Abstract
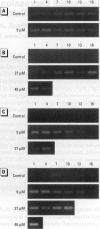
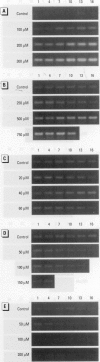
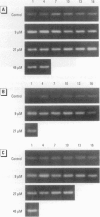
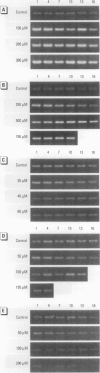
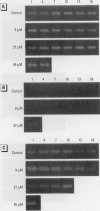
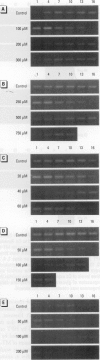
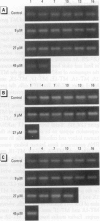
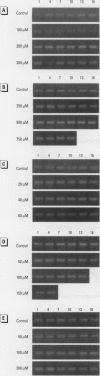
In contrast to the single metallothionein (MT)-1 gene of the mouse, the human MT-1 gene family is composed of seven active genes and six pseudogenes. In this study, the expression of mRNA representing the seven active human MT-1 genes was determined in cultured human proximal tubule (HPT) cells under basal conditions and after exposure to the metals Cd2+, Zn2+, Cu2+, Hg2+, Ag2+, and Pb2+. Basal expression of MT-1X and MT-1E mRNA in HPT cells was similar to expression of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase. In contrast, mRNAs representing the basal expression of MT-1A and MT-1F were a minor transcript in HPT cells. Treatment of HPT cells with Cd2+, Zn2+, or Cu2+ increased the levels of MT-1E and MT-1A mRNA, but not the levels of MT-1X or MT-1F mRNA. The increase in MT-1E mRNA appeared to be influenced mainly by exposure to the various metals, whereas the increase in MT-1A mRNA was influenced more by exposure to a metal concentration eliciting a loss of cell viability. Treatment of HPT cells with the metals Hg2+, Ag2+, and Pb2+ was found to have no effect on the level of MT-1 mRNA at either sublethal or lethal concentrations. Using HPT cells as a model, these results suggest that new features of MT gene expression have been acquired in the human due to the duplication of the MT-1 gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard A., Roels H., Hubermont G., Buchet J. P., Masson P. L., Lauwerys R. R. Characterization of the proteinuria in cadmium-exposed workers. Int Arch Occup Environ Health. 1976 Oct 21;38(1):19–30. doi: 10.1007/BF00378317. [DOI] [PubMed] [Google Scholar]
- Bosco E., Porta N., Diezi J. Renal handling of cadmium: a study by tubular microinjections. Arch Toxicol Suppl. 1984;7:371–373. doi: 10.1007/978-3-642-69132-4_62. [DOI] [PubMed] [Google Scholar]
- Cavigelli M., Kägi J. H., Hunziker P. E. Cell- and inducer-specific accretion of human isometallothioneins. Biochem J. 1993 Jun 1;292(Pt 2):551–554. doi: 10.1042/bj2920551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J. Metallothionein--aspects related to copper and zinc metabolism. J Inherit Metab Dis. 1983;6 (Suppl 1):15–21. doi: 10.1007/BF01811318. [DOI] [PubMed] [Google Scholar]
- Garrett S. H., Somji S., Todd J. H., Sens D. A. Exposure of human proximal tubule cells to cd2+, zn2+, and Cu2+ induces metallothionein protein accumulation but not metallothionein isoform 2 mRNA. Environ Health Perspect. 1998 Sep;106(9):587–595. doi: 10.1289/ehp.98106587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering P. L., Klaassen C. D. Altered subcellular distribution of cadmium following cadmium pretreatment: possible mechanism of tolerance to cadmium-induced lethality. Toxicol Appl Pharmacol. 1983 Sep 15;70(2):195–203. doi: 10.1016/0041-008x(83)90095-9. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hoey J. G., Garrett S. H., Sens M. A., Todd J. H., Sens D. A. Expression of MT-3 mRNA in human kidney, proximal tubule cell cultures, and renal cell carcinoma. Toxicol Lett. 1997 Jul 21;92(2):149–160. doi: 10.1016/s0378-4274(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Jahroudi N., Foster R., Price-Haughey J., Beitel G., Gedamu L. Cell-type specific and differential regulation of the human metallothionein genes. Correlation with DNA methylation and chromatin structure. J Biol Chem. 1990 Apr 15;265(11):6506–6511. [PubMed] [Google Scholar]
- Kägi J. H., Hunziker P. Mammalian metallothionein. Biol Trace Elem Res. 1989 Jul-Sep;21:111–118. doi: 10.1007/BF02917243. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Kojima Y. Chemistry and biochemistry of metallothionein. Experientia Suppl. 1987;52:25–61. doi: 10.1007/978-3-0348-6784-9_3. [DOI] [PubMed] [Google Scholar]
- Mididoddi S., McGuirt J. P., Sens M. A., Todd J. H., Sens D. A. Isoform-specific expression of metallothionein mRNA in the developing and adult human kidney. Toxicol Lett. 1996 Apr;85(1):17–27. doi: 10.1016/0378-4274(96)03632-6. [DOI] [PubMed] [Google Scholar]
- Nartey N. O., Banerjee D., Cherian M. G. Immunohistochemical localization of metallothionein in cell nucleus and cytoplasm of fetal human liver and kidney and its changes during development. Pathology. 1987 Jul;19(3):233–238. doi: 10.3109/00313028709066555. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Findley S. D., Whitmore T. E., Durnam D. M. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels M., van Weyenbergh J., Soumillion A., Proost P., De Ley M. Induction by zinc of specific metallothionein isoforms in human monocytes. Eur J Biochem. 1994 Feb 15;220(1):105–110. doi: 10.1111/j.1432-1033.1994.tb18603.x. [DOI] [PubMed] [Google Scholar]
- Quaife C. J., Findley S. D., Erickson J. C., Froelick G. J., Kelly E. J., Zambrowicz B. P., Palmiter R. D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994 Jun 14;33(23):7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Hamer D. H. Cell specificity and an effect of ras on human metallothionein gene expression. Proc Natl Acad Sci U S A. 1986 May;83(10):3346–3350. doi: 10.1073/pnas.83.10.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. J., Jubier M. F., Hamer D. H. Structure and expression of two human metallothionein-I isoform genes and a related pseudogene. J Biol Chem. 1985 Jun 25;260(12):7731–7737. [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumillion A., Van Damme J., De Ley M. Cloning and specific polymerised-chain-reaction amplification of a third charge-separable human metallothionein isoform. Eur J Biochem. 1992 Nov 1;209(3):999–1004. doi: 10.1111/j.1432-1033.1992.tb17374.x. [DOI] [PubMed] [Google Scholar]
- Stennard F. A., Holloway A. F., Hamilton J., West A. K. Characterisation of six additional human metallothionein genes. Biochim Biophys Acta. 1994 Aug 2;1218(3):357–365. doi: 10.1016/0167-4781(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Varshney U., Jahroudi N., Foster R., Gedamu L. Structure, organization, and regulation of human metallothionein IF gene: differential and cell-type-specific expression in response to heavy metals and glucocorticoids. Mol Cell Biol. 1986 Jan;6(1):26–37. doi: 10.1128/mcb.6.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]