiology relating N-nitroso compounds to human cancer. Eur J Cancer Prev. 1996 Sep;5 (Suppl 1):125–130. [PubMed] [Google Scholar]
- Cicchetti D. V. Multiple comparison methods: establishing guidelines for their valid application in neuropsychological research. J Clin Exp Neuropsychol. 1994 Feb;16(1):155–161. doi: 10.1080/01688639408402625. [DOI] [PubMed] [Google Scholar]
- Forman D., Al-Dabbagh S., Doll R. Nitrates, nitrites and gastric cancer in Great Britain. Nature. 1985 Feb 21;313(6004):620–625. doi: 10.1038/313620a0. [DOI] [PubMed] [Google Scholar]
- Forman D. Dietary exposure to N-nitroso compounds and the risk of human cancer. Cancer Surv. 1987;6(4):719–738. [PubMed] [Google Scholar]
- Gangolli S. D., van den Brandt P. A., Feron V. J., Janzowsky C., Koeman J. H., Speijers G. J., Spiegelhalder B., Walker R., Wisnok J. S. Nitrate, nitrite and N-nitroso compounds. Eur J Pharmacol. 1994 Nov 1;292(1):1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Granli T., Dahl R., Brodin P., Bøckman O. C. Nitrate and nitrite concentrations in human saliva: variations with salivary flow-rate. Food Chem Toxicol. 1989 Oct;27(10):675–680. doi: 10.1016/0278-6915(89)90122-1. [DOI] [PubMed] [Google Scholar]
- Hill M. J. Mechanisms of gastric carcinogenesis. Eur J Cancer Prev. 1993 Jun;2 (Suppl 2):73–78. doi: 10.1097/00008469-199306000-00012. [DOI] [PubMed] [Google Scholar]
- Knight T. M., Leach S., Forman D., Vindigni C., Packer P., Venitt S., Minacci C., Lorenzini L., Tosi P., Frosini G. N-nitrosoproline excretion in the presence and absence of gastric disease. Eur J Cancer. 1991;27(4):456–461. doi: 10.1016/0277-5379(91)90386-r. [DOI] [PubMed] [Google Scholar]
- Lakritz L., Gates R. A., Gugger A. M., Wasserman A. E. Nitrosamine levels in human blood, urine and gastric aspirate following ingestion of foods containing potential nitrosamine precursors or preformed nitrosamines. Food Chem Toxicol. 1982 Aug;20(4):455–459. doi: 10.1016/s0278-6915(82)80112-9. [DOI] [PubMed] [Google Scholar]
- Lin J. K., Ho Y. S. Hepatotoxicity and hepatocarcinogenicity in rats fed squid with or without exogenous nitrite. Food Chem Toxicol. 1992 Aug;30(8):695–702. doi: 10.1016/0278-6915(92)90165-h. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995 Jun 29;93(1):17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- Ohshima H., Bartsch H. Quantitative estimation of endogenous nitrosation in humans by monitoring N-nitrosoproline excreted in the urine. Cancer Res. 1981 Sep;41(9 Pt 1):3658–3662. [PubMed] [Google Scholar]
- Pobel D., Riboli E., Cornée J., Hémon B., Guyader M. Nitrosamine, nitrate and nitrite in relation to gastric cancer: a case-control study in Marseille, France. Eur J Epidemiol. 1995 Feb;11(1):67–73. doi: 10.1007/BF01719947. [DOI] [PubMed] [Google Scholar]
- Schorah C. J., Sobala G. M., Sanderson M., Collis N., Primrose J. N. Gastric juice ascorbic acid: effects of disease and implications for gastric carcinogenesis. Am J Clin Nutr. 1991 Jan;53(1 Suppl):287S–293S. doi: 10.1093/ajcn/53.1.287S. [DOI] [PubMed] [Google Scholar]
- Shapiro K. B., Hotchkiss J. H., Roe D. A. Quantitative relationship between oral nitrate-reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food Chem Toxicol. 1991 Nov;29(11):751–755. doi: 10.1016/0278-6915(91)90183-8. [DOI] [PubMed] [Google Scholar]
- Singer G. M., Lijinsky W. Naturally occurring nitrosatable compounds. I. Secondary amines in foodstuffs. J Agric Food Chem. 1976 May-Jun;24(3):550–553. doi: 10.1021/jf60205a044. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder B., Eisenbrand G., Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976 Dec;14(6):545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder B., Preussmann R. In vivo nitrosation of amidopyrine in humans: use of 'ethanol effect' for biological monitoring of N-nitrosodimethylamine in urine. Carcinogenesis. 1985 Apr;6(4):545–548. doi: 10.1093/carcin/6.4.545. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. R., Weisman M., Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet Toxicol. 1976 Dec;14(6):549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. R., Wishnok J. S., Leaf C. D. Inhibition of nitrosamine formation by ascorbic acid. Am J Clin Nutr. 1991 Jan;53(1 Suppl):247S–250S. doi: 10.1093/ajcn/53.1.247S. [DOI] [PubMed] [Google Scholar]
- Tenovuo J. The biochemistry of nitrates, nitrites, nitrosamines and other potential carcinogens in human saliva. J Oral Pathol. 1986 Jul;15(6):303–307. doi: 10.1111/j.1600-0714.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Tricker A. R., Mostafa M. H., Spiegelhalder B., Preussmann R. Urinary excretion of nitrate, nitrite and N-nitroso compounds in Schistosomiasis and bilharzia bladder cancer patients. Carcinogenesis. 1989 Mar;10(3):547–552. doi: 10.1093/carcin/10.3.547. [DOI] [PubMed] [Google Scholar]
- Tricker A. R., Pfundstein B., Kälble T., Preussmann R. Secondary amine precursors to nitrosamines in human saliva, gastric juice, blood, urine and faeces. Carcinogenesis. 1992 Apr;13(4):563–568. doi: 10.1093/carcin/13.4.563. [DOI] [PubMed] [Google Scholar]
- Tricker A. R., Stickler D. J., Chawla J. C., Preussmann R. Increased urinary nitrosamine excretion in paraplegic patients. Carcinogenesis. 1991 May;12(5):943–946. doi: 10.1093/carcin/12.5.943. [DOI] [PubMed] [Google Scholar]
- Ward M. H., Mark S. D., Cantor K. P., Weisenburger D. D., Correa-Villaseñor A., Zahm S. H. Drinking water nitrate and the risk of non-Hodgkin's lymphoma. Epidemiology. 1996 Sep;7(5):465–471. [PubMed] [Google Scholar]
- Yamamoto M., Yamada T., Tanimura A. Volatile nitrosamines in human blood before and after ingestion of a meal containing high concentrations of nitrate and secondary amines. Food Cosmet Toxicol. 1980 Jun;18(3):297–299. doi: 10.1016/0015-6264(80)90111-x. [DOI] [PubMed] [Google Scholar]
- Zatonski W., Ohshima H., Przewozniak K., Drosik K., Mierzwinska J., Krygier M., Chmielarczyk W., Bartsch H. Urinary excretion of N-nitrosamino acids and nitrate by inhabitants of high- and low-risk areas for stomach cancer in Poland. Int J Cancer. 1989 Nov 15;44(5):823–827. doi: 10.1002/ijc.2910440513. [DOI] [PubMed] [Google Scholar]
- van Maanen J. M., Pachen D. M., Dallinga J. W., Kleinjans J. C. Formation of nitrosamines during consumption of nitrate- and amine-rich foods, and the influence of the use of mouthwashes. Cancer Detect Prev. 1998;22(3):204–212. doi: 10.1046/j.1525-1500.1998.0oa26.x. [DOI] [PubMed] [Google Scholar]
- van Maanen J. M., Welle I. J., Hageman G., Dallinga J. W., Mertens P. L., Kleinjans J. C. Nitrate contamination of drinking water: relationship with HPRT variant frequency in lymphocyte DNA and urinary excretion of N-nitrosamines. Environ Health Perspect. 1996 May;104(5):522–528. doi: 10.1289/ehp.96104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maanen J. M., van Dijk A., Mulder K., de Baets M. H., Menheere P. C., van der Heide D., Mertens P. L., Kleinjans J. C. Consumption of drinking water with high nitrate levels causes hypertrophy of the thyroid. Toxicol Lett. 1994 Jun;72(1-3):365–374. doi: 10.1016/0378-4274(94)90050-7. [DOI] [PubMed] [Google Scholar]
- van Maanen J. M., van Geel A. A., Kleinjans J. C. Modulation of nitrate-nitrite conversion in the oral cavity. Cancer Detect Prev. 1996;20(6):590–596. [PubMed] [Google Scholar]