Abstract
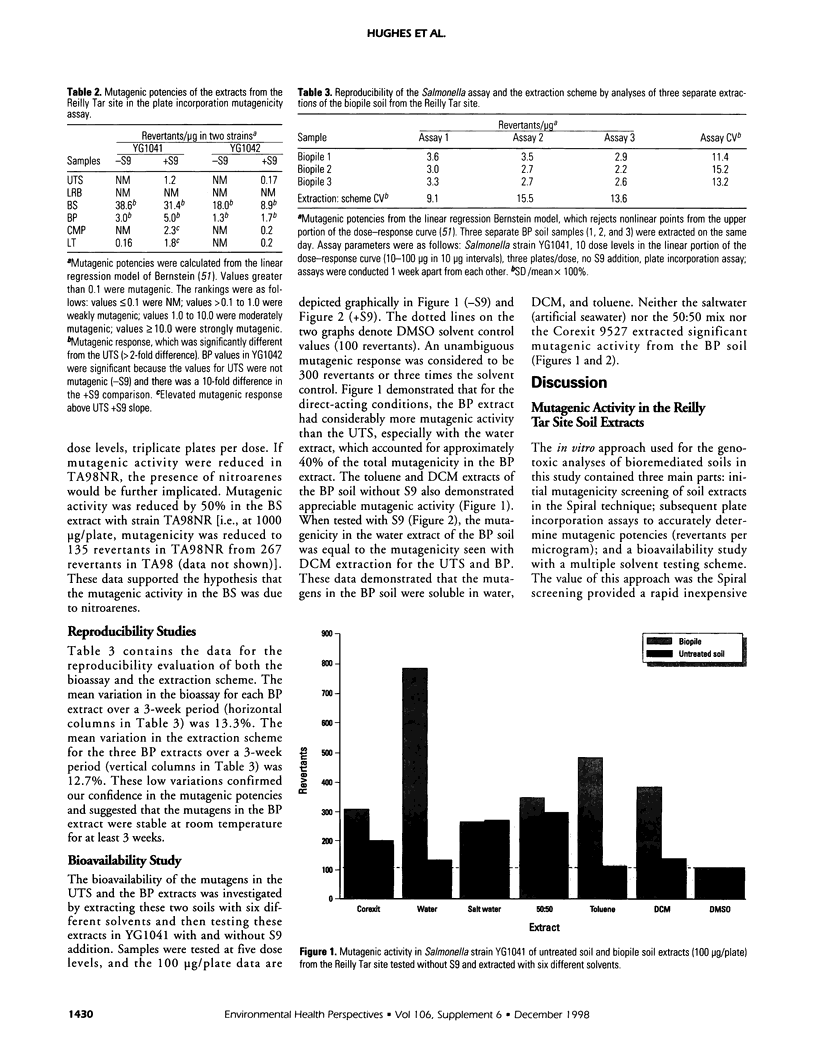
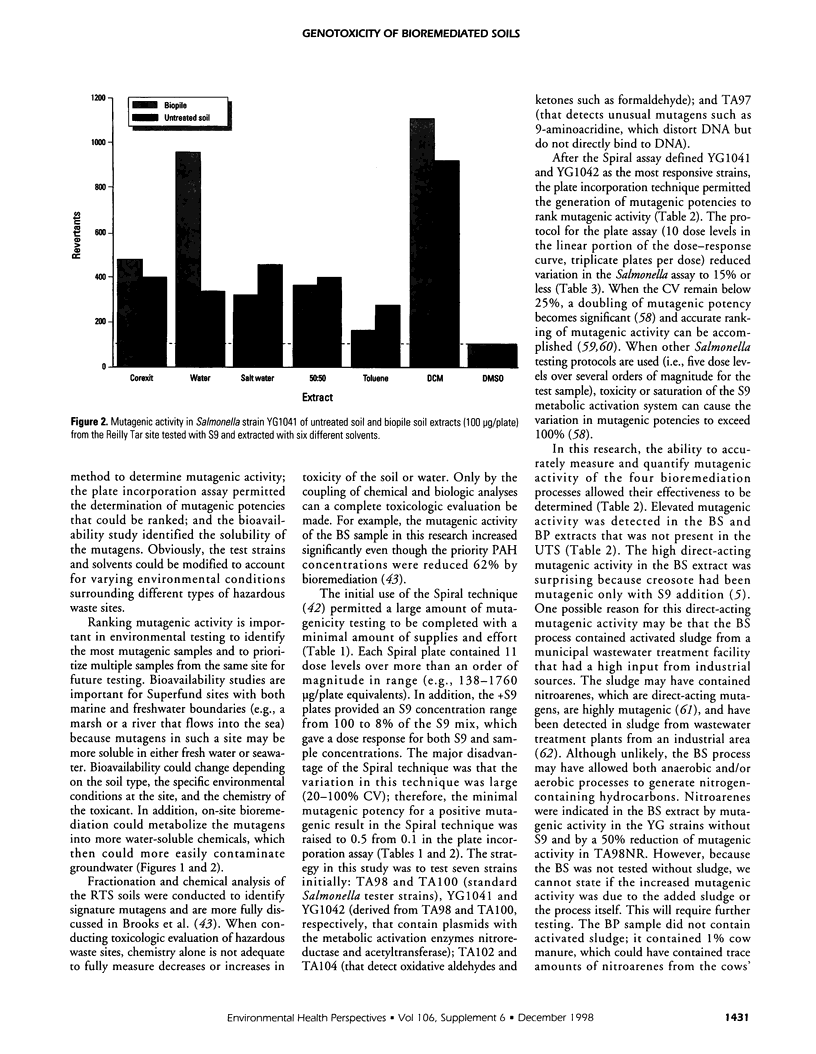
An in vitro approach was used to measure the genotoxicity of creosote-contaminated soil before and after four bioremediation processes. The soil was taken from the Reilly Tar site, a closed Superfund site in Saint Louis Park, Minnesota. The creosote soil was bioremediated in bioslurry, biopile, compost, and land treatment, which were optimized for effective treatment. Mutagenicity profiles of dichloromethane extracts of the five soils were determined in the Spiral technique of the Salmonella assay with seven tester strains. Quantitative mutagenic responses in the plate incorporation technique were then determined in the most sensitive strains, YG1041 and YG1042. Mutagenic potency (revertants per microgram extract) in YG1041 suggested that compost, land treatment, and untreated creosote soil extracts were moderately mutagenic with Arochlor-induced rat liver (S9) but were nonmutagenic without S9. However, the bioslurry extract was strongly mutagenic and the biopile extract was moderately mutagenic either with or without S9. A similar trend was obtained in strain YG1042. The strong mutagenic activity in the bioslurry extract was reduced by 50% in TA98NR, which suggested the presence of mutagenic nitrohydrocarbons. Variation in reproducibility was 15% or less for the bioassay and extraction procedures. Bioavailability of mutagens in the biopile soil was determined with six solvents; water-soluble mutagens accounted for 40% of the total mutagenic activity and they were stable at room temperature. The mutagenic activity in the bioslurry and biopsile samples was due to either the processes themselves or to the added sludge/manure amendments. The in vitro approach was effective in monitoring bioremediated soils for genotoxicity and will be useful in future laboratory and in situ studies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbee G. C., Brown K. W., Thomas J. C., Donnelly K. C., Murray H. E. Mutagenic activity (Ames test) of wood-preserving waste sludge applied to soil. Bull Environ Contam Toxicol. 1996 Jul;57(1):54–62. doi: 10.1007/s001289900155. [DOI] [PubMed] [Google Scholar]
- Barr D. P., Aust S. D. Pollutant degradation by white rot fungi. Rev Environ Contam Toxicol. 1994;138:49–72. doi: 10.1007/978-1-4612-2672-7_3. [DOI] [PubMed] [Google Scholar]
- Bernstein L., Kaldor J., McCann J., Pike M. C. An empirical approach to the statistical analysis of mutagenesis data from the Salmonella test. Mutat Res. 1982 Aug;97(4):267–281. doi: 10.1016/0165-1161(82)90026-7. [DOI] [PubMed] [Google Scholar]
- Bodzek D., Janoszka B., Dobosz C., Warzecha L., Bodzek M. Determination of polycyclic aromatic compounds and heavy metals in sludges from biological sewage treatment plants. J Chromatogr A. 1997 Jul 11;774(1-2):177–192. doi: 10.1016/s0021-9673(97)00079-4. [DOI] [PubMed] [Google Scholar]
- Bogan B. W., Lamar R. T. One-electron oxidation in the degradation of creosote polycyclic aromatic hydrocarbons by Phanerochaete chrysosporium. Appl Environ Microbiol. 1995 Jul;61(7):2631–2635. doi: 10.1128/aem.61.7.2631-2635.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura C., Johnson F. M. Healthy environments for healthy people: bioremediation today and tomorrow. Environ Health Perspect. 1997 Feb;105 (Suppl 1):5–20. doi: 10.1289/ehp.97105s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R. P., Hulshof C. T., Theuws J. L., Henderson P. T. Mutagenicity of creosote in the Salmonella/microsome assay. Mutat Res. 1983 Jan;119(1):21–25. doi: 10.1016/0165-7992(83)90033-7. [DOI] [PubMed] [Google Scholar]
- Bos R. P., Jongeneelen F. J., Theuws J. L., Henderson P. T. Detection of volatile mutagens in creosote and coal tar. Mutat Res. 1985 Jun;156(3):195–198. doi: 10.1016/0165-1218(85)90064-3. [DOI] [PubMed] [Google Scholar]
- Bos R. P., Prinsen W. J., van Rooy J. G., Jongeneelen F. J., Theuws J. L., Henderson P. T. Fluoranthene, a volatile mutagenic compound, present in creosote and coal tar. Mutat Res. 1987 Mar;187(3):119–125. doi: 10.1016/0165-1218(87)90078-4. [DOI] [PubMed] [Google Scholar]
- Bos R. P., Theuws J. L., Leijdekkers C. M., Henderson P. T. The presence of the mutagenic polycyclic aromatic hydrocarbons benzo[a]pyrene and benz[a]anthracene in creosote P1. Mutat Res. 1984 Jun;130(3):153–158. doi: 10.1016/0165-1161(84)90117-1. [DOI] [PubMed] [Google Scholar]
- Brooks L. R., Hughes T. J., Claxton L. D., Austern B., Brenner R., Kremer F. Bioassay-directed fractionation and chemical identification of mutagens in bioremediated soils. Environ Health Perspect. 1998 Dec;106 (Suppl 6):1435–1440. doi: 10.1289/ehp.98106s61435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. J., Shelton M., Grifoll M., Selifonov S. Fossil fuel biodegradation: laboratory studies. Environ Health Perspect. 1995 Jun;103 (Suppl 5):79–83. doi: 10.1289/ehp.95103s479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton L. D., Allen J., Auletta A., Mortelmans K., Nestmann E., Zeiger E. Guide for the Salmonella typhimurium/mammalian microsome tests for bacterial mutagenicity. Mutat Res. 1987 Oct;189(2):83–91. doi: 10.1016/0165-1218(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Claxton L. D., Creason J., Nader J. A., Poteat W., Orr J. D. GeneTox manager for bacterial mutagenicity assays: a personal computer and minicomputer system. Mutat Res. 1995 Mar;342(1-2):87–94. doi: 10.1016/0165-1218(95)90093-4. [DOI] [PubMed] [Google Scholar]
- Claxton L. D., Houk V. S., Monteith L. G., Myers L. E., Hughes T. J. Assessing the use of known mutagens to calibrate the Salmonella typhimurium mutagenicity assay: I. Without exogenous activation. Mutat Res. 1991 Oct;253(2):137–147. doi: 10.1016/0165-1161(91)90127-t. [DOI] [PubMed] [Google Scholar]
- Claxton L. D., Houk V. S., Warner J. R., Myers L. E., Hughes T. J. Assessing the use of known mutagens to calibrate the Salmonella typhimurium mutagenicity assay: II. With exogenous activation. Mutat Res. 1991 Oct;253(2):149–159. doi: 10.1016/0165-1161(91)90128-u. [DOI] [PubMed] [Google Scholar]
- Desai J. D., Banat I. M. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997 Mar;61(1):47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes L., Lafrance P., Villeneuve J. P., Samson R. Adding sodium dodecyl sulfate and Pseudomonas aeruginosa UG2 biosurfactants inhibits polycyclic aromatic hydrocarbon biodegradation in a weathered creosote-contaminated soil. Appl Microbiol Biotechnol. 1996 Dec;46(5-6):638–646. doi: 10.1007/s002530050874. [DOI] [PubMed] [Google Scholar]
- Fayad N. M., Edora R. L., el-Mubarak A. H., Polancos A. B., Jr Effectiveness of a bioremediation product in degrading the oil spilled in the 1991 Arabian Gulf War. Bull Environ Contam Toxicol. 1992 Dec;49(6):787–796. doi: 10.1007/BF00203149. [DOI] [PubMed] [Google Scholar]
- Geiselbrecht A. D., Herwig R. P., Deming J. W., Staley J. T. Enumeration and phylogenetic analysis of polycyclic aromatic hydrocarbon-degrading marine bacteria from Puget sound sediments. Appl Environ Microbiol. 1996 Sep;62(9):3344–3349. doi: 10.1128/aem.62.9.3344-3349.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaswami M., Feldhake D. J., Kinkle B. K., Mindell D. P., Loper J. C. Phylogenetic comparison of two polycyclic aromatic hydrocarbon-degrading mycobacteria. Appl Environ Microbiol. 1995 Sep;61(9):3221–3226. doi: 10.1128/aem.61.9.3221-3226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara Y., Watanabe M., Oda Y., Sofuni T., Nohmi T. Specificity and sensitivity of Salmonella typhimurium YG1041 and YG1042 strains possessing elevated levels of both nitroreductase and acetyltransferase activity. Mutat Res. 1993 Jun;291(3):171–180. doi: 10.1016/0165-1161(93)90157-u. [DOI] [PubMed] [Google Scholar]
- Hanson K. G., Nigam A., Kapadia M., Desai A. J. Bioremediation of crude oil contamination with Acinetobacter sp. A3. Curr Microbiol. 1997 Sep;35(3):191–193. doi: 10.1007/s002849900237. [DOI] [PubMed] [Google Scholar]
- Houk V. S., Early G., Claxton L. D. Use of the spiral Salmonella assay to detect the mutagenicity of complex environmental mixtures. Environ Mol Mutagen. 1991;17(2):112–121. doi: 10.1002/em.2850170208. [DOI] [PubMed] [Google Scholar]
- Hughes J. B., Beckles D. M., Chandra S. D., Ward C. H. Utilization of bioremediation processes for the treatment of PAH-contaminated sediments. J Ind Microbiol Biotechnol. 1997 Feb-Mar;18(2-3):152–160. doi: 10.1038/sj.jim.2900308. [DOI] [PubMed] [Google Scholar]
- Hughes T. J., Lewtas J., Claxton L. D. Development of a standard reference material for diesel mutagenicity in the Salmonella plate incorporation assay. Mutat Res. 1997 Jul 14;391(3):243–258. doi: 10.1016/s1383-5718(97)00075-2. [DOI] [PubMed] [Google Scholar]
- Knapp R. B., Faison B. D. A bioengineering system for in situ bioremediation of contaminated groundwater. J Ind Microbiol Biotechnol. 1997 Feb-Mar;18(2-3):189–197. doi: 10.1038/sj.jim.2900321. [DOI] [PubMed] [Google Scholar]
- Lebkowska M., Karwowska E., Miaśkiewicz E. Isolation and identification of bacteria from petroleum derivatives contaminated soil. Acta Microbiol Pol. 1995;44(3-4):297–303. [PubMed] [Google Scholar]
- Malins D. C., Krahn M. M., Myers M. S., Rhodes L. D., Brown D. W., Krone C. A., McCain B. B., Chan S. L. Toxic chemicals in sediments and biota from a creosote-polluted harbor: relationships with hepatic neoplasms and other hepatic lesions in English sole (Parophrys vetulus). Carcinogenesis. 1985 Oct;6(10):1463–1469. doi: 10.1093/carcin/6.10.1463. [DOI] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Middaugh D. P., Mueller J. G., Thomas R. L., Lantz S. E., Hemmer M. H., Brooks G. T., Chapman P. J. Detoxification of pentachlorophenol and creosote contaminated groundwater by physical extraction: chemical and biological assessment. Arch Environ Contam Toxicol. 1991 Aug;21(2):233–244. doi: 10.1007/BF01055342. [DOI] [PubMed] [Google Scholar]
- Mueller J. G., Devereux R., Santavy D. L., Lantz S. E., Willis S. G., Pritchard P. H. Phylogenetic and physiological comparisons of PAH-degrading bacteria from geographically diverse soils. Antonie Van Leeuwenhoek. 1997 May;71(4):329–343. doi: 10.1023/a:1000277008064. [DOI] [PubMed] [Google Scholar]
- Mueller J. G., Middaugh D. P., Lantz S. E., Chapman P. J. Biodegradation of creosote and pentachlorophenol in contaminated groundwater: chemical and biological assessment. Appl Environ Microbiol. 1991 May;57(5):1277–1285. doi: 10.1128/aem.57.5.1277-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L. E., Adams N. H., Hughes T. J., Williams L. R., Claxton L. D. An interlaboratory study of an EPA/Ames/Salmonella test protocol. Mutat Res. 1987 Jun;182(3):121–133. doi: 10.1016/0165-1161(87)90066-5. [DOI] [PubMed] [Google Scholar]
- Nylund L., Heikkilä P., Hämeilä M., Pyy L., Linnainmaa K., Sorsa M. Genotoxic effects and chemical compositions of four creosotes. Mutat Res. 1992 Feb;265(2):223–236. doi: 10.1016/0027-5107(92)90051-3. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., Mermelstein R. Mutagenicity and genotoxicity of nitroarenes. All nitro-containing chemicals were not created equal. Mutat Res. 1983 Apr;114(3):217–267. doi: 10.1016/0165-1110(83)90034-9. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., Poirier L. A. Evaluation of the mutagenicity and DNA-modifying activity of carcinogens and noncarcinogens in microbial systems. J Natl Cancer Inst. 1979 Apr;62(4):873–892. [PubMed] [Google Scholar]
- Ríha V., Nymburská K., Tichy R., Tríska J. Microbiological, chemical and toxicological characterization of contaminated sites in Czechoslovakia. Sci Total Environ. 1993;Suppl Pt 1:185–193. doi: 10.1016/s0048-9697(05)80018-x. [DOI] [PubMed] [Google Scholar]
- Sayler G. S., Layton A., Lajoie C., Bowman J., Tschantz M., Fleming J. T. Molecular site assessment and process monitoring in bioremediation and natural attenuation. off. Appl Biochem Biotechnol. 1995 Jul-Sep;54(1-3):277–290. doi: 10.1007/BF02787926. [DOI] [PubMed] [Google Scholar]
- Schoket B., Hewer A., Grover P. L., Phillips D. H. Covalent binding of components of coal-tar, creosote and bitumen to the DNA of the skin and lungs of mice following topical application. Carcinogenesis. 1988 Jul;9(7):1253–1258. doi: 10.1093/carcin/9.7.1253. [DOI] [PubMed] [Google Scholar]
- Schoor W. P., Williams D. E., Takahashi N. The induction of cytochrome P-450-IA1 in juvenile fish by creosote-contaminated sediment. Arch Environ Contam Toxicol. 1991 May;20(4):497–504. doi: 10.1007/BF01065838. [DOI] [PubMed] [Google Scholar]
- Shannon M. J., Unterman R. Evaluating bioremediation: distinguishing fact from fiction. Annu Rev Microbiol. 1993;47:715–738. doi: 10.1146/annurev.mi.47.100193.003435. [DOI] [PubMed] [Google Scholar]
- Stead A. G., Hasselblad V., Creason J. P., Claxton L. Modeling the Ames test. Mutat Res. 1981 Feb;85(1):13–27. doi: 10.1016/0165-1161(81)90282-x. [DOI] [PubMed] [Google Scholar]
- Stinson M. K., Skovronek H. S., Ellis W. D. EPA SITE demonstration of the BioTrol soil washing process. J Air Waste Manage Assoc. 1992 Jan;42(1):96–103. doi: 10.1080/10473289.1992.10466973. [DOI] [PubMed] [Google Scholar]
- Stringfellow W. T., Aitken M. D. Competitive metabolism of naphthalene, methylnaphthalenes, and fluorene by phenanthrene-degrading pseudomonads. Appl Environ Microbiol. 1995 Jan;61(1):357–362. doi: 10.1128/aem.61.1.357-362.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swannell R. P., Lee K., McDonagh M. Field evaluations of marine oil spill bioremediation. Microbiol Rev. 1996 Jun;60(2):342–365. doi: 10.1128/mr.60.2.342-365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T. M. Bioaugmentation as a soil bioremediation approach. Curr Opin Biotechnol. 1996 Jun;7(3):311–316. doi: 10.1016/s0958-1669(96)80036-x. [DOI] [PubMed] [Google Scholar]
- Vogelbein W. K., Fournie J. W., Van Veld P. A., Huggett R. J. Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Res. 1990 Sep 15;50(18):5978–5986. [PubMed] [Google Scholar]
- Zhang Y., Miller R. M. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl Environ Microbiol. 1992 Oct;58(10):3276–3282. doi: 10.1128/aem.58.10.3276-3282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]