Abstract
Estrogens play an important role in the male reproductive tract, and this is especially so for the efferent ductules, where α-estrogen receptors (ERα) have been localized. Mice deficient in ERα (αERKO mice) are infertile, and the effect appears to be due in part to retention of water at the level of the efferent ductules. In the present study, we examined the consequences of ERα deletion on the distribution of certain aquaporins (AQPs), water protein channels, in the efferent ductules and on sperm numbers and motility. In addition, the effects of feeding mice a regular lab chow diet, which contains phytoestrogens, known to affect male reproductive tract functions, and a casein diet, which lacks phytoestrogens, were also assessed. Light microscope immunolocalizations of AQP-1 and AQP-9 revealed dramatic reduction and patchier staining in αERKO mice with distal areas of the efferent ductules being more affected than proximal areas. No other changes in immunolocalizations were noted as a consequence of diet. Computer-assisted sperm analyses demonstrated a 62% reduction in cauda epididymal sperm/ml in αERKO mice fed lab chow, whereas 87% fewer sperm/ml were observed in αERKO mice fed casein, suggesting an enhanced role for sperm production and concentration in a diet containing phytoestrogens. All sperm motility parameters were altered to some degree in αERKO mice fed lab chow. Alterations in sperm motility parameters were also detected, but were less dramatic in αERKO mice fed casein. These data suggest that the decrease in AQP expression in the efferent ductules of αERKO mice contributes in part to water retention in this tissue, eventually leading to backflow of water into the testis, with subsequent decreases in sperm concentration and motility. The data also suggest that phytoestrogens, which are present in regular lab chow, can influence the male reproductive tract with and without the presence of ERα, promoting efferent ductule and epididymal functions when ERα is expressed, but inhibiting these same functions when ERα is missing. Taken together the data underscore the importance of estrogens and ERα in maintaining sperm maturation and preventing male infertility.
Keywords: estrogen receptor, water transport, aquaporin, CASA, epididymis, efferent ductules
INTRODUCTION
Many mammalian tissues require rapid transport of water into and out of constituent cells. As a consequence, protein water channels, referred to as aquaporins (AQPs), have evolved to serve this purpose (Preston and Agre, 1991; Wintour, 1997; Verkman and Mitra, 2000; Schrier and Cadnapaphornchai, 2003). AQPs are homologous to the major intrinsic protein superfamily of integral membrane proteins and are assembled in plasma membranes as homotetramers. Each 30 kD monomer consists of six membrane-spanning α-helical domains and has its own distinct pore to allow bidirectional water transport (King and Agre, 1996; Wintour, 1997; Brown et al., 1998; Verkman and Mitra, 2000). Currently, 11 AQPs (0–10) have been identified in various tissues and cells. They have been divided into two groups, based on their permeability properties: the water-selective AQPs and the aqua-glyceroporins, which, in addition to water, are also highly permeable to urea, glycerol, and small uncharged molecules (Preston and Agre, 1991; Deen and van Os, 1998; Borgnia et al., 1999; Hatakeyama et al., 2001; Sansom and Law, 2001).
AQPs are expressed throughout the mammalian body and have been studied extensively (Verkman and Mitra, 2000; Nielsen et al., 2002; Schrier and Cadnapaphornchai, 2003). Many are tissue-, region-, and even cell-specific, but more than one AQP can be expressed in the same cell (King and Agre, 1996; Echevarria and Ilundain, 1998; Verkman and Mitra, 2000; Nielsen et al., 2002). While hormones regulate some AQPs, others are constitutively expressed (Verkman and Mitra, 2000; Nielsen et al., 2002; Schrier and Cadnapaphornchai, 2003). Alterations in the expression of AQPs have been shown to cause a variety of pathological states (King et al., 2000; Verkman and Mitra, 2000; Nielsen et al., 2002; Schrier and Cadnapaphornchai, 2003).
Water plays an important role in the male reproductive tract. In seminiferous tubules of the testis, water is secreted into the lumen by Sertoli cells in order to create a fluid environment essential for maintaining spermatogenesis and transporting sperm out of the testis. In the efferent ductules, linking the rete testis to the epididymis, up to 90% of testicular luminal fluid is reabsorbed. This process appears to concentrate sperm as an initial step to promoting fertility and motility as sperm pass along the epididymis (Hess et al., 2002). In the epididymis, water serves as a vehicle for sperm passing through this convoluted duct (Setchell et al., 1969; Setchell and Brooks, 1988).
The distributions of several members of the AQP family have been characterized in the male reproductive tract (Brown et al., 1998; Andonian and Hermo, 1999; Nihei et al., 2001; Pastor-Soler et al., 2001; Badran and Hermo, 2002; Hermo et al., 2004). In the testis, AQP-9 is localized to Leydig cells of the interstitial space and while AQP-7 is expressed in germ cells, AQP-8 is expressed in Sertoli cells of the seminiferous epithelium (Ishibashi et al., 1997; Nihei et al., 2001; Badran and Hermo, 2002). In the adult rat, AQP-1, -9 and -10 are localized to epithelial cells of efferent ductules, and while AQP-9 is expressed in principal cells of the epididymis in a region-specific manner, AQP-3 is expressed in basal cells (Fisher et al., 1998; Elkjaer et al., 2000; Pastor-Soler et al., 2001; Badran and Hermo, 2002; Hermo et al., 2004). AQP-1 is localized to endothelial cells of vascular channels throughout the efferent ductules and epididymis (Badran and Hermo, 2002).
Some studies on the hormonal regulation of AQPs in the male reproductive tract have been reported. In efferent ductules, expression of AQP-1 over the microvilli of nonciliated cells is not influenced by testosterone, whereas expression of AQP-9 in principal cells in some epididymal regions is dependent on both testosterone and other unidentified luminal testicular factors (Badran and Hermo, 2002) and in efferent ductule nonciliated cells are regulated by estrogen (Oliveira et al., 2005). Recent studies have suggested an important role for estrogen in regulating water transport in the efferent ductules (Hess et al., 2001b, 2002; Hess, 2003).
In males, estrogen is present in low concentrations in blood, but it can be extraordinarily high in semen and rete testis fluids (Ganjam and Amann, 1976; Free and Jaffe, 1979). Estrogen in the rete testis fluid is derived mainly from the conversion of testosterone to estradiol by P450 aromatase present in germ cells and cytoplasmic droplets of sperm traversing the lumen of the efferent ductules, as well as in Leydig and Sertoli cells of the testis (Payne et al., 1976; Carreau et al., 1999; Hess et al., 2001a). It is well-known that male reproductive tissues express estrogen receptors (ERs) (Schleicher et al., 1984; West and Brenner, 1990; Cooke et al., 1991; Greco et al., 1993), and in particular, the efferent ductules, where ERα is abundant and where much of the fluid coming from the testis is reabsorbed (Fisher et al., 1997; Hess, 2000; Zhou et al., 2002). Use of a mouse model that lacks ERα expression, known as the αERKO mouse, has revealed that water is retained at the level of the efferent ductules in these animals resulting in “back flooding” of seminiferous tubules. This dilutes sperm counts in the epididymis and contributes to infertile seen in these animals (Eddy et al., 1996; Hess, 2000; Hess et al., 2000, 2001b, 2002; Oliveira et al., 2001, 2002; Zhou et al., 2001; Cho et al., 2003). Although it has been shown that AQP-1 protein, but not mRNA, expression is partially regulated by ERα, AQP-1 null mice are fertile (Zhou et al., 2001) suggestive that more than one factor contributes to water retention observed in the αERKO mouse and dependent on morphology of the microvillar border (Oliveira et al., 2001, 2002). The specific effects of absence of ERα on AQP-1, as well as on AQP-9 expression along the entire length of the efferent ductules that may contribute in part to the infertility of αERKO mice have not been fully documented. Furthermore, while sperm counts and motility of sperm in αERKO and antiestrogen treated mice are known to be reduced (Eddy et al., 1996; Cho et al., 2003), detailed studies on motility behavior of sperm in the αERKO mice compared to wild type mice are presently lacking.
In assessing the role(s) that estrogens play in water dynamics of the efferent ducts, the presence of estrogen-mimicking substances in the food is a factor that must also be taken into consideration. A typical rodent lab chow diet consists of mixtures of alfalfa and soybeans (Gaido et al., 1997). These plants are known to contain significant levels of phytoestrogens, which exhibit weak estrogenic activity in vitro and in vivo (Makela et al., 1995; Whitten and Naftolin, 1998). Soybean meal, for example, has moderate estrogenic content at 0.039–0.125 μg/g estradiol, due to genistein (Santell et al., 1997; Nishihara et al., 2000; Kato et al., 2004). Hence, the goals of this study were not only to characterize AQP expression and sperm concentrations and motility behavior in wild type and αERKO mice, but also to determine what happens in mice fed lab chow versus an alternative diet such as purified casein, which is phytoestrogen-free.
MATERIALS AND METHODS
Animals
A total of 20 wild type and 20 αERKO (Lubahn et al., 1993) mice (C57bl/6j) at 3–4 months of age were utilized. Both wild type and αERKO mice were randomly assigned to subgroups based on two diets (diet type). Regular lab chow diet (Nestlé Purina, St. Louis) was administered after weaning to one group of ten wild type and ten αERKO mice, while a diet free of soybean and rich in casein, but otherwise containing all the ingredients of lab chow diet (AIN 93G), was administered to a duplicate group of mice. All mice were housed at controlled temperature, had free access to food and water and were maintained on 12 hr light–dark cycles. Experiments involving these mice were approved by the Animal Care Committees of the different universities involved in this project.
Immunocytochemistry
Mice (five per treatment type and diet type) were anesthetized with sodium pentobarbital and the efferent ductules were removed and fixed by immersion in Bouin's solution. After several minutes, the efferent ductules were removed and prepared such that subsequent sections would include the entire length extending from the rete testis to their initial segment. The tissues were left in fixative for 72 hr, after which they were dehydrated and embedded in paraffin.
The Santa Cruz protocol was used for all immunocytochemical procedures (ImmunoCruz Staining System, Santa Cruz Biotechnology, Santa Cruz, CA). Rabbit anti-AQP-1 (Cat# AQP11-A) and anti-AQP-9 (Cat# AQP91-A) antibodies were purchased from Alpha Diagnostics International (San Antonio, TX). Sections at 5 μm thickness were deparaffinized in Histoclear (Diamed Lab Supplies, Inc., Mississauga, ON, Canada) and hydrated in a series of graded ethanol solutions. During hydration, residual picric acid was neutralized in 70% ethanol containing 1% lithium carbonate, and endogenous peroxidase activity was abolished in 70% ethanol containing 1% (vol/vol) H2O2. Once hydrated, the tissue sections were washed in distilled water containing glycine to block free aldehyde groups. Antigen retrieval was performed using a sodium citrate buffer-microwave method (Santa Cruz Biotechnology, Santa Cruz, CA).
Before staining, the tissues were blocked for 1 hr with a solution containing 1% goat serum albumin. The tissues were incubated with primary antibody at 1:100 dilution in blocking buffer overnight at 4°C. Each slide was then incubated with biotinylated secondary antibody followed by Streptavidin-horseradish peroxidase for amplification. The sections were finally incubated with 0.05% diaminobenzidine tetrahydrochloride (Bio Fx Laboratories, Owing's-Mills, MD), rinsed with tap water for 5 min, and counterstained with 0.1% methylene blue. Negative controls were prepared by incubating additional sections in all solutions except primary antibody.
Sperm Motility Analyses
Mice (five per treatment type and diet type) were weighed and lightly anesthetized with isofurane and killed by cervical dislocation. The left cauda epididymides were removed and frozen (−20°) for subsequent sperm count analysis. The right cauda epididymides were clamped proximally and distally prior to excision, rinsed in prewarmed M199 medium (GIBCO) and placed in a Petri dish containing M199 + 0.5% BSA, preheated to 37°C. Each cauda was poked with the tip of a scalpel blade to permit the release of the sperm into the media. The sperm-media suspension was incubated at 37°C for 5 min, after which 100 μl aliquots were diluted with medium and transferred into each of two compartments on a glass cannula for computer assisted sperm analysis (CASA) using the integrated visual optical system (IVOS) motility analyzer (Hamilton-Thorne Research, Inc., Beverly, MA). The operational settings of the IVOS were the standard mouse parameters as recommended by the manufacturer (IVOS, Hamilton-Thorne). For each sample, 3–5 slides, with 5–10 scans per slide were analyzed. Fourteen of 15 measurement parameters (variables) available through software were analyzed (see footnote 1 in Table 1).
TABLE 1.
Sperm Counts and Motility Changes Comparing aERKO to Wild Type Mice
| Parametera | Wild type mice (num obs)b mean ± SD | αERKO mice (num obs)b mean ± SD | Changec | P-valuesd | Powere | |
|---|---|---|---|---|---|---|
| Diet = lab chow | (80) | (123) | ||||
| Sperm counts | 35.0±15.0 | 13.4±8.9 | −62% | 0.0000 | 1.0000 | |
| Raw values | (300) | (252) | ||||
| VAP | 125.5±26.9 | 68.1±24.5 | −46% | 0.0000 | 1.0000 | |
| VSL | 98.8±23.7 | 48.1±19.7 | −51% | 0.0000 | 1.0000 | |
| VCL | 196.1±38.1 | 134.5±40.6 | −31% | 0.0000 | 1.0000 | |
| ALH | 6.1±1.0 | 4.8±2.3 | −21% | 0.0000 | 1.0000 | |
| BCF | 1.5±1.6 | 4.1±4.1 | 173% | 0.0000 | 1.0000 | |
| Motile | 44.0±15.3 | 22.4±19.2 | −49% | 0.0000 | 1.0000 | |
| Prog(ressive) | 17.4±6.9 | 5.2±10.5 | −70% | 0.0000 | 1.0000 | |
| Rapid | 30.5±10.3 | 10.5±9.9 | −66% | 0.0000 | 1.0000 | |
| Medium | 13.5±8.3 | 11.9±10.6 | −12% | 0.0481 | 0.5050 | |
| Slow | 1.7±1.8 | 2.7±3.4 | 59% | 0.0000 | 0.9902 | |
| Static | 11.1±9.3 | 68.8±76.0 | 520% | 0.0000 | 1.0000 | |
| Ratios | ||||||
| STR | 76.3±4.5 | 69.1±9.2 | −9% | 0.0582 | NS | 0.4810 |
| LIN | 50.6±5.6 | 36.7±8.2 | −27% | 0.0011 | 0.9086 | |
| Elong(ation) | 49.5±4.1 | 50.4±6.8 | 2% | 0.8332 | NS | 0.0551 |
| Percentages | ||||||
| %Motile | 78.9±10.1 | 35.1±23.3 | −56% | 0.0000 | 1.0000 | |
| %Prog(ressive) | 31.5±9.5 | 8.1±8.0 | −74% | 0.0000 | 1.0000 | |
| %Rapid | 55.3±11.6 | 15.9±13.1 | −71% | 0.0000 | 1.0000 | |
| %Medium | 23.7±9.9 | 19.2±15.2 | −19% | 0.2015 | NS | 0.2494 |
| %Slow | 2.9±2.8 | 4.0±4.6 | 38% | 0.4779 | NS | 0.1128 |
| %Static | 18.2±10.2 | 60.9±25.1 | 235% | 0.0000 | 1.0000 | |
| Diet=casein | (109) | (37) | ||||
| Sperm counts | 25.5±8.8 | 3.4±2.4 | −87% | 0.0000 | 1.0000 | |
| Raw values | (193) | (107) | ||||
| VAP | 118.5±22.1 | 91.2±28.5 | −23% | 0.0000 | 1.0000 | |
| VSL | 92.8±19.6 | 69.5±24.8 | −25% | 0.0000 | 1.0000 | |
| VCL | 188.1±32.2 | 154.0±41.7 | −18% | 0.0000 | 1.0000 | |
| ALH | 5.8±1.2 | 4.4±1.6 | −24% | 0.0000 | 1.0000 | |
| BCF | 1.7±2.0 | 2.6±3.5 | 53% | 0.0038 | 0.8197 | |
| Motile | 37.7±16.4 | 16.7±9.9 | −56% | 0.0000 | 1.0000 | |
| Prog(ressive) | 15.5±8.4 | 6.1±4.4 | −61% | 0.0000 | 1.0000 | |
| Rapid | 26.7±12.7 | 10.2±6.5 | −62% | 0.0000 | 1.0000 | |
| Medium | 11.0±5.9 | 6.4±4.7 | −42% | 0.0000 | 1.0000 | |
| Slow | 1.4±1.5 | 1.1±1.5 | −21% | 0.0291 | 0.5827 | |
| Static | 10.1±8.7 | 8.6±8.8 | −15% | 0.1703 | NS | 0.2773 |
| Ratios | ||||||
| STR | 76.2±4.9 | 74.2±7.6 | −3% | 0.7007 | NS | 0.0694 |
| LIN | 49.7±5.8 | 46.6±8.7 | −6% | 0.6072 | NS | 0.0816 |
| Elong(ation) | 46.0±4.6 | 45.3±6.7 | −2% | 0.9073 | NS | 0.0515 |
| Percentages | ||||||
| %Motile | 77.9±11.2 | 65.1±19.6 | −16% | 0.0169 | 0.6659 | |
| %Prog(ressive) | 31.8±9.9 | 23.4±12.6 | −26% | 0.1249 | NS | 0.3342 |
| %Rapid | 54.9±11.4 | 40.3±17.0 | −27% | 0.0160 | 0.6769 | |
| %Medium | 23.1±8.6 | 24.7±13.2 | 7% | 0.7550 | NS | 0.0625 |
| %Slow | 3.2±3.2 | 4.4±6.1 | 38% | 0.5948 | NS | 0.0918 |
| %Static | 18.9±11.3 | 30.6±20.6 | 62% | 0.0219 | 0.6240 |
Explanation of parameters: Sperm counts (millions/ml); VAP, smoothed path velocity (μm/sec); VCL, track velocity (μm/sec); VSL, straight line velocity (μm/sec); ALH, amplitude of lateral head displacement (μm); BCF, beat cross frequency (hertz): Number (in millions/ml) or percent of—Motile, Progressively Motile (Prog), Rapid, Medium, Slow, and Static cells; STR, straightness (ratio of VSL/VAP); LIN, linearity (ratio of VSL/VCL); Elongation, head shape (ratio of minor to major axis of sperm head).
Total number of observations (measurements) made from a pool of five mice per group.
For αERKO mice compared to wild type mice.
P-values < 0.05 are considered significantly different (NS, not significant). A Fisher's exact test was used to compare differences between means for “Ratios” and “Percentages.”
This is the power associated with rejecting the null hypothesis the two means are equal. The Z-test for comparing two proportions was used in power calculations for variables listed under “Ratios” and “Percentages.”
Correlation and statistical analyses and power tests of motility data were done using Version 7.0 of the Statistica Data Miner for Windows (Statsoft, Inc., Tulsa, OK). Initial analyses indicated that there were some outliers present in the dataset and these were removed using the Grubb's test. Raw data for some parameters also did not follow normal distributions and these were obtained by doing log10 transformations on “regular” (continuous) variables (e.g., VAP) or arcsine of the square root transformations for ratio variables (e.g., STR) as required. In subsequent Univariate Factorial ANOVA test and Post-hoc unequal N HSD t-tests for continuous variables and Fisher's exact tests for ratio variables, P-values < 0.05 were considered significant.
Sperm Counts
The frozen left cauda epididymides from each animal used for sperm motility were thawed on ice and homogenized in a 50 ml conical tube containing 20 ml of distilled water. Aliquots (100 μl) of the resulting homogenate were diluted with 100 μl of distilled water in 1.5 ml microcentrifuge tubes coated with “IDENT fluorescent dye” (Hamilton-Thorne Biosciences) and incubated at room temperature for 2 min. The solution was mixed and a 5 μl aliquot was placed on a 20 μm sperm analysis chamber (2X Cel; Hamilton-Thorne Biosciences) and quantified with the IVOS semen analyzer under ultraviolet light. Data for sperm counts were analyzed as concentration (106/ml). As with motility data, sperm counts in α ERKO and wild type mice were not normally distributed and log10 transformations of raw values were done prior to carrying out t-tests assuming unequal variances; P-values < 0.05 were considered significant.
RESULTS
Immunocytochemical Localization of AQP-1 and -9 in the Efferent Ductules of Adult Wild Type and αERKO Mice
In the efferent ductules of wild type mice immunostained for anti-AQP-1 antibody, an intense immunoperoxidase reaction was noted over the microvilli of all nonciliated cells, as well as the cilia of the ciliated cells (Fig. 1A). The basolateral plasma membranes between adjacent epithelial cells were also intensely reactive (Fig. 1A). In contrast, in αERKO mice, there was a noticeable absence of reaction over the microvilli and cilia of some nonciliated and ciliated cells, respectively (Fig. 1B). In addition, staining of the basolateral plasma membranes was markedly reduced compared to localizations obtained in wild type mice (Fig. 1B). The finding of alternating strips of reactive versus unreactive epithelial cells in αERKO mice was more prominent in distal than proximal regions of the efferent ductules (not shown).
Fig. 1.

A–D: Immunolocalization of AQP-1 (A, B) and AQP-9 (C, D) in the efferent ductules of wild type (A, C) and αERKO (B, D) mice. In (A), AQP-1 expression is uniformly distributed over microvilli (thick arrows) and cilia (arrowheads) of nonciliated and ciliated cells, respectively. Basolateral staining (thin arrows) is also evident over the epithelium. In (B), there is absence of reaction over microvilli and cilia of many, but not all nonciliated and ciliated cells (curved arrows), and basolateral staining is dramatically reduced. In (C), AQP-9 expression is uniformly distributed over the microvilli (thick arrows) of nonciliated cells, but no staining of ciliated cells (arrowheads). In (D), there is absence of reaction over the microvilli of some nonciliated cells (curved arrows). Note the lumen (Lu) of αERKO mice (B, D) is enlarged compared to wild type mice (A, C). IT interbular space. 420X.
With the anti-AQP-9 antibody, an intense reaction was observed over the microvilli of nonciliated cells of the efferent ductules in wild type mice (Fig. 1C). Ciliated cells were unreactive, as were the basolateral plasma membranes between adjacent epithelial cells (Fig. 1C). In the αERKO mice, microvillar staining was maintained over some nonciliated cells, whereas others were unreactive, giving the epithelium a patchy appearance of strips of reactivity versus unreactivity (Fig. 1D). Distal efferent ductules were more affected than the proximal ductules (not shown).
While differences in staining patterns and intensities were noted between wild type and αERKO mice, no major differences in the staining pattern of AQP-1 and -9 were evident by LM immunocytochemistry relative to the diet these animals consumed. Control sections in which the primary antibody was eliminated demonstrated an absence of reaction over the epithelium, luminal contents or intertubular spaces (not shown).
Sperm Counts and Motility Analyses: Lab Chow Diet
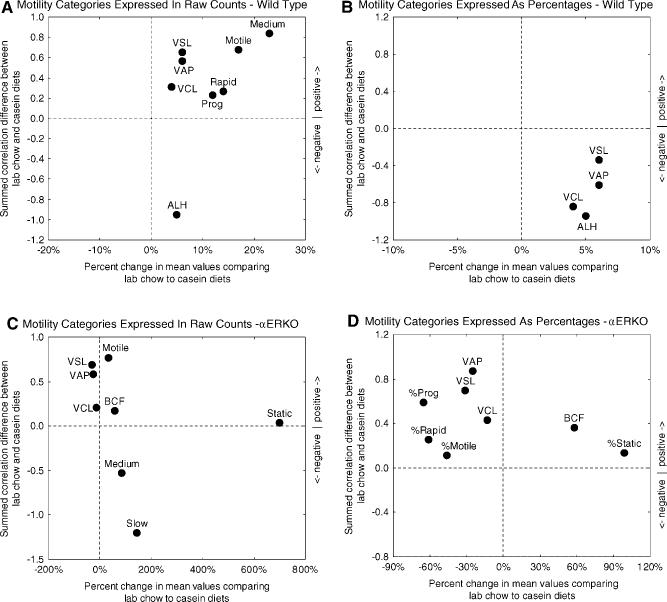
Sperm counts from the cauda epididymidis were 62% lower in αERKO mice compared to wild type mice fed the lab chow diet (Table 1, lab chow, top). CASA measurements indicated that both the raw numbers and relative percentages of sperm subclassified as Motile, Progressive, Rapid, And Medium were noticeably lower, whereas sperm subclassified as Slow and especially Static were much higher, in the αERKO mice (Table 1, lab chow, top; raw counts and percentages; Fig. 2). The data further indicated that the movement velocities of sperm (VAP, VSL, and VCL), the linearity of their motion (LIN; ratio of VSL/VCL), and the amplitudes of their lateral head displacements (ALH) were all greatly reduced in the αERKO mice (Table 1, lab chow, top). The beat cross frequency (BCF) of sperm in αERKO mice, in contrast, was much greater than in controls (Table 1, lab chow, top). Both the head elongation ratios of sperm (Elong) and straightness of sperm movement paths (STR; ratio of VSL/VAP) showed no significant differences in mean values between αERKO mice and controls (Table 1, lab chow, top). There was a trend, however, for increasingly more negative correlations of the parameter Elong to occur in αERKO mice compared to wild type mice fed the lab chow diet (Fig. 2).
Fig. 2.

Scatter plots summarizing changes in the motility behavior of sperm from αERKO mice compared to wild type controls for animals fed lab chow. In Panels A and B, differences in means determined for each of the 14 motility parameters analyzed by CASA are plotted as percentages along the abscissa (from column 4 of lab chow group in Table 1; MeanαERKO − Meanwild type/Meanwild type × 100%), and differences between the sums of correlation coefficients computed for each parameter are plotted along the ordinate (Σ Pearson r for ParameterA across ParametersA-N in αERKO mice—Σ Pearson r for ParameterA across ParametersA-N in wild type mice for lab chow). Panel A shows results for correlation coefficients computed from raw sperm counts (Table 1, raw values, lab chow) whereas Panel B shows results for correlation coefficients computed from motility data expressed as percentages of total sperm cell counts (Table 1, percentages, lab chow). If there were no differences in the motility behavior of sperm from αERKO mice and wild type mice then all points should plot near the “0” x-axis and “0” y-axis position (which they do not). Panel A: motility analyses based on raw counts show a single cluster of nine mildly altered parameters and five additional parameters residing at more outlying positions representing (1) slight decrease (Medium) or increase (Slow) in mean value and correlations much more strongly positive overall in αERKO mice, (2) no change of mean (Elong) and correlations more highly negative overall in αERKO mice, (3) twofold increase in mean value (BCF) and correlations slightly more positive overall in αERKO mice, and (4) fivefold increase in mean value (static) and correlations slightly more negative overall in αERKO mice. Panel B: motility parameters computed as percentages when plotted show clustering and outlier distribution very similar to Panel A. This indicates that changes in motility behavior in mice fed lab chow are uniform and affect sperm equally in all categories. Taken together these graphs provide a visual “fingerprint” of changes in sperm numbers and behavior that characterize the αERKO condition in mice fed lab chow that contains phytoestrogens. [See color version online at www.interscience.wiley.com.]
Sperm Counts and Motility Analyses: Casein Diet
Similar trends for reduced motility values in αERKO mice compared to wild type mice were observed for sperm sampled from animals fed the casein diet (Table 1, casein, bottom). As well, sperm showed increases in BCF and in the relative number of sperm that moved slowly or were static in the αERKO mice (Table 1, casein, bottom; BCF, %Slow, %Static). Features of sperm behavior that were noticeably distinct included the findings that: (1) the degree of changes between αERKO and control mice for the other motility parameters were generally less dramatic for animals fed casein as compared to those fed lab chow (Table 1, other parameters, top group versus bottom group; Fig. 3), and (2) raw sperm counts in the Medium, Slow, and Static categories and their expressions as percentages did not show exactly the same trends or the same proportional amount of change between αERKO and control mice fed casein versus the lab chow diets (Table 1, Medium–%Medium, Slow–%Slow, Static–%Static, top group vs. bottom group; compare Figs. 2 and 3). In addition, sperm counts were much lower between αERKO mice and controls in animals fed the casein diet compared to those fed lab chow diet (Table 1, sperm counts, top group vs. bottom group).
Fig. 3.
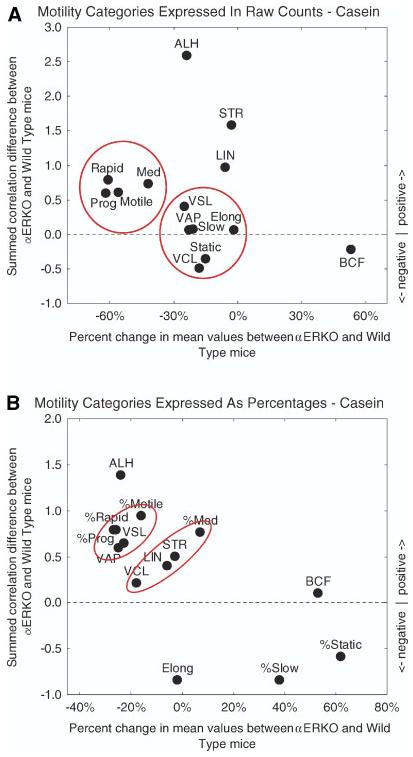
Scatter plots summarizing changes in the motility behavior of sperm from αERKO mice compared to wild type controls for animals fed casein. In Panels A and B, differences in means determined for each of the 14 motility parameters analyzed by CASA are plotted as percentages along the abscissa (from column 4 for casein group in Table 1), and differences between the sums of correlation coefficients computed for each parameter are plotted along the ordinate (see legend of Fig. 2 for additional details). Panel A shows results for correlation coefficients computed from raw sperm counts (Table 1, raw values, casein) whereas Panel B shows results for correlation coefficients computed from motility data expressed as percentages of total sperm cell counts (Table 1, percentages, casein). Panel A: motility analyses based on raw counts show a much different distribution pattern for parameters compared to the lab chow diet (compare Fig. 3 to Fig. 2). With casein, two main parameter clusters are evident. One cluster contains motility descriptors (Motile, Prog, Rapid, Medium) (means much less and correlations more positive overall in αERKO mice), and the second cluster contains the remaining motility descriptors (Slow, Static), velocity descriptors (VAP, VSL, VCL), and a single sperm feature descriptor (Elong) (means less and correlations slightly more negative, more positive or unchanged in αERKO mice). There are also four additional parameters residing at more outlying positions representing (1) two directional descriptors (LIN, STR) (means slightly less and correlations more positive overall in αERKO mice) and (2) two additional sperm descriptors (ALH, BCF) altered in different ways (mean more positive and correlations more negative in αERKO mice (BCF); mean more negative and correlations considerably more positive in αERKO mice (ALH)). Panel B: motility parameters computed as percentages when plotted show clustering and outlier distribution that is similar to Panel A for six parameters (%Motile, %Prog, %Rapid, VCL, ALH, BCF) and much different for the remaining eight parameters. This indicates that changes in motility behavior in mice fed casein are not uniform and affect sperm in multiple ways. [See color version online at www.interscience.wiley.com.]
Sperm Counts and Motility Analyses: Wild Type and αERKO Responses by Diet
Table 2 shows results of comparisons by diet within the wild type and αERKO groups based on mean values given in Table 1 (results in Table 2 are “vertical” comparisons across groups as opposed to the “horizontal” comparisons made in Table 1). It is evident from Table 2 that diet affected sperm counts and sperm motility in both cases, although more dramatically in the case of αERKO mice than in the case of wild type mice (Table 2, bottom group vs. top group; Fig. 4). In broad terms, the lab chow diet for wild type mice appeared mildly stimulatory resulting in higher sperm counts, higher velocities of sperm movement, greater amplitude of lateral sperm head movement (ALH), as well as increases in the numbers of sperm subclassified as Motile, Prog, Rapid, and Medium without significant changes in their relative proportions (%Motile, %Prog, %Rapid, etc. same for both diets) (Table 2, top group; Fig. 4A,B). αERKO mice fed the lab chow diet showed substantially higher sperm counts relative to αERKO mice fed casein, but the lab chow diet otherwise appeared inhibitory both in terms of sperm movement velocities and in terms of the numbers of sperm subclassified as Medium, Slow, and especially Static (Table 2, bottom group; Fig. 4C). In relative terms, the %Motile, %Prog, and %Rapid sperm in these mice were greatly reduced, whereas the %Static sperm showed a huge increase (Table 2, bottom group; Fig. 4D).
TABLE 2.
Effect of Diet on Sperm Counts and Sperm Motility Comparing Mice
| Fed lab chow to those fed caseina | ||||
|---|---|---|---|---|
| Parameter | Changeb | P-valuesc | Powerd | |
| Group = wild type | ||||
| Sperm counts | 37% | 0.0000 | 0.9990 | |
| Raw values | ||||
| VAP | 6% | 0.0025 | 0.8534 | |
| VSL | 6% | 0.0033 | 0.8315 | |
| VCL | 4% | 0.0162 | 0.6684 | |
| ALH | 5% | 0.0012 | 0.8946 | |
| BCF | −12% | 0.2223 | NS | 0.2301 |
| Motile | 17% | 0.0000 | 0.9897 | |
| Prog(ressive) | 12% | 0.0062 | 0.7782 | |
| Rapid | 14% | 0.0003 | 0.9482 | |
| Medium | 23% | 0.0003 | 0.9521 | |
| Slow | 21% | 0.1384 | NS | 0.3157 |
| Static | 10% | 0.2275 | NS | 0.2261 |
| Ratios | ||||
| STR | 0% | 0.9594 | NS | 0.0503 |
| LIN | 2% | 0.8454 | NS | 0.0544 |
| Elong(ation) | 8% | 0.4353 | NS | 0.1198 |
| Percentages | ||||
| %Motile | 1% | 0.7920 | NS | 0.0588 |
| %Prog(ressive) | −1% | 0.9443 | NS | 0.0506 |
| %Rapid | 1% | 0.9306 | NS | 0.0508 |
| %Medium | 3% | 0.8781 | NS | 0.0521 |
| %Slow | −9% | 0.5171 | NS | 0.0566 |
| %Static | −4% | 0.8380 | NS | 0.0555 |
| Group = αERKO | ||||
| Sperm counts | 294% | 0.0000 | 1.0000 | |
| Raw values | ||||
| VAP | −25% | 0.0000 | 1.0000 | |
| VSL | −31% | 0.0000 | 1.0000 | |
| VCL | −13% | 0.0000 | 0.9807 | |
| ALH | 9% | 0.0678 | NS | 0.4448 |
| BCF | 58% | 0.0012 | 0.8945 | |
| Motile | 34% | 0.0038 | 0.8202 | |
| Prog(ressive) | −15% | 0.1281 | NS | 0.3296 |
| Rapid | 3% | 0.7999 | NS | 0.0574 |
| Medium | 86% | 0.0000 | 0.9985 | |
| Slow | 145% | 0.0000 | 0.9949 | |
| Static | 700% | 0.0000 | 1.0000 | |
| Ratios | ||||
| STR | −7% | 0.3325 | NS | 0.1555 |
| LIN | −21% | 0.0815 | NS | 0.4167 |
| Elong(ation) | 11% | 0.3772 | NS | 0.1432 |
| Percentages | ||||
| %Motile | −46% | 0.0000 | 0.9997 | |
| %Prog(ressive) | −65% | 0.0001 | 0.9602 | |
| %Rapid | −61% | 0.0000 | 0.9976 | |
| %Medium | −22% | 0.2009 | NS | 0.2267 |
| %Slow | −9% | 0.8724 | NS | 0.0570 |
| %Static | 99% | 0.0000 | 0.9998 | |
Means for each parameter are explained and listed in Table 1. Comparisons in this table (Table 2) are made vertically across the diet categories of Table 1 (e.g., sperm counts in Table 1 for wild type compares 25.5±8.8–35.0±15.0 and for αERKO compares 3.4±2.4–13.4±8.9, and so on).
For mice fed regular lab chow compared to mice fed casein.
P-values < 0.05 are considered significantly different (NS, not significant). A Fisher's exact test was used to compare differences between means for “Ratios” and “Percentages.”
This is the power associated with rejecting the null hypothesis the two means are equal. The Z-test for comparing two proportions was used in power calculations for variables listed under “Ratios” and “Percentages.”
Fig. 4.
Scatter plots summarizing changes in the motility behavior of sperm comparing wild type against wild type mice (Panels A, B) and αERKO against αERKO mice (Panels C, D) for animals fed lab chow versus casein diet. In all Panels, only those parameters which showed a significant difference between means by diet (see column 2 of Table 2) are plotted as percentages along the abscissa (from column 1 in Table 2 for wild type (Panels A, B) or for αERKO (Panels C, D) groups), and differences between the sums of correlation coefficients computed for each of these parameters are plotted along the ordinate (see legend of Fig. 2 for additional details). Panels A and C show results for correlation coefficients computed from raw sperm cell counts (Table 2, raw values, wild type and αERKO) whereas Panel B and D show results for correlation coefficients computed from motility data expressed as percentages of total sperm cell counts (Table 2, percentages, wild type and αERKO). Panels A and B: the lab chow diet, containing phytoestrogens, is mildly stimulatory in wild type mice; sperm velocities are greater and more sperm are Motile and Progressive and travel at a rapid or medium speed. This diet also results in more positive correlations for all parameters except ALH, which is more negatively correlated across other parameters with the lab chow diet. In terms of relative motility, velocity descriptors and ALH increase but collectively have more negative inter correlations. Panels C and D: the lab chow diet is mildly stimulatory to two motility descriptors (numbers of Motile and Medium) and one sperm descriptor (BCF), and strongly stimulatory to two measures of sluggish sperm (numbers of Slow and Static) in αERKO mice. Sperm velocities are also slightly depressed in αERKO mice fed lab chow. In relative terms, the percentage of sperm that are Motile and Progressive and moving rapidly is less and the percentage of sperm that are static is many times greater in αERKO mice fed lab chow. Panels C and D validate that a component in the diet (phytoestrogens) is responsible for very large changes in parameters BCF and Static observed between αERKO and wild type mice maintained on a lab chow diet (compare to Fig. 2).
DISCUSSION
AQP Expression in Wild Type and αERKO Mice and Effect of Diet
In the present study, AQP-1 expression in αERKO mice showed patches of reactive versus unreactive epithelial cells in the efferent ductules as compared to the uniform homogeneous staining seen in wild type mice (Table 3). Thus estrogen appears to regulate expression of AQP-1 protein, either directly or indirectly, on microvilli and cilia of some but not all nonciliated and ciliated cells, respectively, suggesting the dependence of these cells on ERα activation for AQP-1 expression. Although ERβ is present in the efferent ductule epithelium (Zhou et al., 2002), it is unlikely that estrogen action through ERβ would maintain AQP-1 expression, as other data have shown that ICI 182,780, which block both receptors, had little effect on AQP-1 expression (Zhou et al., 2001). Alternatively, a factor other than estrogen may regulate AQP-1 expression in the remaining reactive cells of αERKO mice. A recent study suggests that AQP-1 is expressed constitutively in both the efferent ductules and initial segment of the epididymis (Oliveira et al., 2005), similar to its expression in the kidney (Nielsen, 1993; Borgnia et al., 1999). The sporadic loss of this water channel in the apical region of the efferent ductule epithelium in αERKO and ICI 182,780 treated mice (Zhou et al., 2001; Oliveira et al., 2005) could be an indirect effect due to the sporadic loss of the microvillus border in these animal models (Hess et al., 1997, 2000; Zhou et al., 2001; Oliveira et al., 2002; Cho et al., 2003; Hess, 2003). In addition, targeting of AQP-1 to the basolateral membrane was dramatically reduced throughout the epithelium of αERKO mice (Table 3), suggesting the requirement of ERα for maintaining expression on this particular membrane domain. Staining for AQP-9 on the microvilli of nonciliated cells was also reduced in αERKO mice (Table 3), and with ICI 182,780 treatment, AQP-9 disappears from the efferent ductule epithelium, even while microvilli were still intact (Oliveira et al., 2005). Thus AQP-9 expression in some nonciliated cells appears to be closely regulated by estrogen activation of ERα; however, this effect appears to be region specific, as the antiestrogen showed no effect on AQP-9 in the initial segment epididymal epithelium (Oliveira et al., 2005).
TABLE 3.
Expression of Aquaporins (AQPs)-1, and -9 in the Efferent Ducts of Wild Type and αERKO Adult Mice Fed Lab Chow or Casein Diets
| Nonciliated cells (microvilli) |
Nonciliated cells (basolateral) |
Ciliated cells (cilia) |
||||
|---|---|---|---|---|---|---|
| Wild type | αERKO | Wild type | αERKO | Wild type | αERKO | |
| AQP-1d | +a | +/−b | + | +/− | + | +/− |
| AQP-9d | + | +/− | −c | − | − | − |
+ indicates reactivity in all cells.
+/− indicates that reactivity is observed in some but not all cells.
− indicates absence of reaction.
The effect in KO mice is more pronounced in distal than proximal regions of the efferent ducts.
In the case of AQP-1 and -9, a more dramatic reduction of staining was noted in distal rather than proximal regions of the efferent ductules in αERKO mice, suggesting a more prominent role for ERα in distal regions. Regulation of other proteins expressed by the epithelial cells of the efferent ductules on a regional basis has not been carefully analyzed, although it has been well-described that the nonciliated cells of proximal regions differ morphologically from those of distal regions (Robaire and Hermo, 1988; Hess, 2002; Ilio and Hess, 2002). On the other hand, region-specific regulation of proteins expressed by a given epithelial cell type in the epididymis has been documented by several investigators (Robaire and Viger, 1995; Hinton et al., 1998; Cornwall et al., 2002; Hermo and Robaire, 2002).
Mice fed either lab chow or the phytoestrogen-free casein diet showed no major differences in the pattern of staining for AQP-1 or -9. This suggests that the small traces of estrogenic compounds present in the lab chow diet exert minimal effects on AQP expression in the epithelium of the efferent ducts of either wild type or αERKO mice. This is in sharp contrast to the major changes observed for sperm counts and sperm motility as a consequence of diet (discussed below).
Sperm Counts
The concentration of sperm in the cauda epididymidis was markedly lower in αERKO mice (Table 1), which confirms previous reports (Eddy et al., 1996; Cho et al., 2003). In αERKO mice, a dramatic reduction of water reabsorption, occurring at the level of the efferent ductules, results in a highly diluted sperm. In addition, the accumulation of water leads to a backflow into the lumen of seminiferous tubules, and disruption of the integrity of the developing germ cell population (Eddy et al., 1996; Lee et al., 2000; Hess et al., 2002). Thus, two mechanisms are involved in the reduction of sperm counts in the epididymis in the αERKO mouse, one which appears to be directly due to the failure of efferent ductules to reabsorb luminal water (Hess et al., 1997). From this study, it is also noteworthy that sperm counts differed in both wild type and αERKO animals depending upon the diet (Table 1). In both animal groups, there was a marked reduction in sperm counts when fed casein rather than lab chow. This suggests that despite their small amounts, phytoestrogens in the lab chow diet enhance either germ cell production or epididymal function, or both mechanisms, in both wild type and αERKO mice (Table 1).
A possible explanation for the dietary effects could be that phytoestrogens in the lab chow diet augment water reabsorption in the efferent ductules, possibly reducing the backflow of water. Furthermore, while AQP expression was diminished between wild type and αERKO mice, no apparent changes in immunostaining was observed due to the different diets. Taking these points into account, it is suggested that in αERKO mice, phytoestrogens in the lab chow diet may stimulate ERβ, which is expressed constitutively in the male (Oliveira et al., 2004) or elicit a nongenomic effect that indirectly increases the concentration of sperm in the epididymis. The differences in sperm counts in wild type mice between the different diets could be due to a combined effect of the phytoestrogens on both ERα and ERβ and their effects on Sertoli, germ and Leydig cells, respectively, as well as augmentation of efferent ductule functions. Hence, although we cannot rule out the possible effects of phytoestrogens on sperm production at the level of the testis, we cannot overlook the concentrating function of the efferent ductules.
Sperm Motility
While sperm counts were improved in αERKO mice fed lab chow, the results of this study also clearly indicate that this increased concentration of sperm consists of a lower quality sperm (Table 1). The effects of diet were more dramatic in the case of αERKO mice than in wild type mice (Table 2; Fig. 4). Taken together with results from sperm counts, these data suggest that the effects on αERKO mice fed the different diets cannot be explained simply as the consequence of water retention at the level of efferent ducts. Rather it is suggested that altered sperm motilities in αERKO mice are a reflection of diminished epididymal functions, as the epithelial cells of this tissue play a major role in producing motile and fertile sperm (Robaire and Hermo, 1988; Orgebin-Crist, 1996; Cornwall et al., 2002). Furthermore, because sperm motility parameters in αERKO mice that were fed lab chow diet were diminished compared to those fed the casein diet, the data suggest that phytoestrogens in the absence of an ERα may be inhibitory through ERβ activity and adversely influence epididymal function and sperm maturation. However, wild type mice fed lab chow showed significantly improved sperm motility compared to those fed casein, suggesting that phytoestrogens in the presence of ERα may be stimulatory through ERβ activity, enhancing epididymal function and hence sperm motility. In the uterus, ERβ appears to play a role in modulation of ERα functions (Weihua et al., 2000), but whether ERα and ERβ modulate each other's function in the male reproductive tract is not known, despite the fact that both ERs are expressed along the epididymis in mice (Fisher et al., 1997; Couse et al., 2001; Zhou et al., 2002).
The epididymis is regarded as a tissue that plays a role in sperm maturation, whereby sperm acquire their proper motility characteristics (Robaire and Hermo, 1988; Cooper, 1995; Cornwall et al., 2002). The coordinated activities of the epithelial cells of the epididymis monitor the luminal environment by secretion and endocytosis of various substances including proteins, water and ions that leads to sperm maturation (Robaire and Hermo, 1988; Cooper, 1995; Turner, 2002). In the present study, multiple motility parameters were altered in αERKO mice as compared to wild type mice and alterations were noted in both cases depending on the diet fed to the animals. This would suggest that different functions of one or more than one epithelial cell type must play a role in the ultimate production of sperm with diverse motility characteristics, and that the composition of the diet can also affect their functions related to motility. However, despite these findings, we cannot at this time ascribe which specific function(s) of the different epithelial cells are altered that lead to the varying sperm motility parameters noted in αERKO mice and wild type mice fed on different diets. The data would suggest, however, that the diverse motility features gained by sperm as they traverse the epididymis are governed by a host of varying epithelial cell functions. In summary, the present data reveal a role for ERα on expression of AQP-1 and -9 in the efferent ductules. In addition, αERKO mice show reduced sperm counts and motility as compared to wild type mice. A role for the presence of phytoestrogens in the diet is also established for sperm counts and motility in the presence or absence of ERα.
ACKNOWLEDGMENTS
We thank Nadia Jandali for assistance with the LM immunocytochemistry.
Footnotes
Grant sponsor: CIHR; Grant sponsor: CONRAD program (RAH; Arlington, VA, USA); Grant sponsor: NSERC; Grant sponsor: NIH; Grant number: DE013237.
REFERENCES
- Andonian S, Hermo L. Cell- and region-specific localization of lysosomal and secretory proteins and endocytic receptors in epithelial cells of the cauda epididymidis and vas deferens of the adult rat. J Androl. 1999;20:415–429. [PubMed] [Google Scholar]
- Badran HH, Hermo L. Expression and regulation of aquaporins 1, 8, and 9 in the testis, efferent ducts, and epididymis of adult rats and during postnatal development. J Androl. 2002;23:358–373. [PubMed] [Google Scholar]
- Borgnia M, Neilsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- Brown D, Katsura T, Gustafson CE. Cellular mechanisms of aquaporin trafficking. Am J Physiol Renal Physiol. 1998;44:F328–F331. doi: 10.1152/ajprenal.1998.275.3.F328. [DOI] [PubMed] [Google Scholar]
- Carreau S, Genissel C, Bilinska B, Levallet J. Sources of oestrogen in the testis and reproductive tract of the male. Int J Androl. 1999;22:211–223. doi: 10.1046/j.1365-2605.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- Cho HW, Nie R, Carnes K, Zhou Q, Sharief NA, Hess RA. The antiestrogen ICI 182,780 induces early effects on the adult male mouse reproductive tract and long-term decreased fertility without testicular atrophy. Reprod Biol Endocrinol. 2003;1:57. doi: 10.1186/1477-7827-1-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PW, Young P, Hess RA, Cunha GR. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology. 1991;128:2874–2879. doi: 10.1210/endo-128-6-2874. [DOI] [PubMed] [Google Scholar]
- Cooper TG. Role of the epididymis mediating changes in the male gamete during maturation. In: Mukhopadhyay AK, Raizada MK, editors. Tissue renin-angiotensin systems. Plenum Press; New York: 1995. pp. 87–101. [Google Scholar]
- Cornwall GA, Lareyre J-J, Matusik RJ, Hinton BT, Orgebin-Crist M-C. Gene expression and epididymal function. In: Robaire B, Hinton B, editors. The epididymis: From molecules to clinical practice. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 169–199. [Google Scholar]
- Couse JE, Mahato D, Eddy EM, Korach KS. Molecular mechanism of estrogen action in the male: Insights from the estrogen receptor null mice. Reprod Fertil Dev. 2001;13:211–219. doi: 10.1071/rd00128. [DOI] [PubMed] [Google Scholar]
- Deen P, van Os C. Epithelial aquaporins. Curr Opin Cell Biol. 1998;10:435–442. doi: 10.1016/s0955-0674(98)80055-0. [DOI] [PubMed] [Google Scholar]
- Echevarria M, Ilundain AA. Aquaporins. J Physiol Biochem. 1998;54:107–118. [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Elkjaer M, Vajda Z, Nejsum LN, Kwon T, Jensen UB, Amiry-Moghaddam M, Frokiaer J, Nielsen S. Immunolocalization of AQP-9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun. 2000;276:1118–1128. doi: 10.1006/bbrc.2000.3505. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Millar MR, Majdic G, Saunders PT, Fraser HM, Sharpe RM. Immunolocalisation of oestrogen receptor-alpha within the testis and excurrent ducts of the rat and marmoset monkey from perinatal life to adulthood. J Endocrinol. 1997;153:485–495. doi: 10.1677/joe.0.1530485. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Turner KJ, Fraser HM, Saunders PTK, Brown D, Sharpe RM. Immunoexpression of aquaporin-1 in the efferent ducts of the rat and marmoset monkey during development, its modulation by estrogens, and its possible role in fluid resorption. Endocrinology. 1998;139:3935–3945. doi: 10.1210/endo.139.9.6213. [DOI] [PubMed] [Google Scholar]
- Free MJ, Jaffe RA. Collection of rete testis fluid from rats without previous efferent duct ligation. Biol Reprod. 1979;20:269–278. doi: 10.1095/biolreprod20.2.269. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Leonard LS, Lovell S, Gould LC, Babai D, Portier CJ, McDonnell DP. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997;143:205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- Ganjam VK, Amann RP. Steroids in fluids and sperm entering and leaving the bovine epididymis, epididymal tissue, and accessory sex gland secretions. Endocrinology. 1976;99:1618–1630. doi: 10.1210/endo-99-6-1618. [DOI] [PubMed] [Google Scholar]
- Greco TL, Duello TM, Gorski J. Estrogen receptors, estradiol, and diethylstilbestrol in early development: The mouse as a model for the study of estrogen receptors and estrogen sensitivity in embryonic development of male and female reproductive tracts. Endocr Rev. 1993;14:59–71. doi: 10.1210/edrv-14-1-59. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Yoshida Y, Tani T, Koyama Y, Nihei K, Ohshiro K, Kamiie JI, Yaoita E, Suda T, Hatakeyama K, Yamamoto T. Cloning of a new aquaporin (AQP-10) abundantly expressed in duodenum and jejunum. Biochem Biophys Res Comm. 2001;287:814–819. doi: 10.1006/bbrc.2001.5661. [DOI] [PubMed] [Google Scholar]
- Hermo L, Robaire B. Epididymal cell types and their functions. In: Robaire B, Hinton BT, editors. The Epididymis. From molecules to clinical practice. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 81–102. [Google Scholar]
- Hermo L, Krzeczunowicz D, Ruz R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J Androl. 2004;25:494–505. doi: 10.1002/j.1939-4640.2004.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Hess RA. Oestrogen in fluid transport in efferent ducts of the male reproductive tract. Rev Reprod. 2000;5:84–92. doi: 10.1530/ror.0.0050084. [DOI] [PubMed] [Google Scholar]
- Hess RA. The efferent ductules: Structure and functions. In: Robaire B, Hinton B, editors. The epididymis: From molecules to clinical practice. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 49–80. [Google Scholar]
- Hess RA. Estrogen in the adult male reproductive tract: A review. Reprod Biol Endocrinol. 2003;1:52–82. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphologic changes in efferent ductules and epididymis in estrogen receptor-alpha knockout mice. J Androl. 2000;21:107–121. [PubMed] [Google Scholar]
- Hess RA, Bunick D, Bahr J. Oestrogen, its receptors and function in the male reproductive tract-a review. Mol Cell Endocrinol. 2001a;178:29–38. doi: 10.1016/s0303-7207(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Hess RA, Zhou Q, Nie R, Oliveira C, Cho H, Nakaia M, Carnes K. Estrogens and epididymal function. Reprod Fertil Dev. 2001b;13:273–283. doi: 10.1071/rd00100. [DOI] [PubMed] [Google Scholar]
- Hess RA, Zhou Q, Nie R. The role of estrogens in the endocrine and paracrine regulation of the efferent ductules, epididymis and vas deferens. In: Robaire B, Hinton B, editors. The epididymis: From molecules to clinical practice. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 317–337. [Google Scholar]
- Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl. 1998;53:47–57. [PubMed] [Google Scholar]
- Ilio KY, Hess RA. Structure and function of the ductuli efferentes: A review. In: Robaire B, Hinton BT, editors. The Epididymis: From molecules to clinical practice. Kluwer Academic/Plenum Publishers; New York: 2002. pp. 49–80. [Google Scholar]
- Ishibashi K, Kuwahara M, Gu Y, Kageyama Y, Tohsaka F, Suzuki F, Marumo F, Sasaki S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J Biol Chem. 1997;272:20782–20786. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- Kato H, Iwata T, Katsu Y, Watanabe H, Ohta Y, Iguchi T. Evaluation of estrogenic activity in diets for experimenta in vitro assay. J Agric Food Chem. 2004;52:1410–1414. doi: 10.1021/jf034896d. [DOI] [PubMed] [Google Scholar]
- King LS, Agre P. Pathophysiology of the aquaporin water channels. Ann Rev Physiol. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- King LS, Yasui M, Agre P. Aquaporins in health and disease. Mol Med Today. 2000;6:60–65. doi: 10.1016/s1357-4310(99)01636-6. [DOI] [PubMed] [Google Scholar]
- Lee KH, Hess RA, Lubahn D, Taylor J, Bunick D. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol Reprod. 2000;63:1873–1880. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela S, Santti R, Salo L, McLachlan JA. Phytoestrogens are partial estrogen agonists in the adult male mouse. Environ Health Perspect. 1995;103:123–127. doi: 10.1289/ehp.103-1518873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. Endocytosis in proximal tubule cells involves a two-phase membrane- recycling pathway. Am J Physiol. 1993;264:C823–C835. doi: 10.1152/ajpcell.1993.264.4.C823. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Frokler J, Marples D, Kwon T, Agre P, Knepper MA. Aquaporins in the kidney: From molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- Nihei K, Koyama Y, Tani T, Yaoita E, Ohshiro K, Adhikary LP, Kurosaki I, Shirai Y, Hatakeyama K, Yamamoto T. Immuno-localization of aquaporin-9 in rat hepatocytes and Leydig cells. Arch Histol Cytol. 2001;64:81–88. doi: 10.1679/aohc.64.81. [DOI] [PubMed] [Google Scholar]
- Nishihara T, Nishikawa J, Kanayama T, Dakeyama F, Saito K, Imagawa M, Takatori S, Kitagawa Y, Hori S, Utsumi H. Estrogenic activities of 517 chemicals by yeast two-hybrid assay. J Health Sci. 2000;46:282–298. [Google Scholar]
- Oliveira CA, Carnes K, Franca LR, Hess RA. Infertility and testicular atrophy in the antiestrogen-treated adult male rat. Biol Reprod. 2001;65:913–920. doi: 10.1095/biolreprod65.3.913. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Zhou Q, Carnes K, Nie R, Kuehl DE, Jackson GL, Franca LR, Nakai M, Hess RA. ER function in the adult male rat: Short- and long-term effects of the antiestrogen ICI 182,780 on the testis and efferent ductules, without changes in testosterone. Endocrinology. 2002;143:2399–2409. doi: 10.1210/endo.143.6.8873. [DOI] [PubMed] [Google Scholar]
- Oliveira CA, Mahecha GA, Carnes K, Prins GS, Saunders PT, Franca LR, Hess RA. Differential hormonal regulation of estrogen receptors ER alpha and ER beta and androgen receptor expression in rat efferent ductules. Reproduction. 2004;128:3–86. doi: 10.1530/rep.1.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CA, Carnes K, França LR, Hermo L, Hess RA. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol Cell. 2005;97:385–395. doi: 10.1042/BC20040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgebin-Crist M-C. Androgens and epididymal function. In: Bhasin S, Gabelnick H, Spieler G, Swerdloff R, Wang C, editors. Pharmacology, biology, and clinical applications of androgens. Wiley-Liss, Inc.; New York: 1996. pp. 27–38. [Google Scholar]
- Pastor-Soler N, Ganis C, Sabolic I, Tyszkowski R, McKee M, Van Hoek A, Breton S, Brown D. Aquaporin 9 expression along the male reproductive tract. Biol Reprod. 2001;65:384–393. doi: 10.1095/biolreprod65.2.384. [DOI] [PubMed] [Google Scholar]
- Payne AH, Kelch RP, Musich SS, Halpern ME. Intratesticular site of aromatization in the human. J Clin Endocrinol Metab. 1976;42:1081–1087. doi: 10.1210/jcem-42-6-1081. [DOI] [PubMed] [Google Scholar]
- Preston GM, Agre P. Molecular cloning of the red cell integral protein of Mr 28000: A member of an ancient channel family. Proc Natl Acad Sci USA. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B, Hermo L. Efferent ducts, epididymis, and vas deferens: Structure, functions, and their regulation. In: Knobil E, Neill J, editors. The physiology of reproduction. Raven Press; New York: 1988. pp. 999–1080. [Google Scholar]
- Robaire B, Viger RS. Regulation of epididymal epithelial cell functions. Biol Reprod. 1995;52:226–236. doi: 10.1095/biolreprod52.2.226. [DOI] [PubMed] [Google Scholar]
- Sansom M, Law R. Membrane proteins: Aquaporins-channels without ions. Curr Biol. 2001;11:R71–R73. doi: 10.1016/s0960-9822(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland, and the hypothalamic/pituitary axis in rats. J Nutr. 1997;127:263–269. doi: 10.1093/jn/127.2.263. [DOI] [PubMed] [Google Scholar]
- Schleicher G, Drews U, Stumpf WE, Sar M. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. An autoradiographic study. Histochemistry. 1984;81:139–147. doi: 10.1007/BF00490107. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Cadnapaphornchai MA. Renal aquaporin water channels: From molecules to human disease. Prog Biophys Mol Biol. 2003;81:117–131. doi: 10.1016/s0079-6107(02)00049-4. [DOI] [PubMed] [Google Scholar]
- Setchell BP, Brooks DE. Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press; New York, NY: 1988. pp. 753–836. [Google Scholar]
- Setchell BP, Scott TW, Voglmayr JK, Waites GMH. Characteristics of spermatozoa and the fluid which transports them into the epididymis. Biol Reprod. 1969;1:40–66. doi: 10.1095/biolreprod1.supplement_1.40. [DOI] [PubMed] [Google Scholar]
- Turner TT. Necessity's potion: Inorganic ions and small organic molecules in the epididymal lumen. In: Robaire B, Hinton BT, editors. The epididymis: From molecules to clinical practice. Kluwer Academic/Plenum Publishers; New York, NY: 2002. pp. 131–150. [Google Scholar]
- Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278:F13–28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci USA. 2000;97:5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NB, Brenner RM. Estrogen receptor in the ductuli efferentes, epididymis, and testis of rhesus and cynomolgus macaques. Biol Reprod. 1990;42:533–538. doi: 10.1095/biolreprod42.3.533. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Naftolin F. Reproductive actions of phytoestrogens. Baillieres Clin Endocrinol Metab. 1998;12:667–690. doi: 10.1016/s0950-351x(98)80010-4. [DOI] [PubMed] [Google Scholar]
- Wintour EM. Water channels and urea transporters. Clin Exp Pharmacol Physiol. 1997;24:1–9. doi: 10.1111/j.1440-1681.1997.tb01775.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: Roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract functions. Proc Natl Acad Sci USA. 2001;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl. 2002;23:870–881. [PubMed] [Google Scholar]