Abstract
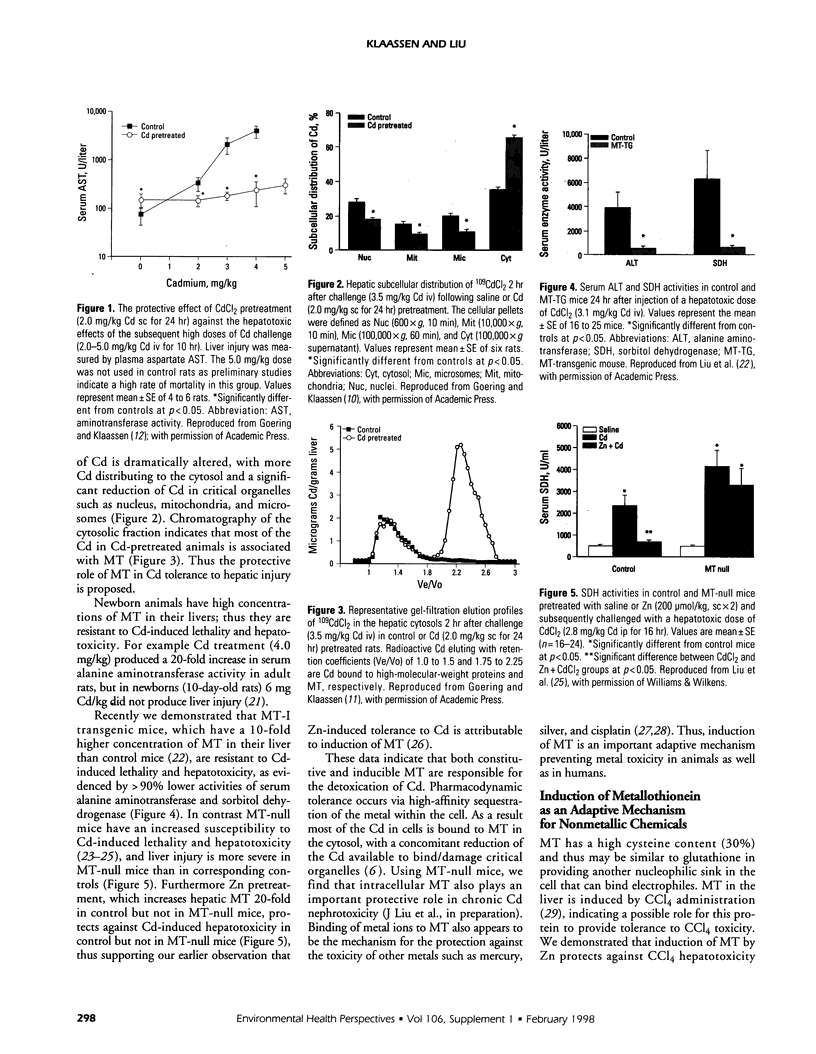
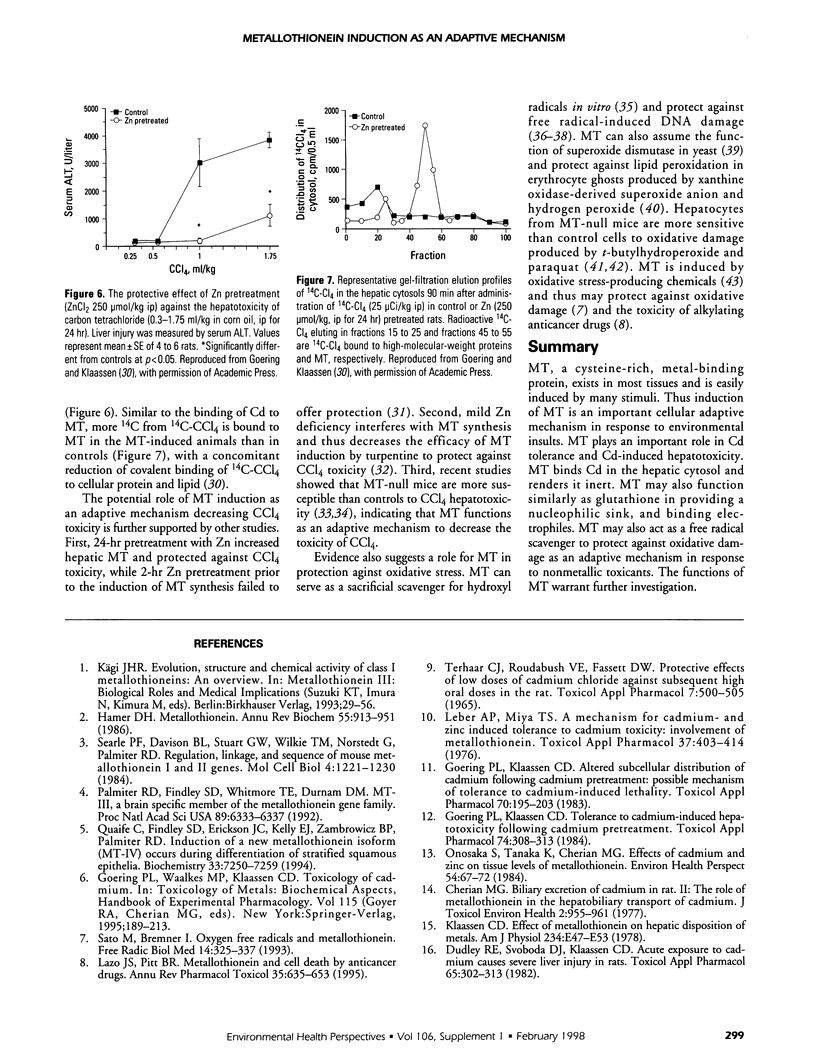
Pretreatment of rats with low doses of Cd produces adaptive tolerance to a subsequent high dose of Cd-induced lethality, thus shifting the dose-response curve to the right. Cd pretreatment of animals also protects against the hepatotoxicity produced by high doses of Cd. This protection is attributable to the 10- to 50-fold induction of hepatic metallothionein (MT) by Cd pretreatment. As a result hepatic subcellular distribution of Cd is significantly altered, with more Cd bound to MT in the cytosol and a concomitant reduction of Cd in other critical organelles. In addition MT-transgenic animals are more resistant, whereas MT-null mice are more sensitive than controls to Cd-induced lethality and hepatotoxicity. This further demonstrates that MT is important in Cd detoxication. Induction of hepatic MT by zinc also protects mice from carbon tetrachloride (CCl4)-induced liver injury, with more 14C-CCl4 bound to MT in the cytosol. MT-null mice are more sensitive to CCl4-induced hepatotoxicity, which supports the hypothesis that induction of MT also plays a protective role for nonmetallic chemicals. These results indicate that MT is a part of cellular adaptive mechanisms affecting the magnitude and progression of toxic insults from metals such as Cd as well as from organic chemicals such as CCl4.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel J., de Ruiter N. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett. 1989 May;47(2):191–196. doi: 10.1016/0378-4274(89)90075-1. [DOI] [PubMed] [Google Scholar]
- Bauman J. W., Liu J., Liu Y. P., Klaassen C. D. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991 Sep 1;110(2):347–354. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- Cagen S. Z., Klaassen C. D. Protection of carbon tetrachloride-induced hepatotoxicity by zinc: role of metallothionein. Toxicol Appl Pharmacol. 1979 Oct;51(1):107–116. doi: 10.1016/0041-008x(79)90013-9. [DOI] [PubMed] [Google Scholar]
- Cherian M. G. Biliary excretion of cadmium in rat. II. The role of metallothionein in the hepatobiliary transport of cadmium. J Toxicol Environ Health. 1977 Mar;2(4):955–961. doi: 10.1080/15287397709529494. [DOI] [PubMed] [Google Scholar]
- Chubatsu L. S., Meneghini R. Metallothionein protects DNA from oxidative damage. Biochem J. 1993 Apr 1;291(Pt 1):193–198. doi: 10.1042/bj2910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke I. S., Lui E. M. Interaction of metallothionein and carbon tetrachloride on the protective effect of zinc on hepatotoxicity. Can J Physiol Pharmacol. 1986 Aug;64(8):1104–1110. doi: 10.1139/y86-188. [DOI] [PubMed] [Google Scholar]
- DiSilvestro R. A., Carlson G. P. Effects of mild zinc deficiency, plus or minus acute phase response, on CCl4 hepatotoxicity. Free Radic Biol Med. 1994 Jan;16(1):57–61. doi: 10.1016/0891-5849(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Dudley R. E., Gammal L. M., Klaassen C. D. Cadmium-induced hepatic and renal injury in chronically exposed rats: likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol Appl Pharmacol. 1985 Mar 15;77(3):414–426. doi: 10.1016/0041-008x(85)90181-4. [DOI] [PubMed] [Google Scholar]
- Dudley R. E., Svoboda D. J., Klaassen C. D. Time course of cadmium-induced ultrastructural changes in rat liver. Toxicol Appl Pharmacol. 1984 Oct;76(1):150–160. doi: 10.1016/0041-008x(84)90038-3. [DOI] [PubMed] [Google Scholar]
- Dudley R. E., Svoboda D. J., Klaassen C. Acute exposure to cadmium causes severe liver injury in rats. Toxicol Appl Pharmacol. 1982 Sep 15;65(2):302–313. doi: 10.1016/0041-008x(82)90013-8. [DOI] [PubMed] [Google Scholar]
- Goering P. L., Klaassen C. D. Altered subcellular distribution of cadmium following cadmium pretreatment: possible mechanism of tolerance to cadmium-induced lethality. Toxicol Appl Pharmacol. 1983 Sep 15;70(2):195–203. doi: 10.1016/0041-008x(83)90095-9. [DOI] [PubMed] [Google Scholar]
- Goering P. L., Klaassen C. D. Resistance to cadmium-induced hepatotoxicity in immature rats. Toxicol Appl Pharmacol. 1984 Jul;74(3):321–329. doi: 10.1016/0041-008x(84)90285-0. [DOI] [PubMed] [Google Scholar]
- Goering P. L., Klaassen C. D. Tolerance to cadmium-induced hepatotoxicity following cadmium pretreatment. Toxicol Appl Pharmacol. 1984 Jul;74(3):308–313. doi: 10.1016/0041-008x(84)90283-7. [DOI] [PubMed] [Google Scholar]
- Goering P. L., Klaassen C. D. Zinc-induced tolerance to cadmium hepatotoxicity. Toxicol Appl Pharmacol. 1984 Jul;74(3):299–307. doi: 10.1016/0041-008x(84)90282-5. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. O., Cook J. A., di Luzio N. R., Coover J. A. The effects of acute cadmium administration in the liver and kidney of the rat. Light and electron microscopic studies. Lab Invest. 1975 May;32(5):655–664. [PubMed] [Google Scholar]
- Klaassen C. D. Effect of metallothionein on hepatic disposition of metals. Am J Physiol. 1978 Jan;234(1):E47–E53. doi: 10.1152/ajpendo.1978.234.1.E47. [DOI] [PubMed] [Google Scholar]
- Kraker A., Schmidt J., Krezoski S., Petering D. H. Binding of cis-dichlorodiammine platinum(II) to metallothionein in Ehrlich cells. Biochem Biophys Res Commun. 1985 Jul 31;130(2):786–792. doi: 10.1016/0006-291x(85)90485-1. [DOI] [PubMed] [Google Scholar]
- Lazo J. S., Kondo Y., Dellapiazza D., Michalska A. E., Choo K. H., Pitt B. R. Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem. 1995 Mar 10;270(10):5506–5510. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- Lazo J. S., Pitt B. R. Metallothioneins and cell death by anticancer drugs. Annu Rev Pharmacol Toxicol. 1995;35:635–653. doi: 10.1146/annurev.pa.35.040195.003223. [DOI] [PubMed] [Google Scholar]
- Leber A. P., Miya T. S. A mechanism for cadmium- and zinc-induced tolerance to cadmium toxicity: involvement of metallothionein. Toxicol Appl Pharmacol. 1976 Sep;37(3):403–414. doi: 10.1016/0041-008x(76)90202-7. [DOI] [PubMed] [Google Scholar]
- Liu J., Kershaw W. C., Klaassen C. D. The protective effect of metallothionein on the toxicity of various metals in rat primary hepatocyte culture. Toxicol Appl Pharmacol. 1991 Jan;107(1):27–34. doi: 10.1016/0041-008x(91)90327-b. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu Y., Michalska A. E., Choo K. H., Klaassen C. D. Metallothionein plays less of a protective role in cadmium-metallothionein-induced nephrotoxicity than in cadmium chloride-induced hepatotoxicity. J Pharmacol Exp Ther. 1996 Mar;276(3):1216–1223. [PubMed] [Google Scholar]
- Liu Y., Liu J., Iszard M. B., Andrews G. K., Palmiter R. D., Klaassen C. D. Transgenic mice that overexpress metallothionein-I are protected from cadmium lethality and hepatotoxicity. Toxicol Appl Pharmacol. 1995 Dec;135(2):222–228. doi: 10.1006/taap.1995.1227. [DOI] [PubMed] [Google Scholar]
- Masters B. A., Kelly E. J., Quaife C. J., Brinster R. L., Palmiter R. D. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska A. E., Choo K. H. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8088–8092. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K. S., Terano Y., Onosaka S., Tanaka K. Induction of metallothionein synthesis by menadione or carbon tetrachloride is independent of free radical production. Toxicol Appl Pharmacol. 1992 Mar;113(1):74–79. doi: 10.1016/0041-008x(92)90010-p. [DOI] [PubMed] [Google Scholar]
- Onosaka S., Tanaka K., Cherian M. G. Effects of cadmium and zinc on tissue levels of metallothionein. Environ Health Perspect. 1984 Mar;54:67–72. doi: 10.1289/ehp.845467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Findley S. D., Whitmore T. E., Durnam D. M. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife C. J., Findley S. D., Erickson J. C., Froelick G. J., Kelly E. J., Zambrowicz B. P., Palmiter R. D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994 Jun 14;33(23):7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- Sato M., Bremner I. Oxygen free radicals and metallothionein. Free Radic Biol Med. 1993 Mar;14(3):325–337. doi: 10.1016/0891-5849(93)90029-t. [DOI] [PubMed] [Google Scholar]
- Schwarz M. A., Lazo J. S., Yalowich J. C., Allen W. P., Whitmore M., Bergonia H. A., Tzeng E., Billiar T. R., Robbins P. D., Lancaster J. R., Jr Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric oxide. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4452–4456. doi: 10.1073/pnas.92.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K. T., Gralla E. B., Ellerby L. M., Valentine J. S., Thiele D. J. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8013–8017. doi: 10.1073/pnas.90.17.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. P., Bachowski G. J., Girotti A. W. Inhibition of cell membrane lipid peroxidation by cadmium- and zinc-metallothioneins. Biochim Biophys Acta. 1986 Dec 10;884(3):448–461. doi: 10.1016/0304-4165(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Thornalley P. J., Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985 Jan 21;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Zheng H., Liu J., Liu Y., Klaassen C. D. Hepatocytes from metallothionein-I and II knock-out mice are sensitive to cadmium- and tert-butylhydroperoxide-induced cytotoxicity. Toxicol Lett. 1996 Oct;87(2-3):139–145. doi: 10.1016/0378-4274(96)03770-8. [DOI] [PubMed] [Google Scholar]