Abstract
Toxin-secreting “killer” yeasts were initially identified >40 years ago in Saccharomyces cerevisiae strains infected with a double-stranded RNA “killer” virus. Despite extensive research conducted on yeast killer toxins, the mechanism of protecting immunity by which toxin-producing cells evade the lethal activities of these proteins has remained elusive. Here, we identify the mechanism leading to protecting immunity in a killer yeast secreting a viral α/β protein toxin (K28) that enters susceptible cells by receptor-mediated endocytosis and, after retrograde transport into the cytosol, blocks DNA synthesis, resulting in both cell-cycle arrest and caspase-mediated apoptosis. We demonstrate that toxin immunity is effected within the cytosol of a toxin-secreting yeast and occurs via the formation of complexes between reinternalized toxin and unprocessed precursor moieties that are subsequently ubiquitinated and proteasomally degraded, eliminating the active form of the toxin. Interference with cellular ubiquitin homeostasis, either through overexpression of mutated ubiquitin (Ub-RR48/63) or by blocking deubiquitination, prevents ubiquitination of toxin and results in an impaired immunity and the expression of a suicidal phenotype. The results presented here reveal the uniquely elegant and efficient strategy that killer cells have developed to circumvent the lethal effects of the toxin they produce.
Keywords: α/β protein toxin, preprotoxin, ubiquitination
The killer phenomenon in yeast, originally discovered in certain killer strains of Saccharomyces cerevisiae in 1963, is associated with the secretion of a protein toxin that kills sensitive target cells in a receptor-mediated fashion without direct cell-to-cell contact (1, 2). In baker's yeast, the killer phenotype depends on the presence of cytoplasmic dsRNA killer viruses encoding the unprocessed toxin precursor protein (3). Until now, three killer toxins have been identified, of which K1 and K28 have been studied most extensively. Each killer toxin is translated as a preprotoxin (pptox) precursor that is imported into the yeast secretory pathway where it is processed to the mature and cytotoxic α/β heterodimer, which is then released from the cell and secreted into the medium (4–6).
Although the two “prototypes” of yeast killer toxins K1 and K28 exert their lethal effect in a receptor-mediated fashion, the mechanism of toxin-induced lethality is significantly different. K1 disrupts cytoplasmic membrane function, whereas K28 enters its target cell by receptor-mediated endocytosis and blocks DNA synthesis, leading to both G1/S cell-cycle arrest and caspase-mediated apoptosis (7, 8). Both toxins initially bind to a primary receptor within the yeast cell wall and are then translocated to the plasma membrane, where they interact with a secondary toxin receptor (9, 10). The glycosylphosphatidylinositol-anchored cell-surface protein Kre1p has recently been identified as the plasma membrane receptor for K1 toxin, whereas the membrane receptor for K28 remains unknown (11). After having bound to the yeast cell surface, K1 perturbs plasma membrane function by forming cation-selective ion channels (12, 13), whereas K28, in contrast, enters the cell and is trafficked through the secretory pathway to translocate into the cytosol and reach its final target in the nucleus. Retrograde toxin transport from and through the Golgi apparatus and the endoplasmic reticulum (ER) critically depends on the presence of a short amino acid sequence (HDEL) at the C terminus of the toxin's β subunit that enables the toxin to reach the secretory pathway from an early endosomal compartment (14). Once within the ER, the α/β heterodimer dislocates into the cytosol via Sec61c, the major ER transport channel, subsequently transmitting its lethal signal into the nucleus.
Extensive investigation of the killer phenomenon in yeast has resulted in substantial progress in elucidating many of the intricacies of this phenomenon and, additionally, providing valuable insight into a number of fundamental aspects of eukaryotic cell biology and virus–host cell interactions (3, 6, 15). Furthermore, the detailed analysis of the toxins' mode of action also revealed a number of potential targets against which antifungal agents could potentially be directed. Despite all this, the mechanism by which toxin-producing cells evade the lethal activities of these proteins has remained elusive, especially since the recently proposed model for K1 immunity (16) has, meanwhile, been repeatedly shown to be of little significance in vivo (11, 17, 18). Until the present study, no attempt has been made to explain how K28 toxin-producing cells resist the activity of this protein. In this investigation, we show that K28-producing cells, just like toxin-susceptible target cells, take up toxin and transport it to the cytosol. We demonstrate that immunity to K28 does not rely on inhibition of extracellular toxin uptake and present data revealing that both pptox and reinternalized mature toxin are present in the cytosol of K28-producing cells. Replacement of the N-terminal K28 leader peptide by the cotranslationally active K1 pptox signal peptide indicated that the mechanism of K28 pptox import into the ER is critical in conferring toxin immunity. Partial immunity to K28 could be achieved by cytosolic expression of only the mature α subunit of the toxin, whereas complete protection required a nonspecific sequence extension to the C terminus of the α subunit. Finally, we reveal the formation of a complex between pptox and the reinternalized, mature toxin in the cytosol of K28-producing cells and demonstrate that, in vivo, ubiquitination and proteasomal degradation of this pptox/K28 complex is essential for K28-producing cells to achieve complete protection and immunity from the toxin.
Results and Discussion
Immune Killer Cells Partially Reinternalize Secreted Toxin.
In killer yeast, functional immunity is central and essential for cell survival, because the toxins exclusively target and inhibit eukaryotic cell functions. This situation is in major contrast to protein toxins produced by bacteria (such as cholera toxin and Shiga toxins) which selectively kill eukaryots, thus making immunity and self-defense in a prokaryotic host dispensable.
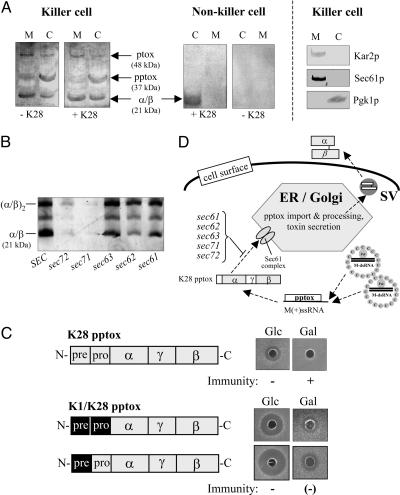
The K28 toxin, in common with cholera toxin, Pseudomonas exotoxin A, and other family members of microbial α/β toxins, uses receptor-mediated endocytosis and retrograde transport to reach its intracellular target compartment (14). In principal, therefore, toxin immunity could be conferred either by preventing reinternalization of secreted, active toxin, or by inactivating reinternalized toxin somewhere within the secretory pathway or further downstream in the yeast cell cytosol. By analyzing the intracellular membrane fraction and the cytosol of a K28-secreting superkiller (ski2–2) mutant, we show that both the pptox precursor protein and the mature and fully processed α/β heterodimer are present in the cytosol, whereas an additional 48-kDa toxin signal is detectable in the intracellular membrane fraction, most likely representing the N-glycosylated protoxin (ptox) resulting from signal peptidase cleavage and coreglycosylation in the ER lumen (Fig. 1A). The small amount of ptox present in the yeast cell cytosol indicates some ptox leakage from the secretory pathway during cell lysis (Fig. 1A); however, this minor amount does not account for the observed pptox enrichment in the cytosol. As expected, no such toxin-specific signal was detectable in intracellular membranes of the corresponding nonkiller derivative obtained after heat-curing the ski2–2 mutant from its toxin-encoding M-dsRNA killer virus (19) (Fig. 1A). Interestingly, when the same killer cells were treated with exogenous toxin, the amount of mature α/β toxin present in the cytosol significantly increased, indicating that K28 killer cells are capable of taking up their own toxin and transporting it retrograde to the cytosol, just like toxin-susceptible nonkiller cells (+K28 in Fig. 1A). This observation also implies that K28 immunity does not rely on inhibition of extracellular toxin uptake; and, vice versa, toxin reinternalization was completely blocked in a K28-secreting end3ts mutant defective in early steps of endocytosis (data not shown). The presence of significant amounts of the α/β heterodimeric toxin in the cytosol of K28-producing cells supports the argument that the dimeric conformation of the mature toxin is rather stable, a finding that is in agreement with recent studies on human superoxide dismutase that similarly appears to have the ability to maintain intrasubunit disulfide bonds in the reducing environment of the cytosol (20). This finding also confirms that K28 toxin's retrotranslocation from the ER to the cytosol occurs in the α/β heterodimeric conformation (14) and is consistent with a recent report that, likewise, showed that protein unfolding is not a prerequisite for ER dislocation (21). The conclusion from these results is that reinternalization of at least a fraction of the active α/β toxin occurs in K28-producing cells and that immunity relies on inactivation of reinternalized toxin rather than its exclusion from the cell.
Fig. 1.
Posttranslational pptox import into the ER and reinternalization of the mature α/β toxin. (A) SDS/PAGE and Western blot analysis of the intracellular membrane fraction (M) and the cytosol (C) of the K28 superkiller mutant (ski2–2) S. cerevisiae MS300b and its heat-cured nonkiller derivative probed with a polyclonal anti-β-subunit antibody. Positions of the unprocessed pptox in the cytosol, the N-glycosylated ptox in the ER lumen, and the α/β heterodimeric K28 toxin in toxin-treated (+K28) and untreated (−K28) cells are indicated. Organellar marker proteins in the same subcellular fractions of the K28 killer strain MS300b were probed with anti-Kar2p (ER lumenal marker), anti-Sec61p (ER membrane marker), and anti-Pgk1p (cytosolic marker). (B) Immunoblot of secreted K28 toxin in cell-free culture supernatants of the temperature-sensitive yeast mutants sec71, sec72, sec62, sec63, and sec61 and their isogenic SEC wild-type equivalent after cultivation at the semirestrictive temperature of 30°C. Positions of the α/β heterodimeric toxin and its tetrameric derivative (α/β)2 (which forms spontaneously under conditions of a nonreducing SDS/PAGE) are indicated. (C) Schematic outline of K28 pptox derivatives containing either the natural N-terminal secretion signal (prepro sequence) of K28 pptox or the corresponding prepro or pre sequence of K1 pptox (shown in a black box) under transcriptional control of the galactose-inducible GAL1 promoter and their phenotypic effect on toxin immunity after in vivo expression in the K28-sensitive strain S. cerevisiae SEY6210. Immunity against exogenously applied K28 toxin was determined on MBA plates (pH 4.7) under repressing or inducing culture conditions in the presence of glucose or galactose as carbon source. (D) Model of posttranslational pptox import into the ER lumen. In the cytosol of a toxin-producing killer yeast, unprocessed pptox encoded by the killer viral M(+)ssRNA transcript enters the secretory pathway through the major ER import channel (Sec61 complex) aided by cytosolic chaperones of the Ssa family (not indicated). Consequently, pptox import into the ER is prevented or at least significantly impaired in the indicated conditional yeast mutants sec61, sec62, sec63, sec71, and sec72. SV, secretory vesicles.
Posttranslational pptox Import into the ER Is Critical for Toxin Immunity.
Our finding of significant amounts of pptox within the yeast cell cytosol implies that this protein is imported posttranslationally into the secretory pathway (representing the predominant mechanism of protein import into the ER in yeast) rather than cotranslationally, as it preferentially occurs in mammalian cells (22). Detailed analysis of the physicochemical properties of the K28 signal peptide as well as calculations of the hydrophobic core values both supported our prediction that pptox import into the ER occurs posttranslationally (data not shown). In support of the in silico data, we found a dramatic reduction of K28 secretion in conditional mutants impaired in essential cellular components of posttranslational protein import into the secretory pathway. As illustrated in Fig. 1B, K28 toxin secretion was almost completely blocked in yeast sec71 and sec72 mutants and significantly decreased in sec62, sec63, and sec61 mutants when cultivated at the semirestrictive temperature. The most pronounced decrease in toxin secretion was seen in sec71, sec72, and sec62 mutants, whose gene products are believed to form a cytosolic receptor complex involved in the signal-recognition particle-independent posttranslational protein import into the ER lumen (23, 24). In addition, toxin secretion was markedly decreased in mutants defective in cytosolic Hsp70 chaperones (such as Ssa1p and Ssa2p) that are known to be required for posttranslational translocation into the ER (data not shown). Interestingly, substitution of either the K28 signal peptide or the entire K28 prepro region with the corresponding sequence(s) of the significantly more hydrophobic K1 signal peptide (thus favoring cotranslational translocation into the secretory pathway) led to a dramatic increase in K28 susceptibility of toxin-producing cells harboring the chimeric K1/K28 construct (Fig. 1C), demonstrating that a certain level of pptox in the cytosol of a killer cell is necessary for the conferrence of immunity and that this level is maintained in vivo by posttranslational pptox translocation into the ER (Fig. 1D). This finding is in agreement with previous findings showing that functional immunity occurs only if pptox expression exceeded a certain critical level (25, 26). Our results provide striking evidence that the mechanism of pptox import into the secretion pathway and its impact on the steady-state concentration of pptox in the cytosol is of critical importance in the ability of cells to exhibit immunity from K28 toxin.
The α Subunit of K28 Is Essential for Immunity.
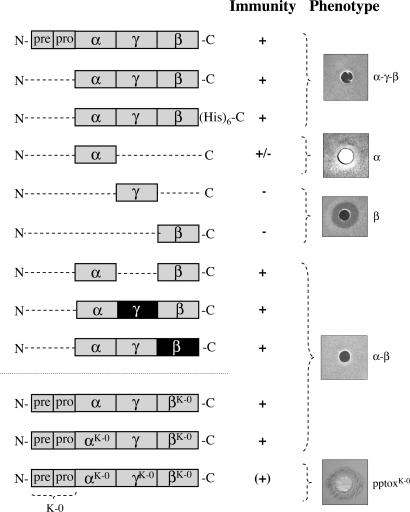
The existence of pptox and reinternalized toxin within the cytosol of K28-immune cells and the finding that cellular uptake of external toxin is unaffected by the immune status of the cell suggested to us that the key element in toxin immunity involves an interaction between pptox and reinternalized mature α/β toxin in the cytosol of toxin-producing cells. To test this hypothesis, an N-terminally truncated K28 pptox derivative lacking its natural prepro sequence and, thus, incapable of entry into the secretory pathway, was expressed in a K28-susceptible strain under transcriptional control of the GAL1 promoter. As illustrated in Fig. 2, the former K28-susceptible cells were immune under induced conditions in the presence of galactose, whereas the same cells remained toxin-sensitive when pptox transcription was repressed (data not shown). To define the pptox domain that is required for protective immunity, we independently introduced a number of constructs expressing different pptox domains into susceptible host cells and assessed the resultant transformants for toxin immunity. As shown in Fig. 2, expression of neither the β subunit nor the γ sequence that bridges α and β had any impact on toxin susceptibility, indicating that both subunits are not involved in conferrence of immunity. This finding bolsters our previous speculation that β functions as intracellular transport “vehicle” solely, ensuring proper uptake and efficient transport of the cytotoxic α toxin to its final site of action, whereas β has no relevance for the expression of protecting immunity. In contrast, sole expression of α rendered cells considerably less susceptible to toxin (Fig. 2), demonstrating that the presence of α is necessary, but not sufficient, to confer complete immunity in vivo. Because, in the native pptox, the α subunit is C-terminally linked to the γ sequence, we investigated whether extending the C terminus of α would increase the level of K28 immunity. An additional toxin derivative in which the intervening γ sequence was removed, resulting in a direct linkage between α and β, conferred complete immunity to K28-susceptible cells (Fig. 2), confirming that a C-terminal extension of α is needed to give full protection. Interestingly, because this construct contained a sequence that never occurs in nature, i.e., the α subunit directly linked to β, the precise sequence of the C-terminal extension of α is apparently irrelevant with respect to the conferrence of immunity. To further confirm this interesting observation, we replaced the γ sequence of K28 with the corresponding sequence of K1. Although both γ sequences share no sequence homology, K28-susceptible transformants expressing γ from K1 were fully immune to K28 just as cells expressing αγ from K28 but containing β from K1 pptox (Fig. 2), substantiating the finding that expression of C-terminal extended α is both necessary and sufficient to confer protective immunity to K28. A similar phenomenon has been described for the expression of immunity from K1, which likewise requires expression of a C-terminally extended α subunit. For expression of K1 immunity, however, this C-terminal extension of α must, at a minimum, constitute the N-terminal 31 residues of the K1 γ subunit (27), whereas the C-terminal extension necessary for immunity from K28 appears to be essentially sequence-nonspecific and is unquestionably independent of γ.
Fig. 2.
The α subunit of K28 is essential but not sufficient to confer protective immunity. Schematic drawing and effect on toxin immunity of K28 wild-type pptox and various truncated variants thereof lacking the N-terminal prepro sequence after in vivo expression in the K28-susceptible strain S. cerevisiae SEY6210. In the K1/K28 chimeric constructs, the corresponding subunits from K1 pptox are indicated by a black box. In each case, pptox expression was driven from the GAL1 promoter, and K28 immunity was determined in a well-plate assay on MBA (pH 4.7) with galactose as carbon source. In the pptox variants α/βK-0, αK-0/βK-0 and pptoxK-0, the single lysine residue in β or all internal lysyl residues in either both subunits or in the entire preprotoxin had been converted to arginine to reduce or prevent lysine-mediated α/β ubiquitination in vivo (see also Fig. 3G). The particular toxin construct producing the exemplarily shown halo on MBA is indicated in the figure. Note that toxin-treated cells of K28-α-expressing yeast show a small but cell-free zone of growth inhibition around the well, whereas pptoxK-0-expressing cells show a dark blue colony staining around the well, indicating that the cells are being killed by the toxin.
Cytosolic pptox Complexes with Reinternalized K28 Toxin.
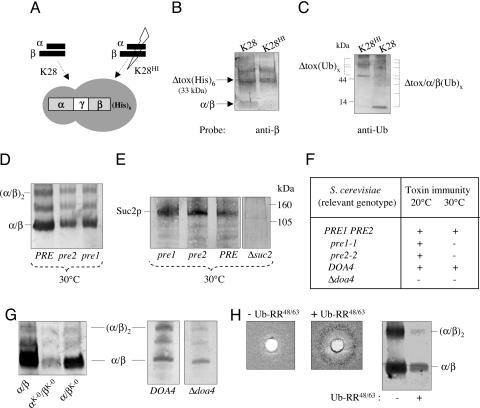
We had shown that expression of unprocessed K28 pptox is sufficient to confer immunity by introducing a K28 cDNA into a susceptible Δkex2 null mutant, incapable of pptox processing, and thereby generating immune nonkiller cells (28, 29). This finding is entirely consistent with, and supportive of, our hypothesis that the formation of a complex between pptox and reinternalized K28 within the cytosol of toxin-producing cells is the key step in immunity, because the formation of such a complex would occur irrespective of the cells' ability to process pptox for secretion. We succeeded in isolating the pptox/K28 complex by copurifying a His-tagged pptox derivative [Δtox(His)6] lacking the N-terminal prepro sequence (Fig. 3A) but still capable of conferring immunity (see Fig. 3), with the mature α/β heterodimer of K28. As shown in Fig. 3B, Δtox(His)6 and heterodimeric toxin were copurified (albeit not in a stoichiometric ratio; see below), providing evidence for the in vivo formation of a complex between K28 pptox and reinternalized toxin. That this complex is formed between Δtox and reinternalized toxin was also evidenced by its absence when heat-inactivated toxin (K28HI in Fig. 3B) was used in lieu of native toxin. In contrast to native toxin, heat-inactivated K28HI is incapable of entering and penetrating a sensitive target cell, because initial toxin binding to both the K28 cell wall and its membrane receptor on the yeast cell surface is completely blocked (J. Spindler and M.J.S., unpublished data). The in vivo formation of a pptox/K28 complex presumably enables highly effective interception of reinternalized α/β in toxin-producing cells immediately after this molecule has been retrograde transported to the cytosol and before the release of the biologically active α subunit from the heterodimer.
Fig. 3.
Functional immunity requires ubiquitination and proteosomal degradation of the pptox/K28 complex. (A) Schematic drawing of a His-tagged K28 pptox derivative lacking its N-terminal prepro sequence [Δtox(His)6] and experimental setup to copurify a Δtox(His)6/toxin complex in vivo. Yeast transformants expressing the His-tagged K28 toxin precursor Δtox(His)6 were spheroplasted and treated with either native toxin (K28) or heat-inactivated toxin (K28HI), the latter being completely blocked in cell entry. Cells were lysed, cell debris and membranes were removed, and the resultant supernatant applied onto a TALON column to purify the His-tagged K28-derivative. (B) Demonstration of a Δtox(His)6/α/β complex purified from the cytosol of toxin-immune cells. After purification, eluted proteins from A were fractionated by SDS/PAGE and probed with polyclonal anti-β subunit antibody. Positions of the His-tagged protoxin [Δtox(His)6] and the heterodimeric α/β toxin are indicated. (C) Evidence that the Δtox(His)6/α/β complex is ubiquitinated and proteasomally degraded. Eluted protein samples from A were fractionated by SDS/PAGE and detected by using polyclonal anti-Ub antibody. Positions of the ubiquitinated toxin precursor [Δtox(Ub)x] and the breakdown products of the ubiquitinated Δtox/α/β(Ub)x complex are indicated. (D) Western blot analysis of secreted K28 toxin and (E) invertase Suc2p in cell-free culture supernatants. (F) Impaired toxin immunity in conditional proteasome mutants pre1–1 and pre2–2 and a Δdoa4 knockout compared with wild-type. As negative control for invertase secretion, the Δsuc2 mutant S. cerevisiae SEY6210 was used. (G) Relative amount of secreted K28 wild-type toxin (α/β) and its two lysine-free variants α/βK-0 and αK-0/βK-0 in S. cerevisiae SEY6210 and decreased α/β toxin secretion in a yeast Δdoa4 mutant defective in protein deubiquitination compared with the isogenic DOA4 wild type. Positions of the α/β heterodimeric toxin and its tetrameric deriative (α/β)2 are indicated. (H) Lack of functional immunity and decrease in toxin secretion in K28 pptox-expressing wild-type cells before and after Cu2+-induced overexpression of mutated Ub (Ub-RR48/63) incapable of forming polyubiquitin chains.
Ubiquitination and Proteasomal Degradation of the Cytosolic pptox/K28 Complex Is an Essential Prerequisite for Functional Immunity.
The formation of cytosolic pptox/K28 complexes raises the question of how toxin-producing cells maintain adequate concentrations of free pptox in the cytosol to ensure ongoing immunity while still secreting sufficient processed toxin to kill susceptible cells. Our experiments indicate that this is accomplished by ubiquitination and proteasomal degradation of, primarily, the K28 component of the pptox/K28 complex and by simultaneously ensuring that sufficient free pptox is available for (i) complex formation with reinternalized α/β toxin and (ii) import into the ER and subsequent toxin secretion. When K28-immune cells were treated with heat-inactivated toxin, several high-molecular-weight protein signals comprising ubiquitinated forms of Δtox(Ub)x were visible (Fig. 3C). Furthermore, the cytosol of immune cells treated with native toxin additionally contained low-molecular-weight toxin signals that most likely represent proteasomal breakdown products of the heterodimeric toxin within the Δtox/K28 complex (Fig. 3C). It appears that ubiquitination and proteasomal degradation are somehow more effective to the toxin component within the Δtox/K28 complex, because ubiquitinated degradation products in the low-molecular-weight range were hardly present in control cells treated with heat-inactivated α/β toxin incapable of cell entry (Fig. 3C). In direct support, cytosolic Δtox complexed with reinternalized α/β toxin was affinity-purified in a nonstoichiometric ratio (Fig. 3B), suggesting that an excess in free cytosolic pptox in vivo exists, which similarly ensures pptox import into the ER and subsequent secretion of mature α/β toxin. Thus, it can be assumed that, once the ubiquitinated α/β component of the pptox/K28 complex has been proteasomally degraded, sufficient nonubiquitinated pptox is still available to either complex with another K28 molecule or enter the yeast secretory pathway to be processed into mature toxin; and, vice versa, it can be presumed that toxin secretion should be negatively affected when proteasomal degradation of the complex is disturbed, because, in this case, pptox primarily has to complex internalized toxin instead of being translocated into the ER. Such a scenario should bias the balance between pptox needed to complex toxin and pptox destined for ER import to pptox/α/β complex formation. Consistent with this hypothesis is our finding that toxin secretion is significantly perturbed and immunity severely impaired in conditional proteasomal yeast mutants (pre1–1 and pre2–2), resulting in the expression of a suicidal phenotype against exogenously applied toxin. At the restrictive temperature, these mutants are severely impaired in the chymotrypsin-like activity of the 20S proteolytic core particle of the proteasome (30), and cells of both pre mutants show significantly decreased levels of toxin secretion and exhibit increased susceptibility to toxin, although protein secretion in general is not negatively affected, as could be shown by SDS/PAGE and Western blot analysis of yeast invertase (Suc2p) secretion in pre1/2 mutants compared with isogenic PRE1/2 wild-type cells (Fig. 3 D–F). This finding indicates that both toxin secretion and immunity are severely impaired when cells are blocked (or at least impaired) in proteasomal degradation of the cytosolic pptox/K28 immunity complex. Interestingly, an entirely analogous phenotype could be seen in cells expressing a mutant K28 variant in which either the single lysine residue in β or all internal lysyl residues in both subunits α and β had been destroyed and converted to arginine to prevent (or at least reduce) lysine-mediated ubiquitination and proteasomal degradation in vivo. In case of αK-0/βK-0, secretion of the mutant toxin was significantly decreased compared with wild-type α/β toxin (Fig. 3G), and functional immunity against exogenous α/β was negatively affected in cells expressing a K28 pptox variant completely avoid of internal lysyl residues (see Fig. 2). In sum, from these findings, it can be deduced that prevention of ubiquitination of the reinternalized α/β toxin within the cytosolic pptox/K28 complex severely affects both toxin secretion and functional K28 immunity. In a compelling parallel experiment, loss of toxin immunity could also be accomplished by interfering with in vivo ubiquitin (Ub) homeostasis. This result was achieved in two ways: first, by copper-induced overexpression of a mutated Ub (Ub-RR48/63) that can no longer form polyubiquitin chains and, second, by deletion of a gene, DOA4, which encodes the deubiquitinating enzyme required for regenerating Ub from proteasome-bound ubiquitinated intermediates. Both scenarios resulted in the expected loss in toxin immunity and decrease in toxin secretion (Fig. 3 G and H), demonstrating that ubiquitination and proteasomal degradation of cytosolic pptox/K28 complexes is not simply a process to eliminate waste byproducts of K28-toxin biosynthesis but, rather, is an essential and integral component in maintaining immunity to toxin. However, in comparison with the immunity-compromised yeast mutants Δdoa4 and/or pre1/2, the observed decrease in toxin secretion was significantly more pronounced in wild-type yeast expressing either mutant Ub (Ub-RR48/63) or a lysine-free K28 derivative (pptoxK-0), indicating that the levels of toxin secretion and toxin immunity do not strongly correlate in vivo.
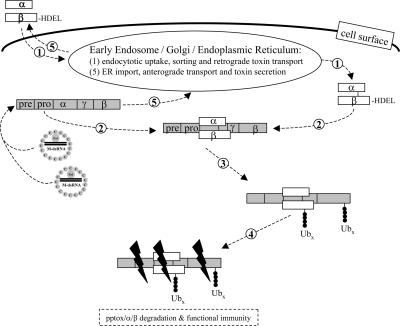
Based on the data presented here, we can now provide a mechanistic model of the events resulting in immunity of K28-secreting cells from the action of this toxin (Fig. 4). We present evidence that immunity from K28 toxin, one of the three best characterized toxins produced by virus-infected killer strains of S. cerevisiae, occurs via a specific interaction between reinternalized toxin and its precursor protein within the cytosol of toxin-producing cells. This interaction results in the generation of a pptox/K28 complex that is a target for ubiquitination and subsequent proteasomal degradation. This elegant, efficient, and entirely self-contained mechanism enables K28-producing cells to inactivate toxin before the cytotoxic α toxin reaches its ultimate intracellular target. The mechanism of posttranslational pptox import into the yeast secretory pathway ensures that free cytosolic pptox is in significant excess over reinternalized α/β toxin, and, thus, the formation of a pptox/K28 immunity complex in the cytosol does not negatively impact toxin production.
Fig. 4.
Mechanistic model of toxin immunity in a K28-secreting killer yeast. In the cytosol of a toxin-producing killer cell, the unprocessed preprotoxin encoded by the M-dsRNA killer virus complexes with mature α/β toxin that has been reinternalized and retrogradely transported through the secretory pathway (1, 2). Within the subsequently generated complex of pptox and mature α/β toxin (3), the β-subunit is (poly)ubiquitinated (Ubx) and degraded by the proteasome (4). Because of an excess in the noncomplexed and nonubiquitinated pptox in the cytosol, the toxin precursor can also be imported into the ER, processed, and secreted as active toxin (5).
Materials and Methods
Yeast Strains and Culture Media.
For the in vivo expression of the K1/K28 pptox constructs, S. cerevisiae strain SEY6210 (MATα leu2–3 his3-Δ200 lys2–801 trp1-Δ901 suc2-Δ9) was used. Cells were grown in yeast extract/peptone/dextrose medium at 30°C. Transformation was carried out by the lithium acetate method according to Ito et al. (31), and transformants were selected on synthetic complete medium lacking uracil. Yeast proteasomal mutants pre1–1 and pre2–2 were kindly provided by Dieter Wolf (University of Stuttgart) (32); wild-type strain BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) and all deletant strains were from the Saccharomyces Genome Deletion Consortium and were obtained from Research Genetics. The yeast Δdoa4 null mutant MHY623 (MATα his3Δ200 leu2–3,112 ura3–52 lys2–801 trp1–1 doa4Δ1::LEU2) and its isogenic wild-type strain MHY501, both originally derived from the yeast strain collection of Mark Hochstrasser (33).
Escherichia coli Strains, Plasmids, and DNA Manipulations.
Standard molecular manipulations were performed as described by Sambrook et al. (34). For cloning, E. coli strain DH5α (F− recA1 endA1 gyr A96 thi hsdR17 supE44 relA1 Δ(argF-lac-ZYA) U169 (Φ80 dlacZΔM15) λ−) was grown at 37°C in LB-medium supplemented with 100 μg of ampicillin·ml−1 when necessary. PCR amplifications were performed by using high-fidelity TaqDNA-polymerase (Roche) according to the instructions of the manufacturer and products routinely sequenced. K28[α/β], K28[α/γK1/β] and K28[SPK1] (the latter containing the prepro-sequence from K1 pptox) were constructed by PCR-mediated overlap extension (35, 36). After PCR amplification, the corresponding DNA fragments were directly cloned into pYES2.1/V5-HIS-TOPO according to the instructions of the manufacturer (Invitrogen), allowing regulated pptox expression under transcriptional control of the galactose-inducible GAL1 promoter. Overexpression of mutated Ub (Ub-RR48/63) was achieved by cotransformation of K28 pptox-expressing wild-type cells with plasmid pWO21, containing the mutated Ub gene under transcriptional control of the Cu2+-inducible CUP1 promoter. The K294R substitution in K28-β (α/βK-0) was introduced by splicing by overlapping extension (SOE)-PCR (see above) by using the K28 pptox-encoding plasmid pPGK-M28-I as template (29) and oligonucleotides K294Rrev and E28 as primers. The resulting PCR fragment was cloned into the yeast expression vector pYX242 (Invitrogen). The lysine-free K28 variant αK-0/βK-0 and the pptox variant lacking any internal lysyl residue (pptoxK-0) were PCR amplified (all primers used throughout this study are shown in Table 1, which is published as supporting information on the PNAS web site).
Yeast Cell Fractionation.
For subcellular toxin-localization, either the K28-susceptible strain S. cerevisiae SEY6210 or the K28-secreting superkiller (ski2–2) mutant MS300b and its heat-cured nonkiller derivative (19) were grown at 30°C in yeast extract/peptone/dextrose medium to early exponential phase, harvested by centrifugation, and used for cell fractionation experiments essentially as described in ref. 14.
Purification of His-Tagged pptox.
Transformants expressing a His-tagged K28 pptox derivative lacking its N-terminal prepro sequence [Δtox(His)6] were grown at 30°C to early exponential phase in synthetic complete medium lacking uracil and then spheroplasted as described in ref. 11. Spheroplasts were subsequently incubated for 1 h in the presence of 104 units of either native or heat-inactivated (15 min, 60°C) K28 toxin·ml−1. After this incubation, cells were lysed (see above), cell debris and membranes were removed by centrifugation at 13,000 × g, and the resulting supernatant applied onto a TALONspin column that had been preequilibrated and purified according to the instructions of the manufacturer (Clontech). In brief, His-tagged proteins were allowed to bind to the matrix (5 min; 20°C), and the column resin was then washed three times (50 mM sodium phosphate and 300 mM sodium chloride, pH 7.0) before bound proteins were eluted by the addition of elution buffer (wash buffer containing 150 mM imidazole, pH 7.0). Eluted proteins were subsequently concentrated by ethanol-precipitation and used for Western blotting.
Immunoblot Analysis.
Protein samples were electrophoretically separated in Tris·tricine SDS polyacrylamide gels (37). After electrotransfer of the proteins to a poly(vinylidene difluoride) membrane, blots were incubated with a polyclonal antibody directed against the β subunit of K28 toxin (14). Alternatively, blots were probed with a monoclonal antibody directed against either a C-terminal His-tag (Invitrogen) or a polyclonal anti-Ub antibody (Sigma). Invertase (Suc2p) secretion was determined in cell-free culture supernatants of Suc2+ wild-type strains and the Δsuc2 mutant SEY6210, which had been included as negative control. In each case, aliquots of the cell-free culture supernatants were separated by SDS/PAGE and probed with polyclonal anti-Suc2p (kindly provided by Ludwig Lehle, Regensburg University, Regensburg, Germany).
Killer Assay and Immunity Tests.
K28-specific immunity was determined in an agar diffusion assay on methylene blue agar plates (MBA) (pH 4.7) as described in ref. 19. Toxin sensitivity/immunity tests were performed on MBA plates by using an overlay (105 cells per plate) of the toxin-sensitive strain S. cerevisiae SEY6210 carrying the indicated plasmid; a cell-free concentrated culture supernatant of a K28 killer strain was used as the toxin source. Briefly, concentrated culture supernatants (100 μl) were pipetted into wells (10-mm diameter) cut into the agar, and plates were then incubated for 4 days at 20°C. In this assay, yeast transformants expressing a pptox derivative that is incapable of conferring immunity show a sensitive nonkiller phenotype, resulting in a zone of growth inhibition around the well.
Supplementary Material
Acknowledgments
We thank Charles P. Cartwright for valuable comments and for critically reading the manuscript; Mark Hochstrasser (Yale University, New Haven, CT) and Dieter Wolf for providing various yeast mutants (Δdoa4, pre1–1, and pre2–2) and plasmid pWO21; Randy Schekman and Ludwig Lehle for kindly providing aliquots of polyclonal anti-Sec61p, anti-Kar2p, and anti-Suc2p antiserum; and Beate Schmitt and Nadine Laßotta for technical assistance. This work was kindly supported by Deutsche Forschungsgemeinschaft Grants Schm 541/11-2 and Graduiertenkolleg 845-1.
Abbreviations
- ER
endoplasmic reticulum
- pptox
preprotoxin
- ptox
protoxin
- Δtox
pptox variant lacking its N-terminal prepro sequence
- Ub
ubiquitin
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bevan E. A., Makower M. In: Genetics Today, XIth Int. Congr. Genet. Geerts S. J., editor. Vol. 1. Oxford: Pergamon; 1963. pp. 202–203. [Google Scholar]
- 2.Bevan E. A., Herring A. J., Mitchell D. J. Nature. 1973;245:81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- 3.Wickner R. B. Microbiol. Rev. 1996;60:250–265. doi: 10.1128/mr.60.1.250-265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostian K. A., Elliott Q., Bussey H., Burn V., Smith A., Tipper D. J. Cell. 1984;36:741–751. doi: 10.1016/0092-8674(84)90354-4. [DOI] [PubMed] [Google Scholar]
- 5.Riffer F., Eisfeld K., Breinig F., Schmitt M. J. Microbiology. 2002;148:1317–1328. doi: 10.1099/00221287-148-5-1317. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt M. J., Breinig F. FEMS Microbiol. Rev. 2002;26:257–276. doi: 10.1111/j.1574-6976.2002.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt M. J., Klavehn P., Wang J., Schonig I., Tipper D. J. Microbiology. 1996;142:2655–2662. doi: 10.1099/00221287-142-9-2655. [DOI] [PubMed] [Google Scholar]
- 8.Reiter J., Herker E., Madeo F., Schmitt M. J. J. Cell Biol. 2005;168:353–358. doi: 10.1083/jcb.200408071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussey H. Mol. Microbiol. 1991;5:2339–2343. doi: 10.1111/j.1365-2958.1991.tb02079.x. [DOI] [PubMed] [Google Scholar]
- 10.Tipper D. J., Schmitt M. J. Mol. Microbiol. 1991;5:2331–2338. doi: 10.1111/j.1365-2958.1991.tb02078.x. [DOI] [PubMed] [Google Scholar]
- 11.Breinig F., Tipper D. J., Schmitt M. J. Cell. 2002;108:395–405. doi: 10.1016/s0092-8674(02)00634-7. [DOI] [PubMed] [Google Scholar]
- 12.Martinac B., Zhu H., Kubalski A., Zhou X. L., Culbertson M., Bussey H., Kung C. Proc. Natl. Acad. Sci. USA. 1990;87:6228–6232. doi: 10.1073/pnas.87.16.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Pena P., Barros F., Gascon S., Lazo P. S., Ramos S. J. Biol. Chem. 1981;256:10420–10425. [PubMed] [Google Scholar]
- 14.Eisfeld K., Riffer F., Mentges J., Schmitt M. J. Mol. Microbiol. 2000;37:926–940. doi: 10.1046/j.1365-2958.2000.02063.x. [DOI] [PubMed] [Google Scholar]
- 15.Wickner R. B. Annu. Rev. Microbiol. 1992;46:347–375. doi: 10.1146/annurev.mi.46.100192.002023. [DOI] [PubMed] [Google Scholar]
- 16.Sesti F., Shih T. M., Nikolaeva N., Goldstein S. A. Cell. 2001;105:637–644. doi: 10.1016/s0092-8674(01)00376-2. [DOI] [PubMed] [Google Scholar]
- 17.Page N., Gerard-Vincent M., Menard P., Beaulieu M., Azuma M., Dijkgraaf G. J., Li H., Marcoux J., Nguyen T., Dowse T., Sdicu A. M., Bussey H. Genetics. 2003;163:875–894. doi: 10.1093/genetics/163.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertl A., Ramos J., Ludwig J., Lichtenberg-Frate H., Reid J., Bihler H., Calero F., Martinez P., Ljungdahl P. O. Mol. Microbiol. 2003;47:767–780. doi: 10.1046/j.1365-2958.2003.03335.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt M. J., Tipper D. J. Mol. Cell. Biol. 1990;10:4807–4815. doi: 10.1128/mcb.10.9.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindberg M. J., Normark J., Holmgren A., Oliveberg M. Proc. Natl. Acad. Sci. USA. 2004;101:15893–15898. doi: 10.1073/pnas.0403979101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirosh B., Furman M. H., Tortorella D., Ploegh H. L. J. Biol. Chem. 2003;278:6664–6672. doi: 10.1074/jbc.M210158200. [DOI] [PubMed] [Google Scholar]
- 22.Groll M., Heinemeyer W., Jager S., Ullrich T., Bochtler M., Wolf D. H., Huber R. Proc. Natl. Acad. Sci. USA. 1999;96:10976–10983. doi: 10.1073/pnas.96.20.10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker J., Walter W., Yan W., Craig E. A. Mol. Cell. Biol. 1996;16:4378–4386. doi: 10.1128/mcb.16.8.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. Nature. 1988;332:800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- 25.Bostian K. A., Jayachandran S., Tipper D. J. Cell. 1983;32:169–180. doi: 10.1016/0092-8674(83)90507-x. [DOI] [PubMed] [Google Scholar]
- 26.Boone C., Bussey H., Greene D., Thomas D. Y., Vernet T. Cell. 1986;46:105–113. doi: 10.1016/0092-8674(86)90864-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y. S., Kane J., Zhang X. Y., Zhang M., Tipper D. J. Yeast. 1993;9:251–266. doi: 10.1002/yea.320090305. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt M. J. Mol. Gen. Genet. 1995;246:236–246. doi: 10.1007/BF00294687. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt M. J., Tipper D. J. Virology. 1995;213:341–351. doi: 10.1006/viro.1995.0007. [DOI] [PubMed] [Google Scholar]
- 30.Brodsky J. L., Goeckeler J., Schekman R. Proc. Natl. Acad. Sci. USA. 1995;92:9643–9646. doi: 10.1073/pnas.92.21.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito H., Fukuda Y., Murata K., Kimura A. J. Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemeyer W., Gruhler A., Mohrle V., Mahe Y., Wolf D. H. J. Biol. Chem. 1993;268:5115–5120. [PubMed] [Google Scholar]
- 33.Amerik A. Y., Nowak J., Swaminathan S., Hochstrasser M. Mol. Biol. Cell. 2000;11:3365–3380. doi: 10.1091/mbc.11.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J., Fritsch E. F., Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 35.Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 36.Breinig F., Schmitt M. J. Appl. Microbiol. Biotechnol. 2002;58:637–644. doi: 10.1007/s00253-002-0939-2. [DOI] [PubMed] [Google Scholar]
- 37.Schagger H., von Jagow G. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.