Abstract
Reactive oxygen species (ROS) have been implicated in the pathogenesis of many clinical disorders such as adult respiratory distress syndrome, ischemia-reperfusion injury, atherosclerosis, neurodegenerative diseases, and cancer. Genetically engineered animal models have been used as a tool for understanding the function of various antioxidant enzymes in cellular defense mechanisms against various types of oxidant tissue injury. Transgenic mice overexpressing three isoforms of superoxide dismutase, catalase, and the cellular glutathione peroxidase (GSHPx-1) in various tissues show an increased tolerance to ischemia-reperfusion heart and brain injury, hyperoxia, cold-induced brain edema, adriamycin, and paraquat toxicity. These results have provided for the first time direct evidence demonstrating the importance of each of these antioxidant enzymes in protecting the animals against the injury resulting from these insults, as well as the effect of an enhanced level of antioxidant in ameliorating the oxidant tissue injury. To evaluate further the nature of these enzymes in antioxidant defense, gene knockout mice deficient in copper-zinc superoxide dismutase (CuZnSOD) and GSHPx-1 have also been generated in our laboratory. These mice developed normally and showed no marked pathologic changes under normal physiologic conditions. In addition, a deficiency in these genes had no effects on animal survival under hyperoxida. However, these knockout mice exhibited a pronounced susceptibility to paraquat toxicity and myocardial ischemia-reperfusion injury. Furthermore, female mice lacking CuZnSOD also displayed a marked increase in postimplantation embryonic lethality. These animals should provide a useful model for uncovering the identity of ROS that participate in the pathogenesis of various clinical disorders and for defining the role of each antioxidant enzyme in cellular defense against oxidant-mediated tissue injury.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar N., Finkelstein J. N., Horowitz S., Willey J. C., Coy E., Frampton M. W., Watkins R. H., Khullar P., Xu Y. L., Cohen H. J. Extracellular glutathione peroxidase in human lung epithelial lining fluid and in lung cells. Am J Physiol. 1996 Feb;270(2 Pt 1):L173–L182. doi: 10.1152/ajplung.1996.270.2.L173. [DOI] [PubMed] [Google Scholar]
- Avraham K. B., Schickler M., Sapoznikov D., Yarom R., Groner Y. Down's syndrome: abnormal neuromuscular junction in tongue of transgenic mice with elevated levels of human Cu/Zn-superoxide dismutase. Cell. 1988 Sep 9;54(6):823–829. doi: 10.1016/s0092-8674(88)91153-1. [DOI] [PubMed] [Google Scholar]
- Bar-Peled O., Korkotian E., Segal M., Groner Y. Constitutive overexpression of Cu/Zn superoxide dismutase exacerbates kainic acid-induced apoptosis of transgenic-Cu/Zn superoxide dismutase neurons. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8530–8535. doi: 10.1073/pnas.93.16.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwal R. S., Bahuguna A. Zinc, copper and selenium in reproduction. Experientia. 1994 Jul 15;50(7):626–640. doi: 10.1007/BF01952862. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Hill K. E. Regulation of selenoproteins. Annu Rev Nutr. 1993;13:65–81. doi: 10.1146/annurev.nu.13.070193.000433. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Yang G. Y., Chen S. F., Carlson E., Epstein C. J. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing CuZn-superoxide dismutase. Ann Neurol. 1991 May;29(5):482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- Chen E. P., Bittner H. B., Davis R. D., Folz R. J., Van Trigt P. Extracellular superoxide dismutase transgene overexpression preserves postischemic myocardial function in isolated murine hearts. Circulation. 1996 Nov 1;94(9 Suppl):II412–II417. [PubMed] [Google Scholar]
- Cheng W. H., Ho Y. S., Valentine B. A., Ross D. A., Combs G. F., Jr, Lei X. G. Cellular glutathione peroxidase is the mediator of body selenium to protect against paraquat lethality in transgenic mice. J Nutr. 1998 Jul;128(7):1070–1076. doi: 10.1093/jn/128.7.1070. [DOI] [PubMed] [Google Scholar]
- Chu F. F., Doroshow J. H., Esworthy R. S. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem. 1993 Feb 5;268(4):2571–2576. [PubMed] [Google Scholar]
- Chu F. F., Esworthy R. S. The expression of an intestinal form of glutathione peroxidase (GSHPx-GI) in rat intestinal epithelium. Arch Biochem Biophys. 1995 Nov 10;323(2):288–294. doi: 10.1006/abbi.1995.9962. [DOI] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Danciger E., Dafni N., Bernstein Y., Laver-Rudich Z., Neer A., Groner Y. Human Cu/Zn superoxide dismutase gene family: molecular structure and characterization of four Cu/Zn superoxide dismutase-related pseudogenes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3619–3623. doi: 10.1073/pnas.83.11.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. J., Avraham K. B., Lovett M., Smith S., Elroy-Stein O., Rotman G., Bry C., Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J., Conkie D., Chambers I., McBain W., Dexter M., Harrison P. Changes in minor transcripts from the alpha 1 and beta maj globin and glutathione peroxidase genes during erythropoiesis. Nucleic Acids Res. 1987 May 11;15(9):3671–3688. doi: 10.1093/nar/15.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gladyshev V. N., Jeang K. T., Stadtman T. C. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc Natl Acad Sci U S A. 1996 Jun 11;93(12):6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
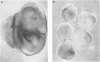
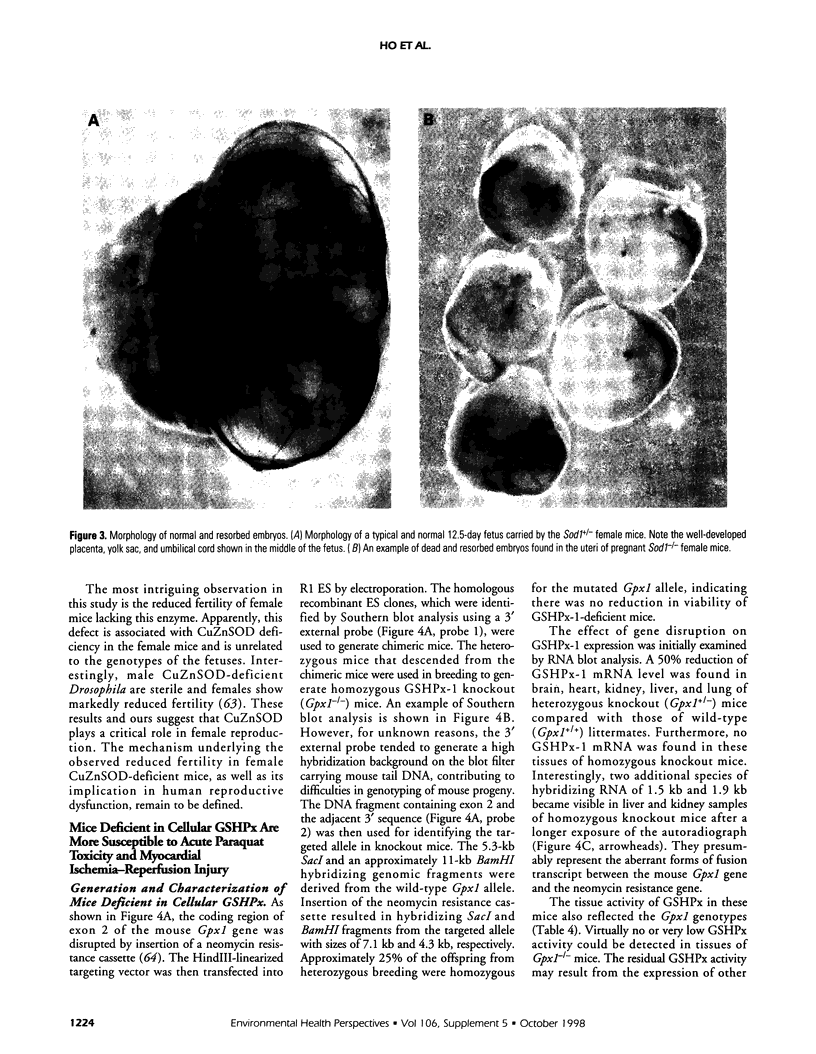
- Ho Y. S., Gargano M., Cao J., Bronson R. T., Heimler I., Hutz R. J. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998 Mar 27;273(13):7765–7769. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
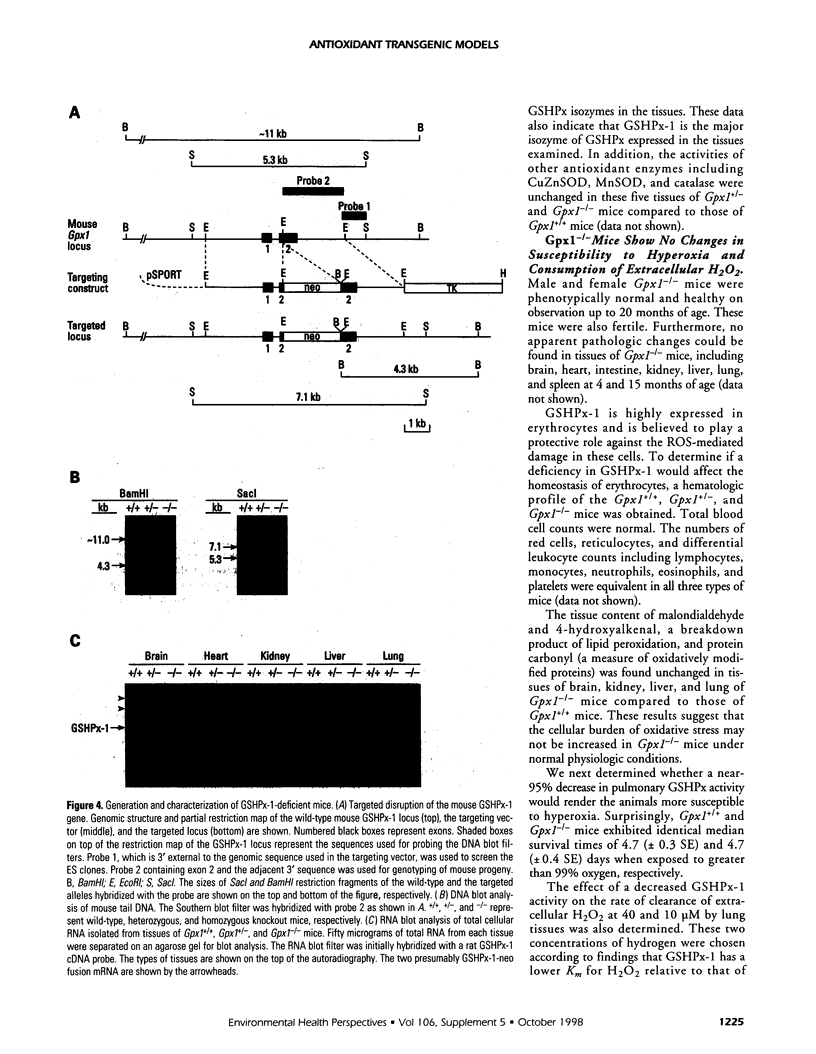
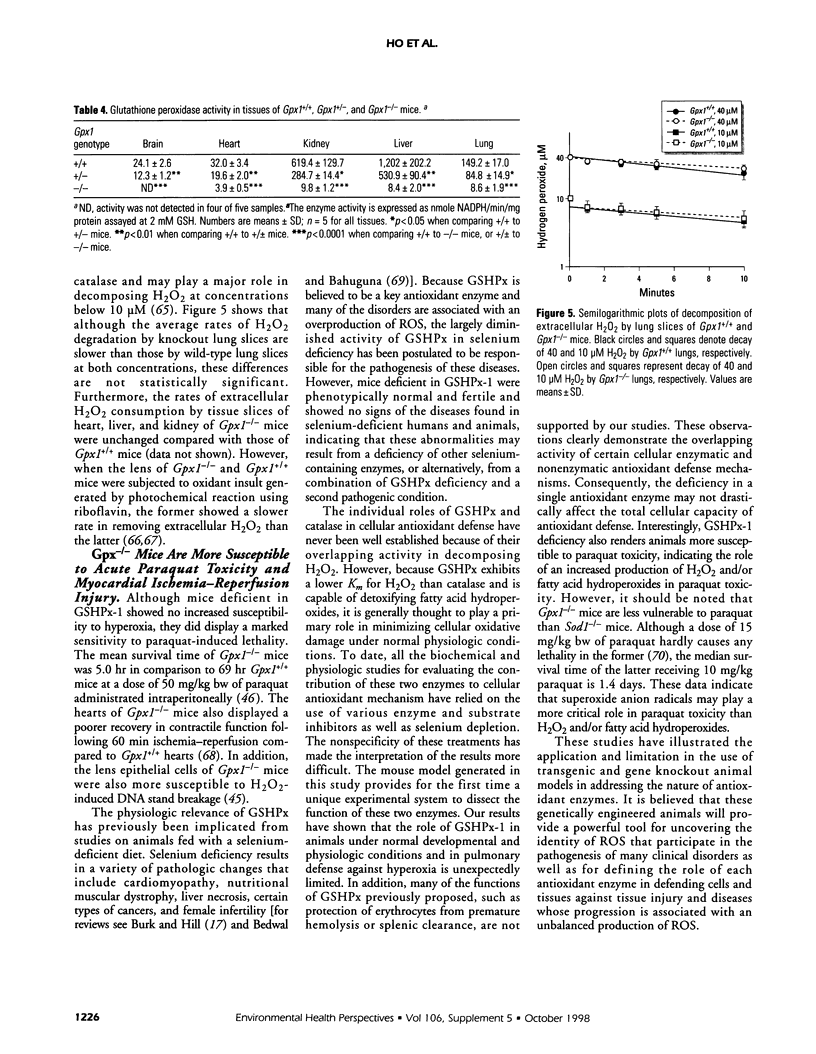
- Ho Y. S., Magnenat J. L., Bronson R. T., Cao J., Gargano M., Sugawara M., Funk C. D. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997 Jun 27;272(26):16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Vincent R., Dey M. S., Slot J. W., Crapo J. D. Transgenic models for the study of lung antioxidant defense: enhanced manganese-containing superoxide dismutase activity gives partial protection to B6C3 hybrid mice exposed to hyperoxia. Am J Respir Cell Mol Biol. 1998 Apr;18(4):538–547. doi: 10.1165/ajrcmb.18.4.2959. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Kang Y. J., Chen Y., Epstein P. N. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996 May 24;271(21):12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- Kinouchi H., Epstein C. J., Mizui T., Carlson E., Chen S. F., Chan P. H. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Reaume A. G., Huang T. T., Carlson E., Murakami K., Chen S. F., Hoffman E. K., Scott R. W., Epstein C. J., Chan P. H. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997 Jun 1;17(11):4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch H. M., Wang J., Luche R., Carlson E., Bray T. M., Epstein C. J., Phillips J. P. Transgenic copper/zinc superoxide dismutase modulates susceptibility to type I diabetes. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9956–9959. doi: 10.1073/pnas.91.21.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R. M., Zhang H., Vogel H., Cartwright J., Jr, Dionne L., Lu N., Huang S., Matzuk M. M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., Noble L. J., Yoshimura M. P., Berger C., Chan P. H. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995 Dec;11(4):376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Lu Y. P., Lou Y. R., Yen P., Newmark H. L., Mirochnitchenko O. I., Inouye M., Huang M. T. Enhanced skin carcinogenesis in transgenic mice with high expression of glutathione peroxidase or both glutathione peroxidase and superoxide dismutase. Cancer Res. 1997 Apr 15;57(8):1468–1474. [PubMed] [Google Scholar]
- Makino N., Mochizuki Y., Bannai S., Sugita Y. Kinetic studies on the removal of extracellular hydrogen peroxide by cultured fibroblasts. J Biol Chem. 1994 Jan 14;269(2):1020–1025. [PubMed] [Google Scholar]
- Marklund S. L., Bjelle A., Elmqvist L. G. Superoxide dismutase isoenzymes of the synovial fluid in rheumatoid arthritis and in reactive arthritides. Ann Rheum Dis. 1986 Oct;45(10):847–851. doi: 10.1136/ard.45.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984 Sep 15;222(3):649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Holme E., Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982 Nov 24;126(1):41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minc-Golomb D., Knobler H., Groner Y. Gene dosage of CuZnSOD and Down's syndrome: diminished prostaglandin synthesis in human trisomy 21, transfected cells and transgenic mice. EMBO J. 1991 Aug;10(8):2119–2124. doi: 10.1002/j.1460-2075.1991.tb07745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirochnitchenko O., Palnitkar U., Philbert M., Inouye M. Thermosensitive phenotype of transgenic mice overproducing human glutathione peroxidases. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8120–8124. doi: 10.1073/pnas.92.18.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Rossant J., Nagy R., Abramow-Newerly W., Roder J. C. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley T. D., Coursin D. B., Cihla H. P., Oberley L. W., el-Sayyad N., Ho Y. S. Immunolocalization of manganese superoxide dismutase in normal and transgenic mice expressing the human enzyme. Histochem J. 1993 Apr;25(4):267–279. doi: 10.1007/BF00159118. [DOI] [PubMed] [Google Scholar]
- Oury T. D., Ho Y. S., Piantadosi C. A., Crapo J. D. Extracellular superoxide dismutase, nitric oxide, and central nervous system O2 toxicity. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9715–9719. doi: 10.1073/pnas.89.20.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury T. D., Piantadosi C. A., Crapo J. D. Cold-induced brain edema in mice. Involvement of extracellular superoxide dismutase and nitric oxide. J Biol Chem. 1993 Jul 25;268(21):15394–15398. [PubMed] [Google Scholar]
- Peled-Kamar M., Lotem J., Okon E., Sachs L., Groner Y. Thymic abnormalities and enhanced apoptosis of thymocytes and bone marrow cells in transgenic mice overexpressing Cu/Zn-superoxide dismutase: implications for Down syndrome. EMBO J. 1995 Oct 16;14(20):4985–4993. doi: 10.1002/j.1460-2075.1995.tb00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled-Kamar M., Lotem J., Wirguin I., Weiner L., Hermalin A., Groner Y. Oxidative stress mediates impairment of muscle function in transgenic mice with elevated level of wild-type Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A. 1997 Apr 15;94(8):3883–3887. doi: 10.1073/pnas.94.8.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S., Kostic V., Jackson-Lewis V., Naini A. B., Simonetti S., Fahn S., Carlson E., Epstein C. J., Cadet J. L. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992 May;12(5):1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume A. G., Elliott J. L., Hoffman E. K., Kowall N. W., Ferrante R. J., Siwek D. F., Wilcox H. M., Flood D. G., Beal M. F., Brown R. H., Jr Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996 May;13(1):43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Reddy V. N., Lin L. R., Ho Y. S., Magnenat J. L., Ibaraki N., Giblin F. J., Dang L. Peroxide-induced damage in lenses of transgenic mice with deficient and elevated levels of glutathione peroxidase. Ophthalmologica. 1997;211(3):192–200. doi: 10.1159/000310788. [DOI] [PubMed] [Google Scholar]
- Roveri A., Casasco A., Maiorino M., Dalan P., Calligaro A., Ursini F. Phospholipid hydroperoxide glutathione peroxidase of rat testis. Gonadotropin dependence and immunocytochemical identification. J Biol Chem. 1992 Mar 25;267(9):6142–6146. [PubMed] [Google Scholar]
- Schickler M., Knobler H., Avraham K. B., Elroy-Stein O., Groner Y. Diminished serotonin uptake in platelets of transgenic mice with increased Cu/Zn-superoxide dismutase activity. EMBO J. 1989 May;8(5):1385–1392. doi: 10.1002/j.1460-2075.1989.tb03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A., Ma W., Wang R. R., Yang Y., Ho Y. S. The contribution of GSH peroxidase-1, catalase and GSH to the degradation of H2O2 by the mouse lens. Exp Eye Res. 1997 Mar;64(3):477–485. doi: 10.1006/exer.1996.0250. [DOI] [PubMed] [Google Scholar]
- Spector A., Yang Y., Ho Y. S., Magnenat J. L., Wang R. R., Ma W., Li W. C. Variation in cellular glutathione peroxidase activity in lens epithelial cells, transgenics and knockouts does not significantly change the response to H2O2 stress. Exp Eye Res. 1996 May;62(5):521–540. doi: 10.1006/exer.1996.0063. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Avissar N., Whitin J., Cohen H. Purification and characterization of human plasma glutathione peroxidase: a selenoglycoprotein distinct from the known cellular enzyme. Arch Biochem Biophys. 1987 Aug 1;256(2):677–686. doi: 10.1016/0003-9861(87)90624-2. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Maiorino M., Ursini F., Girotti A. W. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem. 1990 Jan 5;265(1):454–461. [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Levitt J. G., Crapo J. D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982 Sep;217(2):401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Ursini F., Maiorino M., Valente M., Ferri L., Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982 Feb 15;710(2):197–211. doi: 10.1016/0005-2760(82)90150-3. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]
- White C. W., Avraham K. B., Shanley P. F., Groner Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest. 1991 Jun;87(6):2162–2168. doi: 10.1172/JCI115249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wispé J. R., Warner B. B., Clark J. C., Dey C. R., Neuman J., Glasser S. W., Crapo J. D., Chang L. Y., Whitsett J. A. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992 Nov 25;267(33):23937–23941. [PubMed] [Google Scholar]
- Yen H. C., Oberley T. D., Vichitbandha S., Ho Y. S., St Clair D. K. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996 Sep 1;98(5):1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Maulik N., Engelman R. M., Ho Y. S., Magnenat J. L., Rousou J. A., Flack J. E., 3rd, Deaton D., Das D. K. Glutathione peroxidase knockout mice are susceptible to myocardial ischemia reperfusion injury. Circulation. 1997 Nov 4;96(9 Suppl):II–216-20. [PubMed] [Google Scholar]
- Yoshida T., Watanabe M., Engelman D. T., Engelman R. M., Schley J. A., Maulik N., Ho Y. S., Oberley T. D., Das D. K. Transgenic mice overexpressing glutathione peroxidase are resistant to myocardial ischemia reperfusion injury. J Mol Cell Cardiol. 1996 Aug;28(8):1759–1767. doi: 10.1006/jmcc.1996.0165. [DOI] [PubMed] [Google Scholar]