Abstract
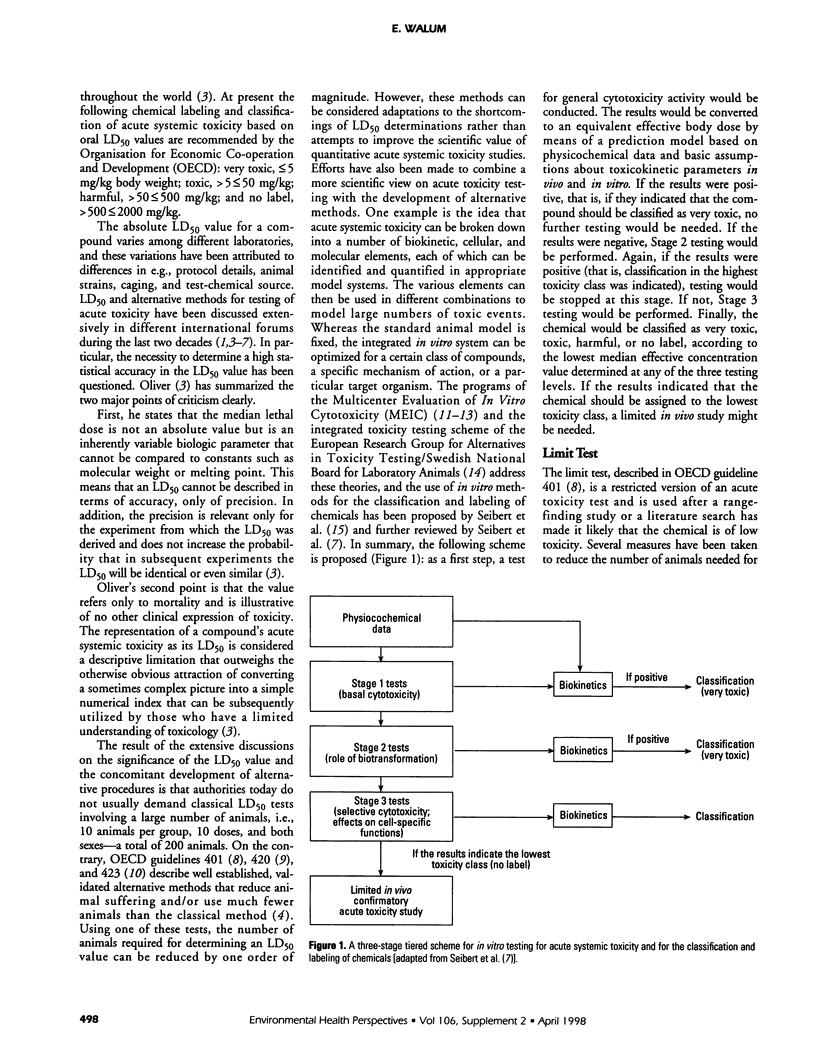
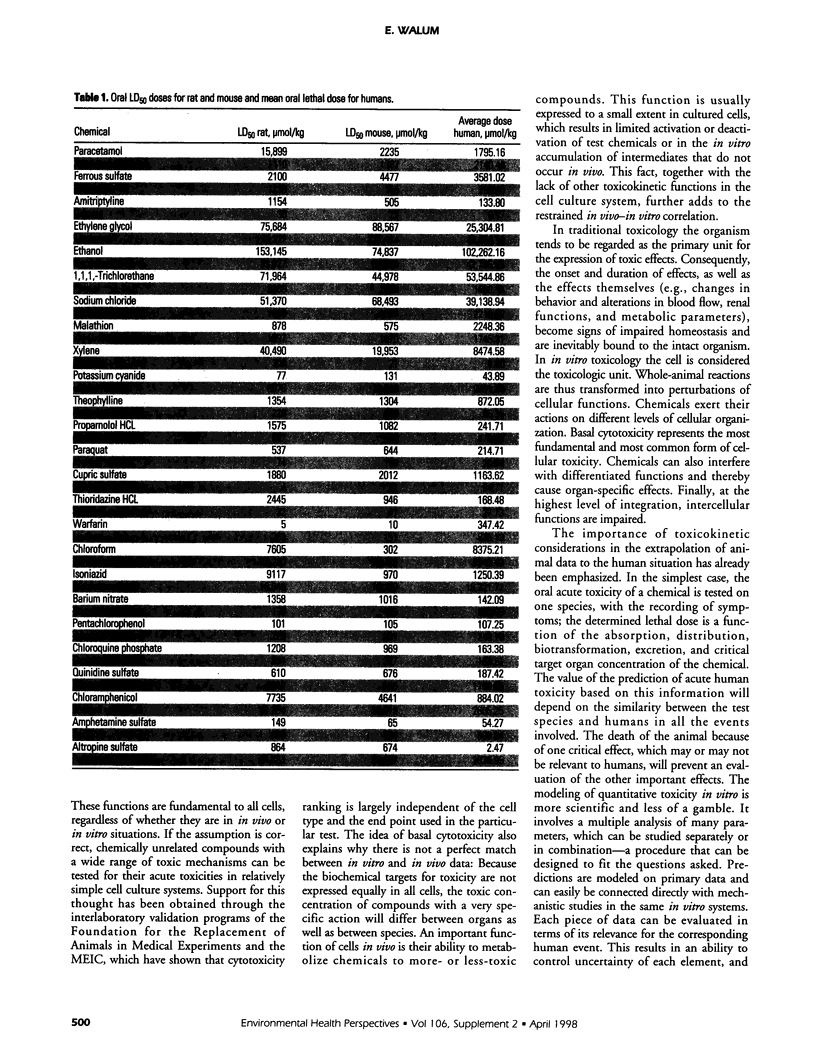
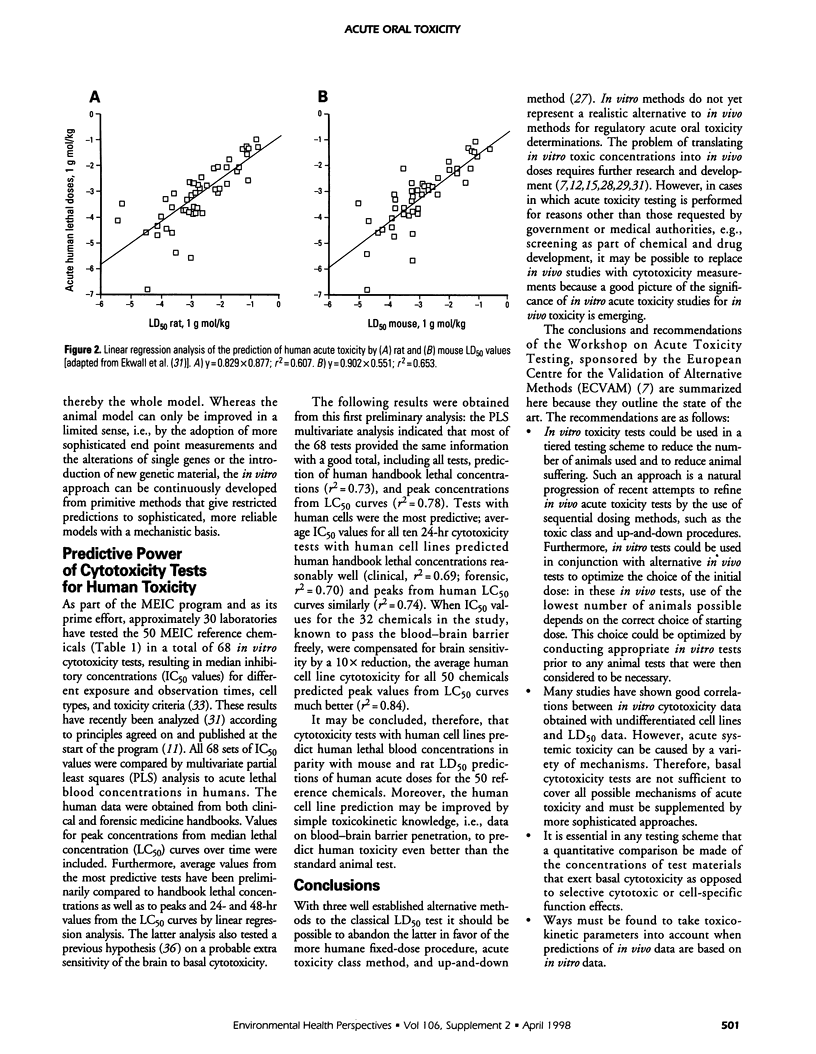
The purposes of acute toxicity testing are to obtain information on the biologic activity of a chemical and gain insight into its mechanism of action. The information on acute systemic toxicity generated by the test is used in hazard identification and risk management in the context of production, handling, and use of chemicals. The LD50 value, defined as the statistically derived dose that, when administered in an acute toxicity test, is expected to cause death in 50% of the treated animals in a given period, is currently the basis for toxicologic classification of chemicals. For a classical LD50 study, laboratory mice and rats are the species typically selected. Often both sexes must be used for regulatory purposes. When oral administration is combined with parenteral, information on the bioavailability of the tested compound is obtained. The result of the extensive discussions on the significance of the LD50 value and the concomitant development of alternative procedures is that authorities today do not usually demand classical LD50 tests involving a large number of animals. The limit test, the fixed-dose procedure, the toxic class method, and the up-and-down methods all represent simplified alternatives using only a few animals. Efforts have also been made to develop in vitro systems; e.g., it has been suggested that acute systemic toxicity can be broken down into a number of biokinetic, cellular, and molecular elements, each of which can be identified and quantified in appropriate models. The various elements may then be used in different combinations to model large numbers of toxic events to predict hazard and classify compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bondesson I., Ekwall B., Hellberg S., Romert L., Stenberg K., Walum E. MEIC--a new international multicenter project to evaluate the relevance to human toxicity of in vitro cytotoxicity tests. Cell Biol Toxicol. 1989 Nov;5(3):331–347. doi: 10.1007/BF01795360. [DOI] [PubMed] [Google Scholar]
- Clothier R. H., Hulme L. M., Smith M., Balls M. Comparison of the in vitro cytotoxicities and acute in vivo toxicities of 59 chemicals. Mol Toxicol. 1987;1(4):571–577. [PubMed] [Google Scholar]
- Diener W., Siccha L., Mischke U., Kayser D., Schlede E. The biometric evaluation of the acute-toxic-class method (oral). Arch Toxicol. 1994;68(10):599–610. doi: 10.1007/BF03208339. [DOI] [PubMed] [Google Scholar]
- Ekwall B. Preliminary studies on the validity of in vitro measurement of drug toxicity using HeLa cells. III. Lethal action to man of 43 drugs related to the HeLa cell toxicity of the lethal drug concentrations. Toxicol Lett. 1980 Apr;5(5):319–331. doi: 10.1016/0378-4274(80)90033-8. [DOI] [PubMed] [Google Scholar]
- Garattini S. Notes on xenobiotic metabolism. Ann N Y Acad Sci. 1983;407:1–25. doi: 10.1111/j.1749-6632.1983.tb47810.x. [DOI] [PubMed] [Google Scholar]
- Garattini S. Toxic effects of chemicals: difficulties in extrapolating data from animals to man. Crit Rev Toxicol. 1985;16(1):1–29. doi: 10.3109/10408448509041323. [DOI] [PubMed] [Google Scholar]
- Lipnick R. L., Cotruvo J. A., Hill R. N., Bruce R. D., Stitzel K. A., Walker A. P., Chu I., Goddard M., Segal L., Springer J. A. Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem Toxicol. 1995 Mar;33(3):223–231. doi: 10.1016/0278-6915(94)00136-c. [DOI] [PubMed] [Google Scholar]
- Paget E. The LD50 test. Acta Pharmacol Toxicol (Copenh) 1983;52 (Suppl 2):6–19. doi: 10.1111/j.1600-0773.1983.tb02680.x. [DOI] [PubMed] [Google Scholar]
- Schlede E., Mischke U., Diener W., Kayser D. The international validation study of the acute toxic class method (oral). Arch Toxicol. 1995;69(10):659–670. doi: 10.1007/s002040050229. [DOI] [PubMed] [Google Scholar]
- Schlede E., Mischke U., Roll R., Kayser D. A national validation study of the acute-toxic-class method--an alternative to the LD50 test. Arch Toxicol. 1992;66(7):455–470. doi: 10.1007/BF01970670. [DOI] [PubMed] [Google Scholar]
- Stallard N., Whitehead A. Reducing animal numbers in the fixed-dose procedure. Hum Exp Toxicol. 1995 Apr;14(4):315–323. doi: 10.1177/096032719501400401. [DOI] [PubMed] [Google Scholar]
- Stallard N., Whitehead A. The fixed-dose procedure and the acute-toxic-class method: a mathematical comparison. Hum Exp Toxicol. 1995 Dec;14(12):974–990. doi: 10.1177/096032719501401206. [DOI] [PubMed] [Google Scholar]
- Tamborini P., Sigg H., Zbinden G. Acute toxicity testing in the nonlethal dose range: a new approach. Regul Toxicol Pharmacol. 1990 Aug;12(1):69–87. doi: 10.1016/s0273-2300(05)80048-0. [DOI] [PubMed] [Google Scholar]
- Walum E., Peterson A. On the application of cultured neuroblastoma cells in chemical toxicity screening. J Toxicol Environ Health. 1984;13(4-6):511–520. doi: 10.1080/15287398409530516. [DOI] [PubMed] [Google Scholar]
- Yam J., Reer P. J., Bruce R. D. Comparison of the up-and-down method and the fixed-dose procedure for acute oral toxicity testing. Food Chem Toxicol. 1991 Apr;29(4):259–263. doi: 10.1016/0278-6915(91)90023-z. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M. J., Clark D. G., Fielder R. J., Koundakjian P. P., Oliver G. J., Pelling D., Tomlinson N. J., Walker A. P. The international validation of a fixed-dose procedure as an alternative to the classical LD50 test. Food Chem Toxicol. 1990 Jul;28(7):469–482. doi: 10.1016/0278-6915(90)90117-6. [DOI] [PubMed] [Google Scholar]


