Abstract
The insulin receptor (IR) and the type-1 insulin-like growth factor receptor (IGF1R) are homologous multidomain proteins that bind insulin and IGF with differing specificity. Here we report the crystal structure of the first three domains (L1–CR–L2) of human IR at 2.3 Å resolution and compare it with the previously determined structure of the corresponding fragment of IGF1R. The most important differences seen between the two receptors are in the two regions governing ligand specificity. The first is at the corner of the ligand-binding surface of the L1 domain, where the side chain of F39 in IR forms part of the ligand binding surface involving the second (central) β-sheet. This is very different to the location of its counterpart in IGF1R, S35, which is not involved in ligand binding. The second major difference is in the sixth module of the CR domain, where IR contains a larger loop that protrudes further into the ligand-binding pocket. This module, which governs IGF1-binding specificity, shows negligible sequence identity, significantly more α-helix, an additional disulfide bond, and opposite electrostatic potential compared to that of the IGF1R.
Keywords: crystal structure, ectodomain, insulin-binding site
The insulin receptor (IR), like the type-1 insulin-like growth factor receptor (IGF1R), is a member of the receptor tyrosine kinase family, and is a large, transmembrane, glycoprotein dimer consisting of several structural domains (1, 2). The N-terminal half of the ectodomain contains two leucine-rich repeat domains (L1 and L2) separated by a cys-rich region (CR) (1, 3). The C-terminal half of the IR ectodomain consists of three fibronectin type III domains, the second of which contains an insert region of ≈120 residues (1, 2).
Although there is no high-resolution structural information available for the IR ectodomain, the three-dimensional structure is known for the first three domains (L1–CR–L2) of the closely related IGF1R (4). This structure has provided a framework to interpret previous studies on receptor chimeras, site-specific mutants, and mutants from patients with defective receptors (see refs. 1 and 2) and has guided subsequent studies on the insulin-binding site using mutational analysis (5, 6). Three regions of the ectodomain are known to be involved in low-affinity binding by the soluble IR ectodomain. These are the L1 domain, the CR region and the last 16 residues of the α-chain (see refs. 1 and 7). Of these, only the first two (L1 and the CR) are important determinants of ligand specificity, because IR/IGF1R chimeras of whole receptors (8) or minireceptors (9) are little affected by swapping the regions that contained the last 16 residues of the α-chain.
The major determinants in L1 for insulin binding specificity lie in the first 68 residues of this domain (10, 11), based on the analysis of receptor chimeras. Twelve residues in this N-terminal segment have been further confirmed as part of the ligand-binding region by site-specific mutagenesis (see Table 1). Surprisingly, nine of these 12 residues are conserved in the IGF1R, the exceptions being Q34/H30 (IR/IGF1R numbering), M38/I34, and F39/S35 (Table 1), and one of these, F39, has been reported to be a major contributor to insulin specificity in IR (19). However, homology modeling of the IR L1 domain, based on the IGF1R structure, does not place F39 in the ligand-binding surface. The equivalent residue in IGF1R, S35, is at the C-terminal end of the second strand in the third β-sheet, which is at right angles to the second β-sheet (the major ligand-binding surface of the L1 domain of IGF1R) and thus outside the putative ligand-binding pocket lined by the L1, CR, and L2 domains (4). Interestingly, F39 was not investigated in subsequent studies of IR binding (6), presumably because the homology models based on the IGF1R structure suggested F39 was not part of the L1 ligand-binding surface.
Table 1.
Effect of L1 domain mutations on IR and IGF1R ligand-binding affinity
| IR relative affinity, % |
IGF1R relative affinity, % |
||||
|---|---|---|---|---|---|
| Mutation* | IR-A† | IR-B† | IR patients | Mutation | IGF1R |
| R14 | 0.1–0.5 | <1 | R10 | 70–142 | |
| N15 | 0.04–0.15 | <1 | N11 | 14–27 | |
| N15K | – | 20 | |||
| F64 | 0.04–0.15 | <1 | F58 | 28–33 | |
| R86P | – | – | <1 | – | – |
| I13 | 5–8 | – | 19 | 100 | |
| Q34 | 7.6–9 | 8 | H30 | 22 | |
| L37 | 5 | 5 | L33 | 17 | |
| F39 | 4–10 | – | S35 | 100 | |
| K121 | 9 | 10.5 | K115 | 300 | |
| D12 | 10–15 | – | D8 | 11–33 | |
| L36 | 10–14 | 14 | L32 | 77–100 | |
| L87 | 11 | 14 | L81 | 100 | |
| L871 | – | – | 400 | – | – |
| L87P | – | – | 15 | – | – |
| N90 | 11–17 | 15 | N84 | 67–300 | |
| E97 | 11 | 12 | E91 | 143 | |
| M38 | 28–33 | – | I34 | – | |
| E44 | 28–34 | – | E38 | 67 | |
| D59G | – | – | 25 | ||
| Y67 | 31–43 | – | Y61 | 77 | |
| F88 | 40 | 43 | F82 | 200 | |
| F89 | 20–22 | 17 | Y83 | 100 | |
| Y91 | 22–33 | 17 | Y85 | 77–300 | |
| E120 | 29 | 29 | E114 | 100 | |
| H32 | 100 | 100 | Y28 | 22 | |
| L62 | 100 | 100 | L56 | 20 | |
| R65 | 100 | 100 | R59 | 20 | |
| S85 | 100 | 100 | W79 | 33 | |
| F96 | – | – | F90 | 4.5 | |
*Mutations were to A except for the natural mutants. Residues that differ between IR and IGF1R are shown in bold. The data are grouped by affinity for IR-A. Data sources (5, 6, 12–18).
†The A- and B-isoforms differ by the absence or presence of a 12 amino acid peptide inserted after P716 near the C terminus of the mature α-chain. This region does not lie within the fragment presented here.
The structure of the L1–CR–L2 fragment from IR (IR485) reported here reveals that there is a major difference in the structures of the L1 domains of the IR and IGF1R in the region involving the second and third β-strands of the second leucine-rich repeat and indicates how F39 contributes to insulin specificity. The data also show that there is a major difference in the structure and electrostatics of the sixth module of the CR domain, the major determinant of IGF1-binding specificity (10, 20). The data in this report allow direct comparison of the regions controlling ligand specificity in these two closely related receptors.
Results and Discussion
Overall Structure.
The truncated IR485 comprises the L1, CR, and L2 domains (residues 1–469) plus the next 16 residues that include part of the first Fn III module. Like the L1–CR–L2 fragment of IGF1R (21), IR485 also does not bind ligand (data not shown) as reported by three other groups (22–24). Unlike IGF1R462, the IR485 crystals contain two receptor molecules in the unit cell, whose structures are very similar to each other but not identical. The comparative structures are shown in Fig. 1 and the amino acid sequences and secondary structural assignments in Fig. 2. Residues 470–485 at the C terminus of the fragment, immediately after the end of the L2 domain, are disordered in both copies of IR485 in the crystal. As shown in Fig. 1, the IR fragment adopts an extended bilobal structure (40 × 49 × 110 Å), very similar to that seen for the corresponding IGF1R462 fragment. N-linked carbohydrate can be seen at eight of the 10 potential sites in the L1–CR–L2 fragment (residues 16, 25, and 111, but not 78 in L1; 215 and 255 but not 295 in CR; 337, 397, and 418 in the L2). Some additional uninterpretable electron density was present in the vicinity of N295. This finding is consistent with chemical analyses which have shown that only N78 is not glycosylated (L. G. Sparrow, J. J. Gorman, P. M. Strike, C. P. Robinson, N.M.M, and C.W.W., unpublished data).
Fig. 1.
Comparison of the structures of the L1–CR–L2 domain fragments of IR (molecules 1 and 2) and IGF1R. Helices are indicated by curled red ribbons, and β-strands are indicated by broad arrows. The blue, green, and yellow β-strands depict the three prominent parallel β-sheets within the L1 and L2 domains. The β-strands in the cys-rich domains are colored orange. The side chains of disulfide-linked cysteine residues are depicted as yellow sticks. IGF1R structure is from ref. 4. Two notable regions of difference between IR and IGF1R are near IR F39 (box) and the larger loop in module 6 of the cys-rich domain (circle).
Fig. 2.
Structure-based sequence alignment of the L1–CR–L2 domains of human IR and IGF1R. Disulfide bond connections are shown as solid lines above the sequences, and the CR modules (M1–M7) are indicated with open bars (two disulfide bonds) or solid bars (one disulfide bond) below. Secondary structure elements are indicated above the sequences as cylinders for α-helices and arrows for β-strands (color-coding is the same as Fig. 1). Regions where the IR and IGF1R structures diverge significantly are shaded. Potential N-linked glycan sites are underlined, and tick marks indicate every tenth residue. Sequences are from refs. 25 (IR) and 26 (IGF1R).
The most notable differences in the overall structure are the relative orientations of the L2 domains, which are rotated 17° (molecule 1) and 32° (molecule 2) relative to the L1 domain of IGF1R462 and 32° between IR molecules 1 and 2 (Fig. 1). The majority of this movement occurs at residue 303 (293 in IGF1R), with a slight difference between modules 3 and 4. Although crystal packing appears responsible for the relative orientations observed here, the structures do reveal that the L2 domain is capable of global movement with respect to the L1-CR domains as suggested (4).
Domain Comparisons.
The largest differences seen in the individual domain comparisons of IR and IGF1R are in the two regions governing ligand specificity, the left side of the ligand-binding surface of the L1 domain as viewed in Fig. 3, and the sixth module of the CR domain (Figs. 1 and 4). The comparative backbone structures of the L1 domains of IR and IGF1R generally follow each other closely owing to their high (70%) sequence identity (rmsd for Cα atoms of IR-1 and IGF1R is 1.2 Å; and for IR-2 and IGF1R is 1.3 Å). There is a major difference between the structures of IR residues 39–48 and the equivalent sequence in IGF1R (residues 35–42) in the second leucine-rich repeat of the L1 domain (Fig. 3), with the largest Cα deviations being >4 Å. A striking feature is the disposition of the side chain of IR F39, which can be seen to contribute to the ligand-binding surface (second β-sheet) of L1. This finding is in contrast to the corresponding residue, S35, at the end of the third β-sheet of IGF1R, whose side chain extends in an orthogonal direction to that of IR F39 (Fig. 3) and is thus in a position that is unlikely to contribute to low-affinity ligand binding by the soluble ectodomain. As shown in Fig. 2, the IR sequence also has an insert of two amino acids in the region K40-P43 compared to IGF1R. F39, and to a lesser extent R42 and P43, have been shown to be the key residues responsible for the increased insulin binding of IR/IGF1R chimeras and this RP insert and the larger side chain at residue 39 are additive in increasing insulin affinity (19). The importance of F39 in insulin binding is further indicated by the data in Table 1. In the region that governs specificity (first 68 residues of L1), five of the seven most important residues for insulin binding to IR are conserved in the IGF1R and thus not responsible for this specificity difference. The two exceptions are Q34 and F39, the importance of the latter being confirmed by site-specific mutagenesis analyses (14).
Fig. 3.
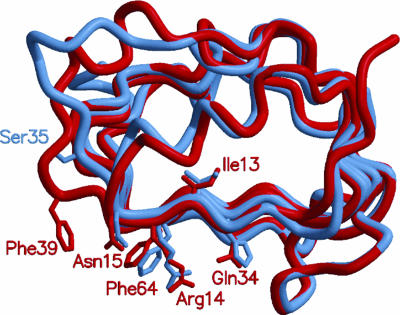
Comparison of main chain traces for the L1 domains of IR and IGF1R. Cα atoms from L1 domains of IR (red) and IGF1R (light blue) have been superposed and show the major backbone differences in the second half of the second leucine-rich repeat. The side chains of key IR residues involved in insulin binding (I13, R14, N15, Q34, F39, and F64) and the IGF1R equivalents are depicted as sticks with IGF1R S35 some distance from the homologous residue, F39, of IR.
Fig. 4.
Electrostatic potentials calculated for the L1 and CR domains of IR and IGF1R. Surface representation colored by electrostatic potential ranging in magnitude from −10 kT/e (dark red) to 10 kT/e (dark blue), where k is the Boltzmann constant, T is the temperature, and e the magnitude of the electron charge. The electrostatic potentials were calculated by using GRASP version 1.3.6 (28), placing partial charges on side chain terminal atoms of Asp, Glu, Arg, and Lys amino acids and all backbone atoms. (A) The ligand binding face of the L1 domain of IR viewed as in Fig. 5. (B) The L1–CR–L2 fragment of IR viewed from the side with the CR region at the back. The electrostatic potential of the CR region lining the putative ligand-binding pocket of IR is predominantly positive. (C) The ligand binding face of the L1 domain of IGF1R viewed as A. (D) The L1–CR–L2 fragment of IGF1R viewed from the side with the CR region at the back. The electrostatic potential of the CR region lining the putative ligand-binding pocket of IGF1R is predominantly negative.
Other differences in L1 involve the insertion of G4 at the start of the L1 domain in IGF1R (Figs. 1 and 2) causing P5 in that structure to extend further (Cα deviation of 2.7 Å) into the central ligand-binding cavity than does P9 in the IR-1 and IR-2 structures. The contribution, if any, of this difference to receptor binding specificity is not known. There is also a backbone deviation around N152 (4.1 Å) at the end of the L1 domain, a region of IR that also contains a single residue insertion compared to IGF1R (see Fig. 2).
The largest difference in the two structures is seen in the CR domain (sequence identity 47%), which governs IGF specificity (10, 20) through its interaction with the C- and D-domains of IGF (27). The backbones of the third, fourth, and fifth modules in IR-1 and IGF1R structures follow each other quite closely, whereas the backbones of the other modules deviate significantly. The largest difference is seen in module 6 in the region 260–276, corresponding to 253–265 in IGF1R (Fig. 2). Here, there is no sequence identity between the two receptors, and IR contains a four-residue insertion and an extra disulfide bond (Fig. 2). Although the size of the insertion is modest, the new sequence forms an α-helix that extends further into the putative binding pocket (see Fig. 1) with the structure being maintained by the additional disulfide bond.
In addition to these structural differences (Figs. 1 and 2), CR module 6 of IR shows very different electrostatic surface potential to IGF1R (Fig. 4), reflecting the abundance of positively charged residues in the IR sequence compared to the acidic residues in the corresponding region of IGF1R (Fig. 2). The electrostatic potential of the CR domain of IR is predominantly positive, whereas that of IGF1R is overwhelmingly negative. The electronegative characteristics of the CR domain of IGF1R are complementarity to the electropositive nature of the C- and D-domains of IGF1 (R36, R37 in C and K65, K68 in D). The CR domain is the region in the receptor known to be important in governing IGF-binding specificity (10, 20) and is the region shown to be particularly responsible for the recognition of IGF1 residues R36 and R37 (27). Furthermore, alanine substitution of R36 and R37 leads not only to a 15-fold loss in binding potency for the IGF1R, but also to a 29-fold increase in binding potency to the IR (27), consistent with the electrostatic differences discussed above.
In contrast to the differences seen between the L1 and CR domain structures in the two receptors, the L2 domains of IR and IGF1R are very similar. There are no insertions or deletions in the L2 domain of IR relative to IGF1R (Fig. 2), and the sequence identity for the L2 domains is high (65%). Consequently, the backbones of the L2 domains of IR-1 (0.9 Å) and IR-2 (0.8 Å) superimpose on the L2 domain of IGF1R better than do the L1 domain comparisons.
Ligand Binding Region of L1.
The central (second) β-sheet of the L1 domain is a major contributor to ligand binding, based on studies of naturally occurring receptor mutants and receptors subjected to alanine scanning mutagenesis (Table 1). Most of the mutant receptors from patients with defects in insulin binding (13, 18) have mutations in this face. Similarly, of the 47 single site and six double site IR alanine mutations, the 14 mutations with defects in insulin binding (14) were all in this central β-sheet. The location of these key residues on the L1-binding face of IR are shown in Fig. 5D.
Fig. 5.
The ligand binding face of the L1 domain of IR. (A) Cartoon showing the main chain trace and the location of key residues. The hydrophobic patch is indicated by the dashed trace when viewed in the same direction as B. (B) Stereo view of the L1 ligand-binding surface with the underlying Cα trace in dark gray and the side chain atoms in brown (carbon), blue (nitrogen), and red (oxygen). (C) View of the surface of insulin that interacts with the binding face of the L1 domain of IR, colored as in B and rotated 180° about a vertical axis relative to D. (D) Surface diagram of the L1 domain of IR. Residues are colored according to relative affinity of insulin for alanine substitutions; red, <5%; orange, 5–15%; yellow, 15–50%; gray, no expression (see Table 1). The potential location of bound insulin is depicted in white, with residues B1–B8 dotted, A1–A20, and B9–B21 as a thin trace and an arrow to indicate the approximate position of residues B22–B30. The view direction is the same direction as in B, and part of the Cys-rich domain trace is in purple. (E) Surface conservation based on sequence alignment of vertebrate insulin receptors. The most conserved surface region in the L1–CR–L2 fragment is the putative ligand-binding site on L1, with strictly conserved residues colored yellow. Thirteen sequences were used from mammals, birds, amphibians, and fish (NCBI accession nos. P06213, AAR04440, P15208, P15127, XP_542108, XP_418250, Q9PVZ4, XP_690534, XP_691069, BAB836677, BAB83668, CAG083667, and CAG08022). View as in B.
As illustrated in Fig. 5, the characteristic feature of this L1 face is a large hydrophobic patch with a cavity at the center, formed by Q34, L36, L62, F64, F88, F89, V94, F96, R118, and E120(Fig. 5B). Clearly, this cavity can bind hydrophobic residues, and two instances of this phenomenon have been observed. In the structure of IGF1R (4), two residues (L and I) from the c-myc purification tag sit in the L1 cavity on an adjacent molecule, and in this study, the side-chain of F89 in IR-2 is swung out and sits in the cavity of IR-1. The hydrophobic patch is ringed by hydrophilic amino acids and, notably, charged residues, some of which are particularly important for insulin binding (Fig. 5D).
This L1 surface is highly conserved in IRs and is the largest conserved patch of surface in the L1–CR–L2 fragment in vertebrates (Fig. 5E). The most important residues for insulin binding in both the A and B isoforms of human IR are R14, N15, and F64, which, when replaced by alanine, resulted in receptors with negligible insulin binding ability (Table 1). The next most important set of L1 residues are L37 (20-fold reduction), F39 (10- to 25-fold), and I13, Q34 and K121 (all 11- to 12-fold) followed by D12, L36, L87, E97, and N90 (6- to 9-fold reduction in affinity). Other residues F89, Y91, M38, E44, E120, Y67, and F88 showed reductions in affinity ranging from 3- to 5-fold (Table 1). The mutation L87I increased binding four-fold (29), whereas the F89 to L, I, S, P, W, or H mutations all abolished insulin binding (30). As shown in Table 1, the seven most important residues for insulin binding, I13, R14, N15, Q34, L37, F39, and F64, plus five of the less important ones (D12, L36, M38, E44, and Y67), occur in the first 68 residues of IR, the region found to confer insulin binding specificity in IR/IGF1R chimeras (10, 11, 19). The distribution of these residues over the central (second) β-sheet of the L1 domain of IR is shown in Fig. 5D and can be seen to be a subset of the highly conserved residues found in the vertebrate IRs (Fig. 5E).
Docking of Insulin on the L1 Domain.
A possible model for the L1 domain-insulin interaction is shown in Fig. 5D and is based on fitting a hydrophobic surface of insulin, the classical surface or site 1 (31) (Fig. 5C), onto the hydrophobic patch of the receptor described above. In the model presented here, we have represented insulin in the R-state (where the B-chain helix extends from B1 to B19), the more active conformation suggested to be adopted by insulin on binding to the receptor (32, 33). In addition, we have truncated the B-chain after residue E21, given the mobility of the B-chain C terminus back to residue G20 (34–36), the increased binding affinity of B-chain despenta-amide insulins (31), the lack of chiral specificity at residue B24 (37), and the low activity of the single-chain, B29–A1 peptide-linked insulin despite its “near native” crystal structure (38). Rotating the B-chain C-terminal peptide (B21–B30) away from its close contact with residues A1 and A2 exposes the hydrophobic surface comprised of A1–A3, A19–B20, B11–B12, B15, and B19 (31, 35, 36, 39).
From the shape and size of these hydrophobic surfaces on the L1 face and the insulin dimer surface and a potential interaction of residue A21 terminal carboxylate with IR R14, our favored orientation is with the B-chain helix running along the length of L1 from the bottom to the top, as viewed in Fig. 5D. The now-exposed A-chain N-terminal residues G1–V3 are placed up against residues F88 and F89, which are in the hydrophobic loop in the fourth LRR rung at the left hand edge of the L1 binding face (see Figs. 1, 2, and 5). The critical insulin residue Y19 in the A-chain (40, 41) could rotate into the top end of the central hydrophobic cavity. Other key residues used to position insulin in the model are the B-chain residues V12 and Y16, the contiguous residues from the B-chain helix, and F24 (40, 42–44). Photoactivatable derivatives of the B-chain residues Y16 and F24 have been shown to cross-link to the L1 domain of IR, whereas V12 could not be modified without destroying its receptor-binding ability (39, 43, 45). In this model, each of these three residues contacts the L1 binding face. The B-chain residue V12 is buried in the interface and sits directly over F64 and the lower end of R65, with F96 underneath and L37 above, whereas Y16 of insulin sits directly between L37 and F39, two of the residues that are highly sensitive to Ala substitution (Table 1). The residue equivalent to the B-chain Y16 in IGF is Q15, and it is interesting to note that swapping IGF1 residues Q15 and F16 with their counterparts Y16 and L17 from insulin’s B-chain, increased the IR binding of the mutant IGF1 10-fold but had no effect on its binding affinity for IGF1R (46).
Although it is not shown in the model, the mobile C-terminal tail of the insulin B-chain (residues 21–30) is envisaged to have moved away from the core of the ligand as indicated in Fig. 5D (see ref. 34). This movement places the B-chain F24 in a position where it can still contact the top rungs of the L1 binding face to which it can be cross-linked (39); it places B25 in an exposed position to interact with the IR α-chain C-terminal 16 aa to which it can be cross-linked (42) and has K29 extending over to the cleft between L1 and CR, a position consistent with the ability to label either of these receptor regions depending on the nature of the K29 side-chain derivative (reviewed in ref. 1). Finally, in the model described, the A-chain residues V3 and T8, derivatives of which, like F25, can be cross-linked to the 13 kDa C-terminal fragment of the IR α-chain, are positioned on the bottom left surface of insulin (Fig. 5E) in a semiexposed position but well away from F25. This finding supports the suggestion (36, 47) that the A-chain residues V3 and T8 may contact a region of the insert domain that is peripheral to the critical site involved in contacting the B-chain residue F25. Such disparate contact points by the IR insert domain are possible given that it appears to be intrinsically disordered based on predictions with the DisProt web server (48). Such an orientation of insulin on the L1 surface results in L17 (B-chain) and L13 (A-chain) (31) and other residues from the hexamer face, the so-called second binding region (7), being positioned where they can readily interact with other parts of the receptor dimer and induce IR signaling. The hydrophobic patch on IR is ringed with charged residues as is the hydrophobic patch on insulin. A number of these hydrophilic residues, E4 and N21 (A-chain) and E13 (B-chain), on the periphery of the insulin hydrophobic patch could form favorable interactions with R118, Q34, and R65 on the IR L1-binding surface and the A chain N terminus could salt link to E120.
In the model described above for insulin binding, hydrophobic interactions contribute a major portion of the ligand-receptor binding energy. However, as discussed earlier with IGF1 binding to the IGF1R, electrostatic complementarity between the electropositive C-domain and the electronegative sixth module of the CR domain would have an additional orientational effect for binding in the IGF1/IGF1R complex. Binding of insulin to the insulin receptor appears to be less electrostatically driven.
Conclusion
Here, we have described atomic resolution details of the ligand-binding surface of the human insulin receptor and compared it with the corresponding surface of IGF1R (4). We report some key differences that are not readily discerned from homology models based on the IGF1R structure and provide detailed descriptions of the regions in the two receptors responsible for ligand specificity. Finally, we present a preliminary model for the way in which insulin might bind the L1–CR–L2 domain fragment. Our orientation for insulin differs substantially from that proposed by Yip and Ottensmeyer (49) based on a three-dimensional reconstruction of ≈700 images selected from scanning transmission electron micrographs. High-resolution structural data are needed to resolve these different models, establish the structural role for the C-terminal region of the α-chain (residues 704–715) in ligand binding, and describe the ligand–receptor interactions that generate the high-affinity complex.
Materials and Methods
Construction of the IR485 Expression Vector, Transfection, Protein Production and Purification.
The construction of the expression plasmid pEE14/IR485 with its c-myc tag, its transfection into Lec8 mutant CHO cells, the production of IR485 in a Celligen Plus bioreactor (New Brunswick Scientific, Edison, NJ), the recovery of soluble IR485 by affinity chromatography on Mab 9E10 columns followed by gel filtration on Superdex 200 HR (Amersham Pharmacia, Uppsala, Sweden), and subsequent chromatography using Uno QP anion exchange column are described in the Supporting Materials and Methods, which is published as supporting information on the PNAS web site, as is the analysis of IR485 by isoelectric focusing polyacrylamide gel electrophoresis and its subsequent treatment with endoproteinase Asp-N (Boehringer, Mannheim, Germany) to yield a stable isoform (the most basic isoform, pI ≈ 7.0) lacking the c-myc tail. As has been reported (22–24), the IR485 fragment did not bind insulin (data not shown).
Crystallization and Data Collection.
Crystallization trials were performed with a factorial screen (50) using the hanging drop method. Initially, small rod-shaped crystals grew within 4–5 days, which diffracted to ≈4 Å. Further crystallization trials using seeding led to the production of large-sized crystals (0.7 mm × 0.1 mm × 0.1 mm) that diffracted to 3.3 Å in the laboratory and 2.3 Å at the synchrotron. The best crystallization conditions were 1.5–1.65 M (NH4)2SO4, 2% PEG 400, pH 8.5. Crystals were cryo-cooled to −170°C in 20% PEG, 20% glycerol. Diffraction data to 3.31 Å resolution were initially recorded as 130 1° exposures on a Rigaku RAXIS IV area detector using RU-300 Rigaku (Tokyo, Japan) generator (Cu Kα radiation) equipped with ellipsoidal glass capillary optics (AXCO, Parkville, Victoria, Australia). Data were integrated and scaled by using DENZO/SCALEPACK (51) giving mosaic spread 0.37°, Rsym = 0.211, 〈I/σI〉 = 5.3, multiplicity 4.6, completeness 99.9%. A second set of diffraction data were recorded to 2.3-Å resolution using a Mar 345 detector at the Advanced Photon Source (APS) beamline 14-BM-D (wavelength 1.037 Å) and integrated in a similar manner, with 116 0.75° exposures, mosaic spread 0.27°, Rsym = 0.072, 〈I/σI〉 = 12.0, multiplicity 3.4, completeness 98%. The space group is P212121 with unit cell dimensions a = 103.86 Å, b = 130.24 Å, c = 160.92 Å. From the apparent molecular mass of 68 kDa per monomer, there could be two, three, or four molecules per asymmetric unit, with a solvent content of 69%, 54%, or 38%, respectively.
Structure Solution and Refinement.
The structure was solved by molecular replacement with AMORE using data (8–4 Å resolution) from the initial data set and the structure of IGF1R 1–300 (L1–CR) and 301–459 (L2) as search models. For L1–CR, solutions corresponded to the two highest peaks from translation functions. Using these solutions, one solution was found for L2 and a second L2 domain was placed by inspection from electron density maps. Four rigid bodies were refined with X-Plor (52), giving R = 0.462 for data 10–4 Å resolution. Despite the low predicted protein content of the crystals (31%, VM = 4.0), electron density was only observed for two molecules of IR485 per asymmetric unit. With data from the APS, structure refinement proceeded with rounds of manual refitting with O (53), alternated with energy minimization, B factor refinement, and sometimes simulated annealing using X-Plor, CNS (54), and REFMAC (55). The resolution was extended in a stepwise manner, and a bulk solvent correction and overall anisotropic thermal parameters were applied. The final model contains 931 aa, 40 carbohydrate residues, one PEG 400 molecule, and 262 solvent molecules, giving R = 0.194, Rfree = 0.235 for data 40–2.317 Å and deviations from ideal geometry of 0.017 Å (bonds) and 1.81° (angles). For residues 1–3 of both molecules and 270–273 from IR-2, the electron density is unclear and there is no density for residues beyond residues 468 in IR-1or 469 in IR-2. Atomic coordinates and structure factors have been deposited in the Protein Data Bank (PDB ID code 2HR7).
Calculation of Electrostatic Potential.
The electrostatic potential was calculated and mapped on to the molecular surface of each of the structures IGF1R and IR-1 by using PARSE charges (56) for each atom, and performed with the GRASP version 1.6 program (28), which computes the electrostatic potentials in a continuum representation of the electrostatics by numerically solving the finite difference Poisson–Boltzmann equation.
Docking of Insulin on the L1 Domain.
Crosslinking, mutational, and structural studies imply that as much as 30% of insulin’s main chain undergoes substantial structural rearrangements upon binding to the receptor. Therefore, a modified insulin model was docked onto the insulin receptor fragment manually by using steric and electrostatic constraints, together with the experimental observations (see Discussion). Coordinates for insulin were based on PDB:4INS with residues B1–B9 from PDB:7INS. The only additional modifications were minor rotations of residues A21 and B1, and change in rotamer for B19 to place it in the receptor’s hydrophobic pocket. For the receptor, the only change made was a rotation of the F64 side chain into the hydrophobic pocket with a concomitant rotation of the F96 phenyl group. Further small modifications could be made to side chain conformation to marginally improve the fit, but this was beyond the scope of this work. The estimated error is ±2 Å and ±10°. For the docked insulin, the total buried accessible surface area is 965 Å2 for insulin and 960 Å2 for the receptor.
Combining this docking with the putative assignment of domains in the EM reconstruction of whole IR dimers (49), insulin would present a second hydrophobic face (site 2, centered on leucines A16 and B11) toward the hydrophobic patch of the dimer-related L1 domain. Insulin’s site 2 is concave in the R state, and this cleft could be occupied, potentially, by the side chains of F88 and F89 of the other receptor. However, there is no direct evidence for this contact, because mutation studies would be confounded by interactions at the classical binding site (site 1).
Supplementary Material
Acknowledgments
We thank the Australian Synchrotron Research Program and the BioCars sector at the Advanced Photon Source (Chicago, IL) for access to synchrotron radiation, Mr. Albert van Donkelaar and Ms. Kim Jachno for valuable contributions, and Dr. P. M. Colman for support and discussions. Financial support was provided under the generic Technology component of the Industry Research and Development Act 1986, from Biota Diabetes Research Pty Ltd., Commonwealth Scientific and Industrial Research Organization, and the National Health and Medical Research Council of Australia.
Glossary
Abbreviations
- IR
insulin receptor
- IGF1R
type-I insulin-like growth factor receptor
- CR
cys-rich.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2HR7).
References
- 1.Adams T. E., Epa V. C., Garrett T. P. J., Ward C. W. Cell. Mol. Life Sci. 2000;57:1050–1093. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Meyts P., Whittaker J. Nat. Rev. Drug. Discov. 2002;1:769–783. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- 3.Ward C. W., Garrett T. P. J. BMC Bioinformatics. 2001;2:4. doi: 10.1186/1471-2105-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrett T. P. J., McKern N. M., Lou M., Frenkel M. J., Bentley J. D., Lovrecz G. O., Elleman T. C., Cosgrove L. J., Ward C. W. Nature. 1998;394:395–399. doi: 10.1038/28668. [DOI] [PubMed] [Google Scholar]
- 5.Whittaker J., Groth A. V., Mynarcik D. C., Pluzek L., Gadsboll V. L., Whittaker L. J. J. Biol. Chem. 2001;276:43980–43986. doi: 10.1074/jbc.M102863200. [DOI] [PubMed] [Google Scholar]
- 6.Whittaker J., Sorensen H., Gadsboll V., Hinrichsen J. J. Biol. Chem. 2002;277:47380–47384. doi: 10.1074/jbc.M208371200. [DOI] [PubMed] [Google Scholar]
- 7.De Meyts P. BioEssays. 2004;26:1351–1362. doi: 10.1002/bies.20151. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher R., Soos M. A., Schlessinger J., Brandenburg D., Siddle K., Ullrich A. J. Biol. Chem. 1993;268:1087–1094. [PubMed] [Google Scholar]
- 9.Kristensen C., Kjeldsen T., Wiberg F. C., Schaffer L., Hach M., Havelund S., Bass J., Steiner D. F., Andersen A. S. J. Biol. Chem. 1997;272:12978–12983. doi: 10.1074/jbc.272.20.12978. [DOI] [PubMed] [Google Scholar]
- 10.Kjeldsen T., Andersen A. S., Wiberg F. C., Rasmussen J. S., Schaffer L., Balschmidt P., Moller K. B., Moller N. P. Proc. Natl. Acad. Sci. USA. 1991;88:4404–4408. doi: 10.1073/pnas.88.10.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen A. S., Kjeldsen T., Wiberg F. C., Vissing H., Schaffer L., Rasmussen J. S., De-Meyts P., Moller N. P. H. J. Biol. Chem. 1992;267:13681–13686. [PubMed] [Google Scholar]
- 12.Grønskov K., Vissing H., Shymko R. M., Tornqvist H, De Meyts P. Biochem. Biophys. Res. Commun. 1993;192:905–911. doi: 10.1006/bbrc.1993.1501. [DOI] [PubMed] [Google Scholar]
- 13.Taylor S. I., Wertheimer E., Accili D., Cama A., Hone J., Roach P., Quon M. J., Suzuki Y., Levy-Toledano R., Taouis M., et al. Endocrine Rev. 1994;2:58–65. [Google Scholar]
- 14.Williams P. F., Mynarcik D. C., Yu G. Q., Whittaker J. J. Biol. Chem. 1995;270:3012–3016. doi: 10.1074/jbc.270.7.3012. [DOI] [PubMed] [Google Scholar]
- 15.Mynarcik D. C., Yu G. Q., Whittaker J. J. Biol. Chem. 1996;271:2439–2442. doi: 10.1074/jbc.271.5.2439. [DOI] [PubMed] [Google Scholar]
- 16.Mynarcik D. C., Williams P. F., Schaffer L., Yu G. Q., Whittaker J. J. Biol. Chem. 1997;272:2077–2081. doi: 10.1074/jbc.272.4.2077. [DOI] [PubMed] [Google Scholar]
- 17.Mynarcik D. C., Williams P. F., Schaffer L., Yu G. Q., Whittaker J. J. Biol. Chem. 1997;272:18650–18655. doi: 10.1074/jbc.272.30.18650. [DOI] [PubMed] [Google Scholar]
- 18.Rouard M., Bass J., Grigorescu F., Garrett T. P. J., Ward C. W., Lipkind G., Jaffiole C., Steiner D. F., Bell G. I. J. Biol. Chem. 1999;274:18487–18491. doi: 10.1074/jbc.274.26.18487. [DOI] [PubMed] [Google Scholar]
- 19.Kjeldsen T., Wiberg F. C., Andersen A. S. J. Biol. Chem. 1994;269:32942–32946. [PubMed] [Google Scholar]
- 20.Hoyne P. A., Elleman T. C., Adams T. E., Richards K. M., Ward C. W. FEBS Lett. 2000;469:57–60. doi: 10.1016/s0014-5793(00)01237-0. [DOI] [PubMed] [Google Scholar]
- 21.McKern N. M., Lou M., Frenkel M. J., Verkuylen A., Bentley J. D., Lovrecz G. O., Ivancic N., Elleman T. C., Garrett T. P., Cosgrove L. J., Ward C. W. Protein Sci. 1997;6:2663–2666. doi: 10.1002/pro.5560061223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaefer E. M., Siddle K., Ellis L. J. Biol. Chem. 1990;265:13248–13253. [PubMed] [Google Scholar]
- 23.Molina L., Marino-buslje C., Quinn D. R., Siddle K. FEBS Lett. 2000;467:226–230. doi: 10.1016/s0014-5793(00)01161-3. [DOI] [PubMed] [Google Scholar]
- 24.Kristensen C., Andersen A. S., Ostergaard S., Hansen P. H., Brandt J. J. Biol. Chem. 2002;277:18340–18345. doi: 10.1074/jbc.M112249200. [DOI] [PubMed] [Google Scholar]
- 25.Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M., et al. Nature. 1985;313:756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- 26.Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E., et al. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang W., Gustafson T. A., Rutter W. J., Johnson J. D. J. Biol. Chem. 1994;269:10609–10613. [PubMed] [Google Scholar]
- 28.Nicholls A., Sharp K., Honig B. Proteins Struct. Funct. Genet. 1991;11:281–295. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 29.Nakae J., Morioka H., Ohtsuka E., Fujieda K. J. Biol. Chem. 1995;270:22017–22022. doi: 10.1074/jbc.270.37.22017. [DOI] [PubMed] [Google Scholar]
- 30.De Meyts P., Gu J.-L., Shymko R. M., Kaplan B. E., Bell G. I., Whittaker J. Mol. Endocrinol. 1990;4:409–416. doi: 10.1210/mend-4-3-409. [DOI] [PubMed] [Google Scholar]
- 31.Schaffer L. Eur. J. Biochem. 1994;221:1127–1132. doi: 10.1111/j.1432-1033.1994.tb18833.x. [DOI] [PubMed] [Google Scholar]
- 32.Derewenda U., Derewenda Z., Dodson E. J., Dodson G. G., Reynolds C. D., Smith G. D., Sparks C., Swenson D. Nature. 1989;338:594–596. doi: 10.1038/338594a0. [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa S. H., Zhao M., Hua Q. X., Hu S. Q., Wan Z. L., Jia W., Weiss M. A. Biochemistry. 2005;44:4984–4999. doi: 10.1021/bi048025o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua Q. X., Shoelson S. E., Kochoyan M., Weiss M. A. Nature. 1991;354:238–241. doi: 10.1038/354238a0. [DOI] [PubMed] [Google Scholar]
- 35.Ludvigsen S., Olsen H. B., Kaarsholm N. C. J. Mol. Biol. 1998;279:1–7. doi: 10.1006/jmbi.1998.1801. [DOI] [PubMed] [Google Scholar]
- 36.Wan Z. L., Huang K., Xu B., Hu S. Q., Wang S., Chu Y. C., Katsoyannis P. G., Weiss M. A. Biochemistry. 2005;44:5000–5016. doi: 10.1021/bi047585k. [DOI] [PubMed] [Google Scholar]
- 37.Mirmira R. G., Tager H. S. J. Biol. Chem. 1989;264:6349–6354. [PubMed] [Google Scholar]
- 38.Derewenda U., Derewenda Z., Dodson E. J., Dodson G. G., Bing X., Markussen J. J. Mol. Biol. 1991;220:425–433. doi: 10.1016/0022-2836(91)90022-x. [DOI] [PubMed] [Google Scholar]
- 39.Xu B., Hu S. Q., Chu Y. C., Huang K., Nakagawa S. H., Whittaker J., Katsoyannis P. G., Weiss M. A. Biochemistry. 2004;43:8356–8372. doi: 10.1021/bi0497796. [DOI] [PubMed] [Google Scholar]
- 40.Pullen R. A., Lindsay D. G., Wood S. P., Tickle I. J., Blundell T. L., Wollmer A., Krail G., Brandenburg D., Zahn H., Gliemann J., Gammeltoft S. Nature. 1976;259:369–373. doi: 10.1038/259369a0. [DOI] [PubMed] [Google Scholar]
- 41.Kristensen C., Wiberg F. C., Andersen A. S. J. Biol. Chem. 1999;274:37351–37356. doi: 10.1074/jbc.274.52.37351. [DOI] [PubMed] [Google Scholar]
- 42.Kurose T., Pashmforoush M., Yoshima Y., Carroll R., Schwartz G. P., Burke G. T., Katsoyannis P. G., Steiner D. F. J. Biol. Chem. 1994;269:29190–29197. [PubMed] [Google Scholar]
- 43.Huang K., Xu B., Hu S. Q., Chu Y. C., Hua Q. X., Qu Y., Li B., Wang S., Wang R. Y., Nakagawa S. H., et al. J. Mol. Biol. 2004;341:529–550. doi: 10.1016/j.jmb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Xu B., Hua Q., Nakagawa S. H., Jia W., Chu Y.-C., Katsoyannis P. G., Weiss M. A. J. Mol. Biol. 2002;316:435–441. doi: 10.1006/jmbi.2001.5377. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa S. H., Tager H. S., Sterner D. S. Biochemistry. 2000;39:15826–15835. doi: 10.1021/bi001802+. [DOI] [PubMed] [Google Scholar]
- 46.Bayne M. L., Applebaum J., Chicchi G. G., Hayes N. S., Green B. G., Cascieri M. A. J. Biol. Chem. 1988;263:6233–6239. [PubMed] [Google Scholar]
- 47.Wan Z., Xu B., Huang K., Chu Y. C., Li B., Nakagawa S. H., Qu Y., Hu S. Q., Katsoyannis P. G., Weiss M. A. Biochemistry. 2004;43:16119–16133. doi: 10.1021/bi048223f. [DOI] [PubMed] [Google Scholar]
- 48.Peng K., Vucetic S., Radivojac P., Brown C. J., Dunker A. K., Obradovic Z. J. Bioinformatics Comput. Biol. 2005;3:35–60. doi: 10.1142/s0219720005000886. [DOI] [PubMed] [Google Scholar]
- 49.Yip C. C., Ottensmeyer P. J. Biol. Chem. 2003;278:27329–27332. doi: 10.1074/jbc.R300021200. [DOI] [PubMed] [Google Scholar]
- 50.Jancarik J., Kim S.-H. J. Appl. Crystallogr. 1991;24:409–411. [Google Scholar]
- 51.Otwinowski Z., Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 52.Brunger A. T. X-PLOR Reference Manual 3.851. New Haven, CT: Yale Univ.; 1996. [Google Scholar]
- 53.Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 54.Brunger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., et al. Acta Crystallogr. D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 55.Murshudov G. N., Vagin A. A., Dodson E. J. Acta Crystallogr. D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 56.Sitkoff D., Lockhart D. J., Sharp K. A., Honig B. Biophys. J. 1994;67:2251–2260. doi: 10.1016/S0006-3495(94)80709-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.