Abstract
Grape (Vitis vinifera) heterotrophic suspension-cultured cells were used as a model system to study glucose (Glc) transport and its regulation. Cells transported d-[14C]Glc according to simple Michaelis-Menten kinetics superimposed on first-order kinetics. The saturating component is a high-affinity, broad-specificity H+-dependent transport system (Km = 0.05 mm). Glc concentration in the medium tightly regulated the transcription of VvHT1 (Vitis vinifera hexose transporter 1), a monosaccharide transporter previously characterized in grape berry, as well as VvHT1 protein amount and monosaccharide transport activity. All the remaining putative monosaccharide transporters identified so far in grape were poorly expressed and responded weakly to Glc. VvHT1 transcription was strongly repressed by Glc and 2-deoxy-d-Glc, but not by 3-O-methyl-d-Glc or Glc plus mannoheptulose, indicating the involvement of a hexokinase-dependent repression. 3-O-Methyl-d-Glc, which cannot be phosphorylated, and Glc plus mannoheptulose induced a decrease of transport activity caused by the reduction of VvHT1 protein in the plasma membrane without affecting VvHT1 transcript levels. This demonstrates hexokinase-independent posttranscriptional regulation. High Glc down-regulated VvHT1 transcription and Glc uptake, whereas low Glc increased those parameters. Present data provide an example showing control of plant sugar transporters by their own substrate both at transcriptional and posttranscriptional levels. VvHT1 protein has an important role in the massive import of monosaccharides into mesocarp cells of young grape berries because it was localized in plasma membranes of the early developing fruit. Protein amount decreased abruptly throughout fruit development as sugar content increases, consistent with the regulating role of Glc on VvHT1 expression found in suspension-cultured cells.
Phloem transport of assimilates provides the materials needed for the buildup of herbaceous plants and trees and has long been recognized as a major determinant in crop yield. Indeed, 80% of the carbon photosynthetically fixed in the leaf is exported through the plant vascular system to the roots, reproductive structures, and storage and developing organs, depending on the supply of sugars for their growth and development (Chiou and Bush, 1998; Williams et al., 2000). Past improvements in yield potential have resulted more from an increase in the proportion of accumulated carbon in the harvestable organs than from genetic increases in photosynthesis (Gifford et al., 1984). It is therefore important to understand the mechanisms and regulations of sugar transport into sink tissues.
In sink organs, Suc from the phloem can be imported from the apoplast via direct Suc transporters (DSTs). Alternatively, it can be hydrolyzed to Glc and Fru by cell wall-bound invertases and taken up via monosaccharide transporters (MSTs; for review, see Williams et al., 2000). Many MSTs and DSTs have been characterized from a molecular and functional standpoint in herbaceous plants, but much less has been done on the sugar transporters from lignous species. A Glc transporter from grape (Vitis vinifera; Fillion et al., 1999; Vignault et al., 2005), a polyol transporter from sour cherry (Prunus avium; Gao et al., 2003), and sorbitol transporters from apple (Malus domestica) leaves (Watari et al., 2004) assumed to function as proton-sugar transporters were recently cloned and expressed in yeast (Saccharomyces cerevisiae).
Expression of DSTs and MSTs may be affected by various parameters, including light, water and ion status, wounding, fungal and bacterial attacks, and hormones, and they are generally not expressed in the same tissues or at the same developmental stages (Kühn et al., 1997). In the model plant Arabidopsis (Arabidopsis thaliana), AtSTP1 is expressed in leaves and other organs, including stems, flowers, and roots (Sauer et al., 1990), suggesting redundant functions, whereas AtSTP4 is sink specific (Truernit et al., 1996) and AtSTP2 is expressed in developing pollen (Truernit et al., 1999). The mechanisms of these regulations are still poorly understood (Delrot et al., 2000). Light and diurnal rhythm may control the expression and activity of some transporters either directly as physical signals and/or because they affect the sugar content of the cell. This control can occur at the level of gene expression or it may affect mRNA and protein turnover (Kühn et al., 1997). Expression of LeSUT1 in tomato (Lycopersicon esculentum; Kühn et al., 1997) and of DcSUT1 in carrot (Daucus carota; required for Suc loading into the phloem) is affected by light. On the contrary, DcSUC2 expression is not diurnally regulated in the storage root (Shakya and Sturm, 1998). Expression of AtSTP1 is repressed by light and under the control of diurnal rhythm in guard cells of Arabidopsis (Stadler et al., 2003), and AtSTP4 is regulated in response to environmental factors, such as wounding or pathogen infection (Truernit et al., 1996).
Besides their role as carbon and energy sources, sugars synthesized during the light phase can act as regulatory signals affecting gene expression. The ability to sense altered sugar concentrations is important in the context of resource allocation, allowing the plant to tailor its metabolism in source tissues to face the demands in sinks. Because sugar transporters play such a key role in source-sink interactions, it is likely that their expression and activity are tightly regulated by sugar levels (Roitsch, 1999). However, the mechanisms underlying regulation by sugars in plants are not as well understood as in yeast and there is some discrepancy about the effect of sugars on the control of sugar transporters. The Suc transporter BvSUT1 is repressed by Suc (Chiou and Bush, 1998; Vaughn et al., 2002; Ransom-Hodgkins et al., 2003), whereas OsSUT1 is up-regulated by Suc (Matsukura et al., 2000). Down-regulation of monosaccharide transport was also observed in suspension-cultured cells of olive (Olea europaea; Oliveira et al., 2002). By contrast, MST genes are constitutively expressed in Chenopodium rubrum and not regulated by sugar (Roitsch and Tanner, 1994). The expression of the grape VvHT1 MST has been reported to be induced by Suc and palatinose in grape cell suspensions (Atanassova et al., 2003). Sorbitol uptake by peach (Prunus persica) tree buds is inhibited by Glc via a hexokinase (HXK)-dependent pathway, but the steps (transcription, translation, targeting, and activity) affected by this process were not investigated in detail (Maurel et al., 2004). The effect (induction or repression) of sugars on transporter genes may depend on the concentration of the sensed sugar. This is the case for VfSUT1, a Suc transporter expressed in broad bean (Vicia faba) cotyledons (Weber et al., 1997) and for yeast hexose transporter gene expression (Rolland et al., 2001). Eventually, sugars may affect sugar transport not only at the transcriptional level, but also by acting on mRNA stability and protein biosynthesis and activity, as documented in yeast (Boles and Hollenberg, 1997).
The mechanisms and regulation of monosaccharide transport have not been characterized so far in grapevine. Most insight has been given into the cloning of grape hexose transporter genes VvHT. VvHT1 (AJ001061; Fillion et al., 1999) was characterized as a MST by heterologous expression in tobacco (Nicotiana tabacum; Leterrier et al., 2003) and yeast (Vignault et al., 2005). In developing berries, VvHT1 transcript amount is high shortly after fruit set and then decreases until véraison. Although preliminary studies by reverse transcription-PCR reported that VvHT1 expression slightly increases after véraison in Ugni-Blanc berries (Fillion et al., 1999), detailed microarray analysis suggested that the second peak of expression does not occur in berries from Chardonnay, Shiraz, and Cabernet Sauvignon varieties (Terrier et al., 2005). Expression of the VvHT1 promoter-reporter gene construct in tobacco cells showed that VvHT1 expression is enhanced by sugars (Atanassova et al., 2003). A grape abscisic acid, stress, ripening-induced protein was isolated by means of a one-hybrid approach using as a target a small fragment of the VvHT1 promoter containing two sugar-responsive elements. This abscisic acid, stress, ripening-induced protein may be part of the transcriptional complex mediating the sugar-inducible expression of VvHT1 (Cakir et al., 2003).
In this article, we report that in grape cell suspensions VvHT1 operates as a high-affinity, broad-specificity monosaccharide-proton cotransport system repressed by Glc. Accordingly, in grape berries, VvHT1 protein was detected only in the early stages of fruit development when sugar is almost absent. High Glc repression of VvHT1 involves both a HXK-dependent decrease in the VvHT1 transcript level and a HXK-independent decrease in the VvHT1 protein amount in the plasma membrane.
RESULTS
Growth in Batch Cultures with Suc
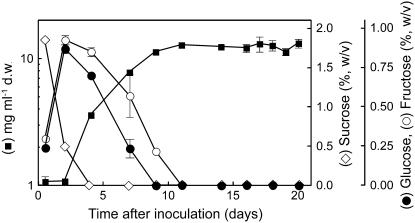
In this work, heterotrophic suspension-cultured cells obtained from grape were used as a model system to study monosaccharide transport into sink cells. Cells were cultivated in liquid mineral medium with Suc as the sole carbon and energy source. Extracellular Suc was completely hydrolyzed within 4 d and growth occurred along with Glc and Fru consumption (Fig. 1). The maximal specific growth rate (μmax) was 0.27 d−1. Prior to reaching the maximal population size, sugar deficiency caused a restriction in the specific growth rate and growth arrest occurred after monosaccharide depletion. This led us to characterize in more detail the hexose transport system and its regulation by Glc in this experimental model.
Figure 1.
Dry weight and sugar concentration in grape suspension-cultured cells grown with an initial concentration of 2% Suc.
Monosaccharide Transport
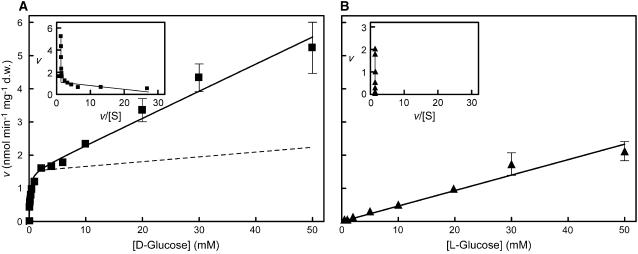
Transport studies were performed with grape suspension-cultured cells grown with 2% Suc (initial concentration) and collected 10 to 12 d after subculture when the monosaccharides resulting from Suc hydrolysis had declined to residual levels (see Fig. 1). The method used to measure Glc transport in olive suspension-cultured cells (Oliveira et al., 2002) proved to be suitable for grape cells and uptake of d-[14C]Glc was linear up to 180 s (data not shown). Most of the transport experiments were done at an external pH value of 5.0 to impose a transmembrane proton gradient. The initial uptake rates of d-[14C]Glc over a concentration range of 0.02 to 50 mm are shown in Figure 2A. The Eadie-Hofstee plot was biphasic, suggesting the involvement of two transport modes for Glc. Computer-assisted nonlinear regression analysis (GraphPad Prism software) showed Michaelis-Menten kinetics superimposed on first-order kinetics. For the saturable phase of transport, the kinetic parameters were Km = 0.05 ± 0.15 mm Glc and Vmax = 1.45 ± 0.04 nmol Glc min−1 mg−1 dry weight; for the diffusion-like component, kd = 0.08 μL min−1 mg−1 dry weight. When the uptake of the Glc analog l-[14C]Glc was measured over the same concentration range, only the linear, diffusion-like component was apparent (Fig. 2B). Carrier-mediated transport was calculated as the difference between d- and l-Glc uptake (Fig. 2A, dotted line) and the values for the kinetic parameters did not differ much from those estimated by GraphPad software: Km = 0.045 mm Glc and Vmax = 1.39 nmol Glc min−1 mg−1 dry weight.
Figure 2.
Glc transport by grape suspension-cultured cells cultivated with 2% Suc as in Figure 1. Initial uptake rates of d-[14C]Glc (A) and l-[14C]Glc (B) at pH 5.0 by cells collected at the end of the exponential growth phase when total sugar concentration had fallen to around 0.1%. Dotted line represents the difference between d-[14C]Glc and l-[14C]Glc uptake. Insets, Eadie-Hofstee plots of the initial Glc uptake rates.
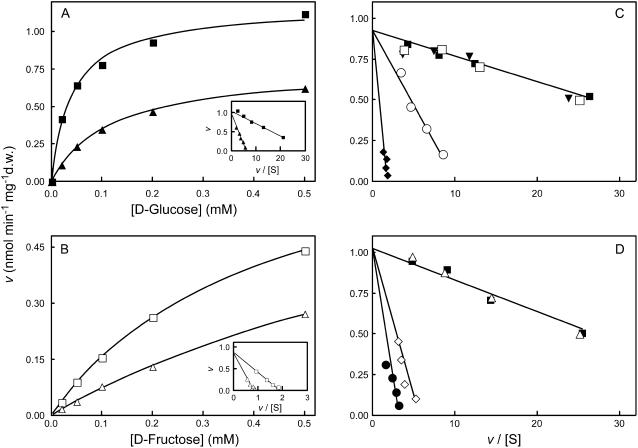
The competitive kinetics of Glc uptake were made in the presence of various unlabeled sugars to test the specificity of the transport system. Figure 3A shows the initial uptake rates of d-[14C]Glc in the presence or absence of 20 mm unlabeled d-Fru. Eadie-Hofstee plots indicated that d-Fru behaves as a competitive inhibitor, although high concentrations had to be used to achieve significant inhibition of d-[14C]Glc uptake (400-fold the Km value for Glc). When initial uptake rates of 0.02 to 0.5 mm d-[14C]Fru were measured, Michaelis-Menten kinetics were also obtained (Fig. 3B), suggesting carrier-mediated transport. The kinetic parameters were Km = 0.5 mm Fru and Vmax = 1.43 nmol Fru min−1 mg−1 dry weight. This Vmax value was similar to that estimated for Glc uptake, suggesting the involvement of the same transport system for both substrates. In addition, a clear inhibition of d-[14C]Fru uptake was obtained with a low concentration (1 mm) of unlabeled Glc.
Figure 3.
Specificity of the grape monosaccharide transport system. A, Initial uptake rates of d-[14C]Glc in the absence (▪) and in the presence (▴) of 20 mm Fru. B, Initial uptake rates of d-[14C]Fru in the absence (□) and in the presence (▵) of 1 mm Glc. Insets, Eadie-Hofstee plots of the initial uptake rates of d-[14C]Glc and d-[14C]Fru. C, Eadie-Hofstee plots of the initial uptake rates of d-[14C]Glc in the absence of other sugars (▪) and in the presence of 5 mm Xyl (○), 5 mm Gal (♦), 5 mm mannitol (□), and 5 mm Ara (▾), or in the presence (D) of the following Glc analogs: 8 mm l-Glc (▵), 0.5 mm 2-dG (⋄), and 0.5 mm 3-O-MG (•). Transport was measured at pH 5.0 with cells cultivated with 2% Suc, as in Figure 1, and collected at the end of the exponential growth phase when total sugar concentration had fallen to around 0.1%, as in Figure 2.
d-Gal and d-Xyl appear to be transported by the same monosaccharide transport system as Glc and Fru (Fig. 3C). The disaccharide Suc (data not shown), the polyol d-mannitol, and the pentose d-Ara did not affect d-[14C]Glc transport, thus appearing not to be recognized. The effects of the d-Glc analogs 2-deoxy-d-Glc (2-dG), 3-O-methyl-d-Glc (3-O-MG), and l-Glc on d-[14C]Glc transport are shown in Figure 3D. 2-dG and 3-O-MG also competitively inhibited d-[14C]Glc uptake, meaning that they are also recognized as substrates by the monosaccharide transport system. As expected, l-Glc did not inhibit d-[14C]Glc transport.
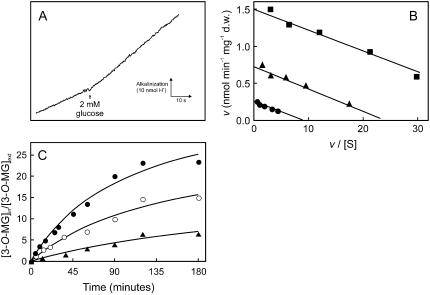
Measurements of Glc-induced proton fluxes in grape cell suspensions were made to test directly the hypothesis of a proton-Glc cotransport mechanism. A transient alkalinization of the extracellular medium occurred upon addition of 2 mm d-Glc (final concentration), suggesting that Glc uptake is dependent on the proton gradient (Fig. 4A). The initial velocity of proton uptake was 1.5 nmol H+ min−1 mg−1 dry weight, similar to the maximal capacity of the carrier-mediated d-Glc transport. Initial velocities of proton uptake induced by the addition of 2 mm d-Glc decreased by 75% from pH 4.5 to 5.5 (data not shown). The protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) inhibited Glc transport up to 80%, consistent with the hypothesis that a H+-dependent active transport system was involved (Fig. 4B). Because proton-sugar cotransport mechanisms are associated with a net influx of positive charges into the cells, the effect of dissipating the transmembrane electric potential on Glc uptake was also studied. Addition of the lipophilic and highly permeant cation tetraphenylphosphonium (TPP+) markedly decreased the initial uptake rates of 0.02 to 0.5 mm d-[14C]Glc (Fig. 4B). The nonmetabolizable Glc analog 3-O-MG was used to study the cumulative capacity of the transport system. The analog was concentrated in the intracellular compartment about 25- and 15-fold at pH 5.0 and 7.0, respectively, and CCCP prevented this accumulation (Fig. 4C).
Figure 4.
Energetics of the grape monosaccharide transport system. A, Proton uptake, at pH 5.0, associated with the addition of 2 mm Glc to a weakly buffered cell suspension. B, Eadie-Hofstee plots of the initial uptake rates of d-[14C]Glc, at pH 5.0, in the absence (▪) or in the presence of 0.05 mm CCCP (•) and 10 mm TPP+ (▴). C, Accumulation of labeled 3-O-MG at pH 7.0 (○) and pH 5.0 in the absence (•) and in the presence (▴) of 0.05 mm CCCP. Initial extracellular concentration of labeled 3-O-MG is 0.1 mm. Cells were cultivated with 2% Suc as in Figure 1 and collected at the end of the exponential growth phase when total sugar concentration had fallen to around 0.1% as in Figure 2.
Glc Regulation of MSTs
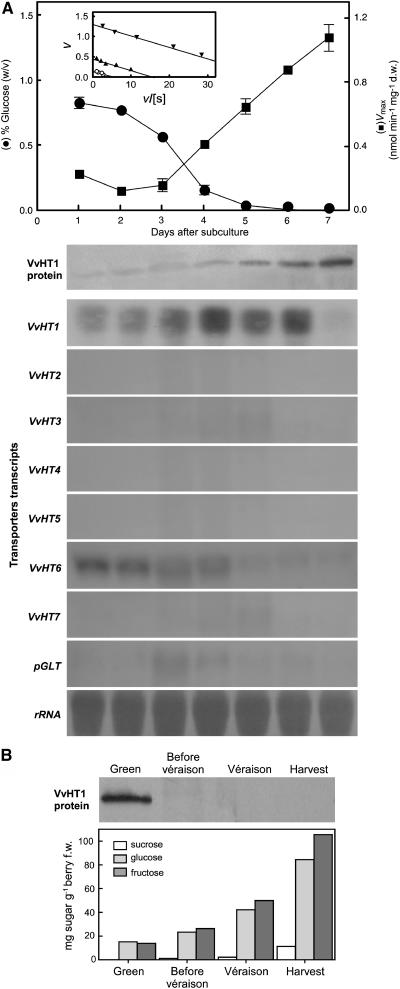
Several plasma membrane MST homologs (VvHTs) and a plastidic Glc transporter (pGLT) have been cloned from grape berries. Specific probes were designed in the 3′ noncoding sequences of VvHT1 (accession no. AJ001061), VvHT2 (AY663846), VvHT3 (AY538259), VvHT4 (AY538260), VvHT5 (AY538261), VvHT6 (AY861386), VvHT7 (AY854146), and pGLT (AY608701) and used to test the corresponding transcript amounts. Monosaccharide transport activity and expression patterns of those transporters were studied with cells grown in mineral medium in the presence of 1% Glc (Fig. 5). The most intense signal on RNA blots was observed after hybridization with VvHT1, with little expression of VvHT2, VvHT3, VvHT4, VvHT5, VvHT7, and pGLT. The amounts of VvHT1 transcripts reached a maximal level at day 4 when the [Glc]medium declined below 10 mm, and a high transcription was maintained up to day 6. VvHT6 transcript amounts constantly decreased after subculture. Transport activity increased abruptly from basal levels at day 4, reaching maximal activity 7 d after subculture. The amount of VvHT1 protein in the plasma membrane was monitored with a polyclonal antibody directed against the C-terminal part of VvHT1 (Vignault et al., 2005). Plasma membranes were purified by a discontinuous Suc gradient from a microsomal fraction prepared from grape cell suspensions collected at various times throughout the culture. The data (Fig. 5A) indicate that the amount of VvHT1 protein present in the plasma membrane began to increase strongly after day 4. The strong expression of VvHT1 and the correlation of VvHT1 protein with Glc uptake suggest a major contribution of this transporter in uptake. VvHT1 transcript levels decreased at day 7 probably due to a too-long Glc starvation period, whereas both transporter protein and uptake activity remain high. Altogether, these results suggest that VvHT1 expression and monosaccharide transport activity are regulated by Glc levels in the culture medium. To check whether these data may be extended to intact plant tissue, VvHT1 protein amount in plasma membrane of berry mesocarp through maturation was investigated. The amount of the MST decreased abruptly throughout fruit development as sugar content increased, consistent with the repressing role of Glc on VvHT1 expression (Fig. 5B).
Figure 5.
A, Activity of the monosaccharide transport system (Vmax), western-blot analysis of VvHT1 levels, and northern analysis of VvHT and pGLT genes in grape suspension-cultured cells along with sugar depletion. Inset, Eadie-Hofstee plots of the initial uptake rates of d-[14C]Glc, at pH 5.0, in cell aliquots harvested from the culture at day 1 (○), day 4 (▴), and day 7 (▾). B, Sugar content and western-blot analysis of VvHT1 levels in the plasma membrane of berry flesh cells at different stages of grape berry development.
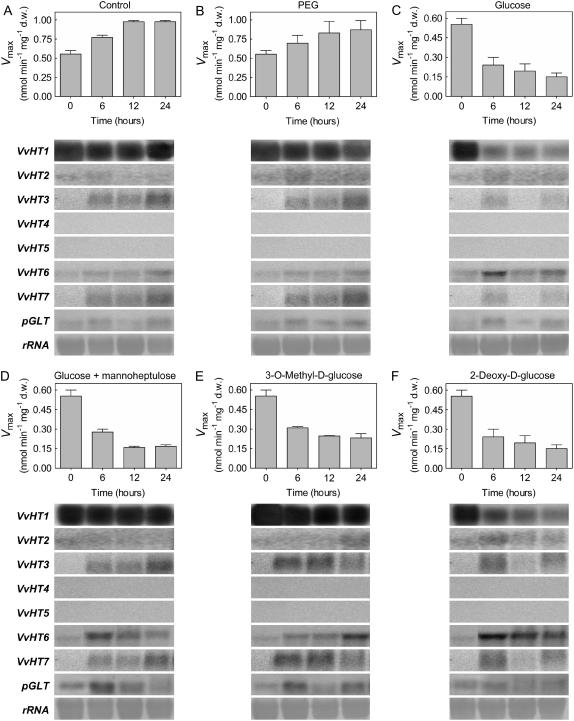
To test this hypothesis more directly, 150 mm Glc was added to cells collected at day 5 when they displayed high transport activity and high levels of VvHT1 transcripts together with low levels of expression of VvHT2 to VvHT7 and pGLT. VvHT1 was by far the most strongly expressed transporter at time 0. Addition of Glc induced a rapid decrease of VvHT1 transcripts, and transport activity reached basal levels within 12 h (Fig. 6C). In the control cells, VvHT1 transcripts were maintained at a high level and an increase of Glc transport activity was observed (Fig. 6A). Similarly, after the addition of the same concentration of polyethylene glycol, Glc uptake activity increased and high VvHT1 transcription was maintained, demonstrating that Glc repression is not due to an osmotic effect (Fig. 6B). Although there was a slight expression of some other VvHT homologs in all treatments, they were not repressed by sugar addition and did not prevent the decrease of Glc transport activity induced by Glc. Thus, although it cannot be excluded that some of these transporters contribute to the residual Glc uptake measured after Glc addition, most of the Glc uptake measured in the cells is associated with VvHT1 expression and is strongly inhibited by Glc.
Figure 6.
Repression of the grape monosaccharide transport system by different sugars. d-[14C]Glc uptake (Vmax) and VvHT homolog transcripts were measured in cell aliquots at time periods indicated after the addition of 150 mm polyethylene glycol, 150 mm Glc, 150 mm Glc plus 10 mm MHL, 150 mm 3-O-MG, and 150 mm 2-dG to cultures at day 5 in the conditions described in Figure 5.
To test the possible involvement of the HXK-signaling pathway in Glc-mediated VvHT1 repression, various additions were made to the cells: (1) 150 mm d-Glc in the presence of the HXK inhibitor mannoheptulose (MHL); (2) 3-O-MG, a Glc analog that cannot be phosphorylated; and (3) 2-dG, an analog that can be phosphorylated, but is not further metabolized. Blockage of HXK activity in cells treated with 10 mm MHL resulted in derepressed levels of VvHT1 transcripts (Fig. 6D). Similarly, high levels of VvHT1 transcripts were maintained in cells treated with 150 mm 3-O-MG (Fig. 6E). However, in both situations, transport activity decreased to basal levels, suggesting that VvHT1 expression may be regulated posttranscriptionally. The addition of 150 mm 2-dG strongly decreased the amount of VvHT1 transcripts, as well as transport activity (Fig. 6F). Taken together, these results strongly support the involvement of a HXK-mediated signal responsible for Glc repression of VvHT1 transcription.
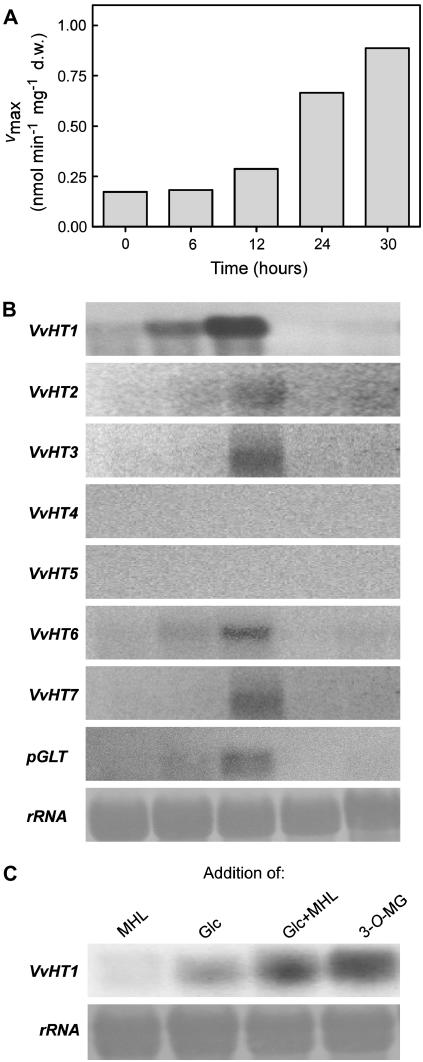
Because VvHT1 appeared to be transcriptionally repressed upon Glc addition, repressing conditions should be relieved by Glc exhaustion in the medium. To test this assumption, 3-d-old cells grown with 2% Glc were collected when total sugar concentration was >1.5% and transferred to sugar-free medium. d-[14C]Glc uptake and expression of MST genes were followed at selected times after transfer. Glc depletion promoted a 4-fold increase in Glc uptake activity within 30 h (Fig. 7A) associated with a transient induction of VvHT1 transcription, with the strongest signal being reached 12 h after sugar removal. Transcript levels of VvHT2, VvHT3, VvHT6, VvHT7, and pGLT were also detected 12 h after sugar starvation, although in much lower amounts than VvHT1 transcripts (Fig. 7B). The prolonged absence of Glc in the medium resulted in the complete disappearance of all VvHT transcripts 24 h after sugar removal. This is in agreement with the absence of VvHT transcription at day 7 after subculture (Fig. 5A).
Figure 7.
Activity of the monosaccharide transport system (Vmax; A) and VvHT homolog transcription (B) in sugar-starved suspension-cultured cells. Cells were grown with 2% Glc and transferred at day 3 to the same medium without sugar. C, VvHT1 transcription 12 h after addition of 10 mm MHL, 150 mm Glc, 150 mm Glc plus 10 mm MHL, and 150 mm 3-O-MG to cells cultivated during 30 h in the absence of sugar as indicated in A.
Although high Glc concentrations repress VvHT1 expression, minimal Glc amounts are required for induction of VvHT1 transcription. To strengthen the idea that Glc also functions as a positive signal for VvHT1 induction, Glc was added to Glc-starved cells 30 h after Glc removal according to the conditions of Figure 7A. Figure 7C shows that 12 h after Glc addition, VvHT1 transcripts increased to levels observed in Glc-repressing conditions (see Fig. 5A up to day 3; Fig. 6C upon Glc addition). In addition, in cells treated with either Glc plus MHL or 3-O-MG, a stronger accumulation of VvHT1 transcripts was observed possibly as a result of HXK impairment in mediating the Glc repression signal, similar to the data of Figure 6, D and E.
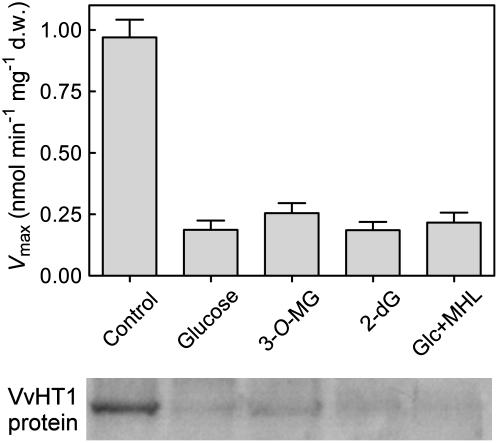
Comparing the data of Figure 6C, when Glc addition promoted a decrease of transport activity and VvHT1 transcription, with those of Figure 6, D and E, when high VvHT1 message levels were maintained although a drop of transport activity was observed, suggests that posttranscriptional mechanisms can be operating. These mechanisms would lead to the inactivation of transporter proteins or a decrease of protein abundance in the membrane. To test this hypothesis, the amount of VvHT1 protein in the plasma membrane was studied in cells collected 24 h after addition of either Glc, 3-O-MG, 2-dG, or Glc plus MHL, as described in Figure 6. The western blot showed that a decrease in transport activity was always correlated with a reduction in the abundance of VvHT1 protein (Fig. 8), even in those cases where the transcript level was not affected.
Figure 8.
Activity of the monosaccharide transport system (Vmax) and VvHT1 protein amount in the plasma membrane. Cells were collected 24 h after Glc, 3-O-MG, 2-dG, and Glc plus MHL had been added to cell suspensions, according to conditions described in Figure 6.
DISCUSSION
Relatively little is known about the regulation of sugar transport activity in plants. Suc transporters may be transcriptionally repressed by phosphorylation-dependent mechanisms (Vaughn et al., 2002; Ransom-Hodgkins et al., 2003) and indirect evidence suggests that Suc transporters may be regulated by phosphorylation (Roblin et al., 1998). The AtSTP4 MST may be induced by fungal and bacterial elicitors (Truernit et al., 1996; Fotopoulos et al., 2003) and the nonhost pathogen Botrytis cinerea enhances Glc transport in Pinus pinaster suspension-cultured cells depending on NADPH oxidase and calcium influx, but not mitogen-activated protein kinase (Azevedo et al., 2006). In contrast, the fungal elicitor cryptogein blocks monosaccharide transport in tobacco cells through a calcium-dependent process (Bourque et al., 2002). Although VvHT1 transcription has been shown to be induced by sugars in grape cell suspensions, the presence in its promoter region of both positive (inducing) and negative (repressing) sugar response cis-elements (Fillion et al., 1999; Atanassova et al., 2003) may suggest that it is under more complex control by sugars. This work provides an extensive description of the hexose transport system operating in grape cell suspensions and investigates in detail the mechanisms and signaling pathways involved in the control of hexose transporters by Glc. A wealth of information is already available on plant sugar transporters in terms of gene structure and expression, whereas much less is known about the regulation of transport activity in relation to gene expression. The problem is that plant organs are often not accessible to such studies. The use of suspension-cultured cells to study sugar uptake and its regulatory mechanisms offers a number of distinct advantages over the intact plant where bulk diffusion, tissue penetration barriers, and cell heterogeneity impair kinetic studies. Additionally, in cell suspensions, the plasma membrane is readily amenable for challenging with exogenous sugars, analogs, and transport inhibitors. Despite the necessary cautions needed to extrapolate the results to a multicellular level, they provide a convenient experimental system that has already yielded a lot of useful information on sugar transport mechanisms and regulation (Roitsch and Tanner, 1994; Ehness and Roitsch, 1997; Oliveira et al., 2002; Cakir et al., 2003), as well as in other key physiological processes, such as cell cycle (Riou-Khamlichi et al., 1999), hormonal signaling (Cakir et al., 2003), and regulation of gene expression (Graham et al., 1994; Cheng et al., 1999). Furthermore, in this work, we demonstrated that when young berries start to accumulate monosaccharides the amounts of VvHT1 protein (Fig. 5B) strongly decrease in accordance with previous results on VvHT1 transcription (Terrier et al., 2005), indicating that the regulations described in grape cell suspensions are physiologically relevant.
Characterization of the Hexose Transport System in Grape Cell Suspensions
Heterotrophic suspension-cultured cells of grape are able to take up d-Glc by a high-affinity saturable component superimposed on a nonsaturating component (Fig. 2). The saturable component involves a proton-Glc transport mechanism as indicated by the following observations: (1) Glc addition to weakly buffered cell suspensions is associated with a transient alkalinization of the extracellular medium (Fig. 4A); (2) the Vmax of proton uptake is similar to the Vmax of carrier-mediated d-Glc uptake, suggesting a stoichiometry of 1 H+ to 1 Glc, and depends on extracellular pH; (3) the analog 3-O-MG is concentrated intracellularly and the accumulation ratio is higher at low pH (Fig. 4B); (4) dissipation of the proton-motive force by CCCP significantly inhibits initial velocities of d-Glc uptake and 3-O-MG accumulation (Fig. 4, B and C); and (5) d-Glc transport is sensitive to TPP+, suggesting that ΔΨ is an important component of proton-motive force involved in Glc accumulation. Grape cultured cells are able to accumulate the nonmetabolizable Glc analog 3-O-MG up to 25-fold. The Nernst-Plank equation may be used to calculate the transmembrane Glc gradient maintained by a proton-Glc symporter with a stoichiometry of 1 H+ to 1 Glc. An accumulation factor between 700 and 2,000 would be expected at 25°C and pHext = 5.0, assuming an intracellular pH of 7.0 and ΔΨ of −50 to −120 mV. The accumulation ratio observed is thus somewhat lower than that theoretically derived from this equation. However, given that the cytosolic volume only represents about 10% of the cell volume, the estimated accumulation ratio is probably much closer to the theoretical value.
The high affinity (Km = 50 μm Glc) measured for the H+-dependent monosaccharide transport may be important for cell growth in media where the sugar supply rapidly becomes limiting. Similar Km values were measured for monosaccharide uptake in suspension-cultured cells of tobacco (Verstappen et al., 1991), carrot (Krook et al., 2000), and olive (Oliveira et al., 2002), in guard cell protoplasts of pea (Pisum sativum; Ritte et al., 1999), and in yeast expressing MSTs from lower and higher plants (Büttner and Sauer, 2000). The Km measured here is also in good agreement with the one measured by heterologous expression of VvHT1 in yeast (Vignault et al., 2005). The monosaccharide transport system of grape exhibited broad specificity given that d-[14C]Glc uptake is competitively inhibited by d-Fru, d-Gal, and d-Xyl, and by the Glc analogs 2-dG and 3-O-MG (Fig. 3). d-Glc and d-Fru are both recognized as substrates by the same transport system, although the affinity for d-Glc is much higher than for d-Fru. The lower affinity for d-Fru than for d-Glc (Fig. 3) may explain that d-Glc is consumed before Fru after Suc hydrolysis (Fig. 1). The higher affinity of the monosaccharide transport system for Glc than for Fru seems a general property also found in suspension-cultured cells of bean (Phaseolus vulgaris; Botha and O'Kennedy, 1998), carrot (Krook et al., 2000), and olive (Oliveira et al., 2002).
Altogether, these results indicate that the grape H+-dependent monosaccharide transport system exhibits properties of the MST family members, which transport a range of hexoses and pentoses with Km values for the preferred substrate around 10 to 100 μm (Büttner and Sauer, 2000). The involvement of high-affinity carrier-mediated transport is adequate for the growth of cells in media containing low sugar levels. However, at extracellular sugar content from 2 to 50 mm, carrier-mediated transport is saturated, contributing only in a small proportion to overall sugar uptake (Fig. 2). Therefore, nonsaturable mechanisms involved in diffusion-like uptake may play important roles in sugar absorption by grape suspension-cultured cells growing at high external sugar levels. Whether the same conclusion may apply to the cells of grape berry in vivo needs further investigation, but may be possible due to the high sugar content of this organ (Coombe, 1992) and in sink tissues in general (Patrick, 1997). Although nonsaturable mechanisms of sugar transport were also reported in other plant cells and tissues (Delrot, 1989; Krook et al., 2000; Oliveira et al., 2002), the underlying mechanisms are still poorly understood. Several mechanisms or a combination of them could account for diffusion-like kinetics: (1) nonspecific permeation of the sugar by free diffusion across the plasma membrane; (2) involvement of carriers with very low affinity; (3) involvement of carriers with Glc channels; and (4) occurrence of a sugar-inducible endocytic process (Etxeberria et al., 2005).
Several lines of evidence indicate that VvHT1 is responsible for the observed monosaccharide/H+ symporter activity in grape cell suspensions, including (1) its higher expression compared to the other transporters tested; (2) the similar properties (Km = substrate specificity) described here for grape cell suspensions and for yeast expressing VvHT1 as the sole MST (Vignault et al., 2005); and (3) the parallel between the amount of VvHT1 transcript and/or protein and the induction (Fig. 5) or repression (Fig. 6) of Glc uptake according to the monosaccharide concentration in the medium. However, the observation that there was a slight expression of some other VvHT homologs so far identified in the Vitis genome suggests that VvHT1 may not be the unique hexose transporter contributing to overall Glc uptake. Some of these transporters, such as VvHT3, VvHT6, and/or VvHT7 could contribute to the residual Glc uptake observed in Glc repression conditions, possibly as a result of different regulation mechanisms and/or kinetic parameters. Indeed, for a given nutrient, multiple transport systems can coexist within a single cell to assure uptake over a broad range of substrate concentrations (Ludewig and Frommer, 2002). In addition, the participation of a yet-unknown Vitis Glc transporter cannot be completely ruled out. Thirteen clusters were recognized in the MST superfamily, with 66 and 22 putative MSTs in the Arabidopsis and rice (Oryza sativa) genomes, respectively (Lalonde et al., 2004). The reason for the apparent redundancy is not clear, but fine tuning is probably involved.
Monosaccharide Transport Activity in Grape Cell Suspension Is Induced or Repressed by Glc Depending on Its Concentration in the Medium
Both Suc and Glc are known to play distinct roles in sugar signaling (Lalonde et al., 1999; Wiese et al., 2004). Our data highlight the dual aspect of VvHT1 expression in response to Glc signals. Indeed, depending on its concentration, Glc may induce or repress VvHT1 transcription. It induces VvHT1 in cells experiencing sugar starvation, but it represses VvHT1 in cells that have been exposed to high sugar levels. Accordingly, we show that monosaccharide uptake is low when the sugar concentration of the medium is high (Figs. 5 and 6) and is induced when this concentration is low. In this regard, higher plant cells resemble the green unicellular algae Chlorella kessleri (Komor et al., 1972). This complex regulation may be one of the reasons why contradictory conclusions have been reached on the regulation of sugar transporters by sugars (see introduction). Furthermore, although other transporters are expressed to some extent in these cells, VvHT1 is the most sensitive to the Glc content of the medium, which led us to investigate the mechanisms of its regulation.
The sharp increase in VvHT1 expression following transfer to Glc-free medium (Fig. 7B) may result from either a simple derepression of transcription or the cumulative effect of derepression and specific induction of transcription by Glc starvation. RNA-blot data support the first hypothesis because, in the prolonged absence of sugar, VvHT1 transcripts were virtually absent (Figs. 5A and 7B). Thus, the simple relief of Glc repression is insufficient for constant high-level VvHT1 expression and a minimal amount of Glc is required for transcriptional activation of the transporter. Indeed, addition of Glc to Glc-starved cells promoted an increase of VvHT1 transcript levels (Fig. 7C).
Such a dual effect of Glc has also been described for the hexose transporters HXT2 of yeast (Wendell and Bisson, 1994) and KHT2 of Kluyveromyces lactis (Milkowski et al., 2001). During cotyledon development, fava bean Suc transporter VfSUT1 expression was also shown to be under dual regulation by sugars (Weber et al., 1997). The dual aspect of VvHT1 Glc regulation may result from the coexistence of several positive sugar-responsive cis-elements (SURE1 and Suc box 3) and one AMYBOX1 and two AMYBOX2 sugar repression motifs (Fillion et al., 1999; Atanassova et al., 2003).
VvHT1 Expression Is Transcriptionally Repressed by High Glc Concentration via a HXK-Signaling Pathway
Down-regulation of VvHT1 expression by Glc was further substantiated by the inhibition observed when several monosaccharides were added to cells exhibiting high Glc transport activity and high VvHT1 transcript levels (Fig. 6). Kinetic analysis of transport activity showed a decrease in Vmax 6 h after addition of 150 mm Glc, reaching a basal level within 12 h. This sugar concentration, which was also used in previous studies on sugar regulation of Suc transporters (Williams et al., 2000; Vaughn et al., 2002) is lower than that usually employed in studies on sugar sensing (up to 7% Glc, i.e. up to 400 mm [Gibson, 2004]) and may be found in sink tissues (Patrick, 1997). Decreased Vmax activity is consistent with less VvHT1 protein in the membrane, although other forms of down-regulation, such as protein modification, cannot be ruled out. Decreased levels of symporter protein in the membrane may result either from increased rates of protein turnover and/or from decreased protein synthesis. RNA gel-blot analysis revealed a decrease in VvHT1 transcript levels, suggesting a drop in transcriptional activity or mRNA stability (Fig. 6). Thus, Glc repression of monosaccharide uptake in grape cell suspensions is at least in part mediated by transcriptional regulation.
Many examples of sugar-induced changes in gene expression have been described (Koch, 2004), but examples of sugar transporter regulation by sugars are rare (Chiou and Bush, 1998; Atanassova et al., 2003; Maurel et al., 2004) and the underlying mechanisms are poorly known. Both HXK-dependent and -independent signaling pathways have been involved in sugar sensing in plants. To investigate the role of HXK in Glc-induced VvHT1 repression, we studied the effects of Glc analogs and of MHL, a competitive inhibitor of HXK. The results showed that Glc-induced repression of VvHT1 is reversed by the addition of the HXK inhibitor MHL (Fig. 6). Furthermore, the nonphosphorylable Glc analog 3-O-MG did not repress VvHT1, whereas 2-dG, which can be phosphorylated and slowly metabolized, decreased the amounts of VvHT1 transcripts. Although 3-O-MG may be phosphorylated by HXK in maize (Zea mays; Cortes et al., 2003), the phosphorylation efficiency was 5 orders of magnitude less than for Glc and Man. Our results show that Glc-induced repression of VvHT1 requires HXK activity, but apparently no further metabolism, which is typical for a HXK-mediated signaling pathway.
The observation that VvHT1 can be transcribed under conditions in which Glc uptake is weak (i.e. in the presence of 3-O-MG) indicates that it may be regulated at a posttranscriptional level. This prompted us to study the amounts of VvHT1 protein under various experimental conditions. Immunoblot analysis showed that VvHT1 is also repressed at a posttranscriptional level in response to high Glc concentrations. Indeed, the decrease of Glc transport activity upon addition of Glc, 2-dG, 3-O-MG, and Glc plus MHL was accompanied by a decrease of VvHT1 protein in the plasma membrane, regardless the levels of VvHT1 transcripts, showing that steady-state protein levels and mRNA for VvHT1 do not always correspond. The same was found under sugar depletion conditions (Fig. 5A, day 7), probably due to a longer half-life of the transporter protein than the corresponding mRNA. A control step affecting either protein translation and/or turnover is most likely involved in the Glc transport reduction induced by 3-O-MG and Glc in the presence of MHL. Although the control of a sugar transporter by its own substrate at both transcriptional and posttranscriptional levels has been described in yeast (Wendell and Bisson, 1994; Boles and Hollenberg, 1997), these data illustrate that the same applies in plants. In the case of the sugar beet (Beta vulgaris) leaf proton-Suc symporter BvSUT1, immunoblot analysis showed that decreased transport activity was caused by a reduction in the abundance of symporter protein (Vaughn et al., 2002) and RNA gel-blot analysis of the leaf symporter revealed that message levels also declined as a result of decreased transcription, demonstrating a clear connection between steady-state mRNA levels and protein amounts. However, other proteins, such as maize invertase (Cheng et al., 1999) and the ATB2/AtbZIP11 transcription factor of Arabidopsis (Rook et al., 1998; Wiese et al., 2004, 2005), are controlled by sugars at both transcriptional and posttranscriptional levels. Altogether, present results suggested that different control steps affecting transcription, mRNA stability, translation, and protein stability or degradation, which do not affect mRNA levels directly, can be operating from the gene to the protein level to coordinate Glc uptake in Vitis cells.
A Model for Glc Regulation of VvHT1 Expression and Glc Uptake
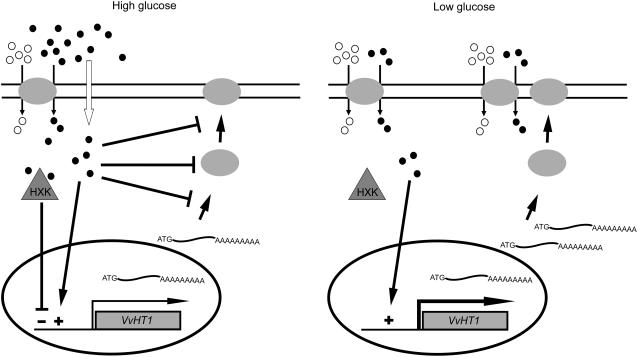
The data may be summarized by the model detailed in Figure 9. When high Glc is present (left), energy-independent, diffusional uptake is the preferred mode of sugar absorption and is sufficient to sustain cell growth and metabolism. Under these conditions, VvHT1 expression is maintained at basal levels due to the balance between a positive induction signal generated by the presence of Glc and a repression signal due to high Glc levels sensed by HXK. Additionally, high Glc levels seem to repress Glc transport activity at the protein level, triggering inactivation, mistargeting, and/or proteolysis of VvHT1. This phenomenon is common for rate-limiting proteins, such as transporters, and has been well demonstrated in yeast where it is called carbon inactivation (Busturia and Lagunas, 1985, 1986; Horak and Wolf, 1997). The mechanism by which high Glc levels trigger proteolysis in yeast is poorly known, but Glc appears to induce the synthesis of proteins required for the degradation process. When external Glc decreases to residual levels (<10 mm Glc, right), the linear transport component no longer sustains Glc transport at a rate sufficient to meet the energy requirements of the cell and the involvement of a concentrative, energy-dependent transport system becomes critical. The absence of the repression signal generated by HXK allows the increase of VvHT1 transcripts to high levels and, in accordance, the number of high-affinity monosaccharide/H+ symporters in the plasma membrane increases (increase of Vmax), ensuring a high-transport capacity at limiting Glc conditions.
Figure 9.
Schematic model of Glc regulation of VvHT1 expression and Glc transport activity. Glc, •; H+, ○.
In conclusion, these data contributed to the understanding of the mechanisms involved in Glc import into the berry. The regulation of the expression of the MST VvHT1 was investigated at transcriptional, translational, and protein activity levels in a sink model. Also, we reported that the VvHT1 transporter is abundant at the green stage of fruit development, consistent with the illustrated regulating role of Glc on VvHT1 expression. Although part of the phloem-translocated Suc may be absorbed directly by mesocarp cells through disaccharide transporters (Davies et al., 1999; Ageorges et al., 2000), VvHT1 should contribute to the early steps of Glc and Fru accumulation in the berry flesh cells after the disaccharide had been hydrolyzed by apoplastic invertases. This high-affinity transporter may be involved in the supply of energy for the intense cell division and growth when low apoplastic sugar is available. As ripening proceeds, repression of VvHT1 is most likely associated with monosaccharide accumulation and other sugar transporters should be involved in sugar import into the mesocarp cells.
MATERIALS AND METHODS
Cell Suspension Culture, Growth Conditions, and Treatments
Cell suspensions of grape (Vitis vinifera) were maintained in 250-mL flasks on a rotatory shaker at 100 rpm in the dark, at 25°C on mineral medium supplemented with 2% (w/v) Suc or 1% (w/v) Glc, as previously described (Descendit et al., 1996). Cells were subcultured weekly by transferring 10-mL aliquots into 70 mL of fresh medium. Maximal specific growth rates (μmax) were determined from dry-weight measurements. Aliquots (1–5 mL) were filtered through preweighed GF/C filters (Whatman). The samples were washed with deionized water and weighed after 24 h at 80°C. Sugar consumption was monitored by HPLC and the Glc oxidase method (test combination; Boehringer Mannheim).
Estimation of Initial Sugar Uptake Rates
Harvested cells were centrifuged, washed twice with ice-cold culture medium without sugar at pH 5.0, and resuspended in the same medium at a final concentration of 5 mg mL−1 dry weight.
To estimate the initial uptake rates of d- or l-[14C]Glc, 1 mL of cell suspension was added to 10-mL flasks under shaking (100 rpm). After 2 min of incubation at 25°C, the reaction was started by the addition of 40 μL of an aqueous solution of radiolabeled sugar at the desired specific activity and concentration. The specific activities were defined according to the final concentration of the sugar in the reaction mixture as follows: 8.33 Bq nmol−1 (0.02–0.5 mm Glc), 1.66 Bq nmol−1 (1–10 mm Glc), and 0.17 Bq nmol−1 (10–50 mm Glc). Sampling times were 0, 60, and 180 s, which ensures linearity of uptake. The reaction was stopped by dilution with 5 mL ice-cold modified Murashige and Skoog medium without sugar and the mixtures were immediately filtered through GF/C filters (Whatman). The filters were washed with 10 mL of the same medium and transferred to vials containing scintillation fluid (OptiPhase HiSafe II; LKB Scintillation Products). Radioactivity was measured in a Packard Tri-Carb 2200 CA liquid scintillation counter (Packard Instruments). The results were corrected for nonspecific binding of labeled sugars to the filters and/or the cells by diluting the cells with 5 mL ice-cold modified Murashige and Skoog medium without sugar before addition of labeled sugar. The values for the nonspecific binding constant of labeled Glc, determined in a range of 0.02 to 50 mm sugar, were 40 ± 3 nL min−1 mg−1 dry weight (mean ± sd; n = 3). d-[U-14C]Glc (11.28 GBq mmol−1) and l-[U-14C]Glc (2.035 GBq mmol−1) were obtained from the Radiochemical Centre (Amersham).
Determination of Substrate Specificity
Competition between Glc and other sugars was tested by running competitive uptake kinetics. The concentration range of labeled sugar varied from 0.02 to 0.5 mm and the final concentration of the unlabeled substrate was at least 10-fold higher than the Km value estimated for the transport system. Initial uptake rates of d-[14C]Fru were estimated as described previously for d- or l-[14C]Glc. The concentration range of labeled Fru varied from 0.02 to 0.5 mm (specific activity = 50 Bq nmol−1). Inhibition of Fru transport by 1 mm Glc was assayed as described for inhibition of Glc transport by nonlabeled sugars.
Accumulation Studies
A 10-mL sample of cell suspension was transferred to a 50-mL Erlenmeyer flask under shaking (100 rpm). After 2-min incubation at 25°C, the reaction was started by the addition of an aqueous solution of radiolabeled 3-O-MG (specific activity = 50 Bq nmol−1) at a final concentration of 0.1 mm. At selected times, 1-mL aliquots were taken from the reaction mixture into 5 mL ice-cold modified Murashige and Skoog medium without sugar and filtered immediately through Whatman GF/C membranes. The filters were washed with 10 mL of the same medium and the radioactivity was counted as indicated above. The intracellular concentrations of 3-O-MG were estimated as the ratio between the intracellular and the extracellular 3-O-MG concentration, using the intracellular volume obtained as indicated below. 3-O-methyl-d-[U-14C]Glc (3.7 MBq mmol−1) was obtained from the Radiochemical Centre (Amersham).
Estimation of Initial Rates of Proton Uptake
To estimate the initial rates of proton uptake upon addition of Glc, a standard pH meter (PHM 82 Radiometer A/S) connected to a recorder (Kipp and Zonen) was used as described earlier (Oliveira et al., 2002). The pH electrode was immersed in a water-jacketed chamber with magnetic stirring. A total of 5 mL of grape cell suspension in 10 mm potassium phosphate buffer (about 4 mg mL−1 dry weight) were added to the chamber. The pH was adjusted to 5.0 and a baseline was obtained. The desired amount of Glc was added and the subsequent alkalinization curve was monitored. The slope of the initial part of the pH trace was used to calculate the initial rates of proton uptake. Calibration was performed with HCl.
Determination of Intracellular Volume
The methodology used to measure intracellular water volume was a modification of the methods previously described (Rottenberg, 1979; De la Peña et al., 1981) and was based on the quantification of the relative distribution of two radioactive compounds in a cellular suspension: [14C]methoxy inulin, to which biomembranes are impermeable, and [3H]H2O that equilibrates across biomembranes. After washing with culture medium without sugar, 2 mL of cell suspension were incubated with 5 μL of 9.25 MBq mL−1 [3H]H2O (Amersham; 185 GBq mL−1), and 5 μL of 0.22 mg mL−1 [14C]methoxy inulin (New England Nuclear; 192 MBq g−1). The mixture was incubated for 30 min and the cells were pelleted by centrifugation at 4,000g for 1 min. The supernatant (100 μL) was added to 5 mL of 1% (w/v) SDS and the same volume of SDS was added to the pellet. After overnight incubation, the mixtures were centrifuged and the radioactivity of 40 μL of each supernatant was measured as described above. Intracellular water volume (Vint) was determined according to the expression Vint = Vsup [(3Hpel/3Hsup) − (14Cpel/14Csup)], where Vsup corresponds to the volume of supernatant, 3Hpel and 3Hsup correspond to the 3H counts in the pellet and in the supernatant, respectively, and 14Cpel and 14Csup correspond to the 14C counts in the pellet and in the supernatant, respectively. A value of 8 ± 3 μL intracellular water mg−1 dry weight (mean ± se, n = 4) was obtained.
Calculation of Kinetic Parameters
Data of the initial uptake rates of labeled Glc were analyzed by computer-assisted nonlinear regression analysis (GraphPad Prism software). By this method, the transport kinetics best fitting to the experimental initial uptake rates were determined and estimates for the kinetic parameters were then obtained. Uptake rates are mean values ± se; n denotes the number of independent experiments.
RNA Gel-Blot Analysis
Total RNAs from grape berry cell suspension samples were isolated by phenol extraction and LiCl 2 m precipitation (adapted from Howell and Hull, 1978). Twenty micrograms of each RNA sample were separated by formaldehyde-agarose gel electrophoresis and transferred onto Hybond N membrane (Amersham Life Science). The 200-bp 3′-specific probes were designed by PCR for each VvHT and pGLT from sequences available in the National Center for Biotechnology Information International Data Bank and used for RNA-blot analysis. Randomly primed 32P probes (prime-a-gene; Promega) were hybridized and RNA blots were revealed on autoradiographic films and by imaging (Bio-Rad personal molecular imager FX).
Plasma Membrane Isolation
Plasma membrane vesicles were isolated from grape suspension-cultured cells by differential centrifugation and Suc gradient (Nagao et al., 1987; Serrano, 1988). Cells (40–50 g fresh weight) were harvested, centrifuged at 3,000g for 1 min, washed twice with distilled water, and suspended in 100 mL of ice-cold buffer containing 250 mm Suc, 20 mm EDTA, pH 8.0, 10 mm dithiothreitol (DTT), 1 mm phenylmethylsulfonyl fluoride, and 50 mm Tris-MES, pH 8.0. The mixture was homogenized with an Ultra-Turrax T25 device (IKA WERKE, Janke and Kumkel IKA) for 3 min at 22,000 rpm, on ice, and the homogenate was strained through a layer of cheesecloth and centrifuged at 3,500g for 10 min. The supernatant was centrifuged once more at 10,000g for 10 min and then at 100,000g for 30 min. The pellet was resuspended in 8 mL ice-cold resuspension buffer (15% [v/v] glycerol, 1 mm DTT, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, and 20 mm Tris-HCl, pH 7.5). The suspension was layered over a 32% and 46% (w/v) discontinuous Suc gradient and centrifuged at 18,000g for 3 h in a Beckman SW 28 rotor. In addition to Suc, the gradient solutions contained 10 mm Tris-HCl buffer (pH 7.6), 1 mm EDTA, and 1 mm DTT. The vesicles sedimenting at the 32%-46% Suc interface were collected, diluted with 3 volumes of ice-cold water, and centrifuged at 100,000g for 30 min. The pellet was resuspended in the resuspension buffer described above. The vesicles were then frozen under liquid nitrogen and stored at −80°C until use. Protein concentration was determined by the method of Lowry (1951), with bovine serum albumin as the standard. ATPase activity, determined by measuring colorimetrically the release of π (Fiske and Subbarow, 1925), was used to measure the purity of plasma membrane preparations. At pH 6.5, 80% to 90% of the ATPase activity was inhibited by 0.1 mm vanadate.
Western-Blot Analysis
Proteins were separated on 10% SDS-polyacrylamide gels by electrophoresis (Laemmli et al., 1970) and transferred onto nitrocellulose membranes. Immunodetection was performed with VvHT1 antibody as previously described (Zhang et al., 2004; Vignault et al., 2005).
Sugar Quantification in Grape Berries, Plasma Membrane Isolation from Mesocarp Cells, and Immunoblot Detection of VvHT1
Berries from grape cv Chardonnay were collected at the following weeks after flowering: 4 (green stage), 6 (before véraison stage), 8 (véraison stage), and 14 (harvesting). They were deseeded, weighed, immediately frozen in liquid nitrogen, and stored at −80°C. To quantify sugar content, berries were ground in liquid nitrogen, the frozen powder was homogenized in ethanol-water (80% [ v/v]), and boiled for 10 min at 80°C to inactivate invertase activity as described by Ageorges et al. (2000). The solution was centrifugated at 15,000g for 5 min and sugars in the supernatant were measured by high performance anion-exchange chromatography as described in Ollé et al. (1996). Plasma membrane fractions were purified from the frozen powders of the berries at the different development stages and were used for western-blot analysis of VvHT1 as described above.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AJ001061 (VvHT1), AY663846 (VvHT2), AY538259 (VvHT3), AY538260 (VvHT4), AY538261 (VvHT5), AY861386 (VvHT6), AY854146 (VvHT7), and AY608701 (pGLT).
Acknowledgments
We thank Paulo Silva (University of Minho) for expert help in figure design and Magali Vachaud and Cécile Gaillard (University of Poitiers) for helpful advice on VvHT1 immunodetection.
This work was supported in part by the Pessoa Program (GRICES/EGIDE), the Conférence des Présidents d'Université, the Conselho de Reitores das Universidades Portuguesas, the Fundação para a Ciência e a Tecnologia (research project no. POCI/AGR/56378/2004; grant no. SFRH/BD/10689/2002 to C.C. and grant no. SFRH/PBD/17166/2004 to A.A.), and the Conseil Régional Poitou-Charentes (to D.G.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hernâni Gerós (geros@bio.uminho.pt).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.080804.
References
- Ageorges A, Issaly N, Picaud S, Delrot S, Romieu C (2000) Identification and functional expression in yeast of a grape berry sucrose carrier. Plant Physiol Biochem 38: 177–185 [Google Scholar]
- Atanassova R, Leterrier M, Gaillard C, Agasse A, Sagot E, Coutos-Thevenot P, Delrot S (2003) Sugar-regulated expression of a putative hexose transport gene in grape. Plant Physiol 131: 326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo H, Conde C, Geros H, Tavares RM (2006) The non-host pathogen Botrytis cinerea enhances glucose transport in Pinus pinaster suspension-cultured cells. Plant Cell Physiol 47: 290–298 [DOI] [PubMed] [Google Scholar]
- Boles E, Hollenberg CP (1997) The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev 21: 85–111 [DOI] [PubMed] [Google Scholar]
- Botha FC, O'Kennedy MM (1998) Carbohydrate utilization by cell suspension cultures of Phaseolus vulgaris. Physiol Plant 102: 429–436 [Google Scholar]
- Bourque S, Lemoine R, Sequeira-Legrand A, Fayolle L, Delrot S, Pugin A (2002) The elicitor cryptogein blocks glucose transport in tobacco cells. Plant Physiol 130: 2177–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busturia A, Lagunas R (1985) Identification of two forms of the maltose transport system in Saccharomyces cerevisiae and their regulation by catabolite inactivation. Biochim Biophys Acta 820: 324–326 [Google Scholar]
- Busturia A, Lagunas R (1986) Catabolite inactivation of the glucose transport system in Saccharomyces cerevisiae. J Gen Microbiol 132: 379–385 [DOI] [PubMed] [Google Scholar]
- Büttner M, Sauer N (2000) Monosaccharide transporters in plants: structure, function and physiology. Biochim Biophys Acta 1465: 263–274 [DOI] [PubMed] [Google Scholar]
- Cakir B, Agasse A, Gaillard C, Saumonneau A, Delrot S, Atanassova R (2003) A grape ASR protein involved in sugar and abscisic acid signaling. Plant Cell 15: 2165–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Taliercio EW, Chourey PS (1999) Sugars modulate an unusual mode of control of the cell-wall invertase gene (Incw1) through its 3′ untranslated region in a cell suspension culture of maize. Proc Natl Acad Sci USA 96: 10512–10517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95: 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe BG (1992) Research on development and ripening of the grape berry. Am J Enol Vitic 43: 101–110 [Google Scholar]
- Cortes S, Gromova M, Evrard A, Roby C, Heyraud A, Rolin DB, Raymond P, Brouquisse RM (2003) In plants, 3-O-methylglucose is phosphorylated by hexokinase but not perceived as a sugar. Plant Physiol 131: 824–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Wolf T, Robinson SP (1999) Three putative sucrose transporters are differentially expressed in grapevine tissues. Plant Sci 147: 93–100 [Google Scholar]
- De la Peña P, Barros F, Gascon S, Lazo PS, Ramos S (1981) Effect of yeast killer toxin of sensitive cells of Saccharomyces cerevisiae. J Biol Chem 256: 10420–10425 [PubMed] [Google Scholar]
- Delrot S (1989) Loading of photoassimilates. In DA Baker, JA Milburn, eds, Transport of Photoassimilates. Longman Scientific, Harlow, UK, pp 167–205
- Delrot S, Atanassova R, Maurousset L (2000) Regulation of sugar, amino acid and peptide plant membrane transporters. Biochim Biophys Acta 1465: 281–306 [DOI] [PubMed] [Google Scholar]
- Descendit A, Ramawat KG, Waffo P, Deffieux G, Badoc A, Merillon JM (1996) Anthocyanins, catechins, condensed tanins and piceid production in Vitis vinifera cell bioreactor cultures. Biotechnol Lett 18: 659–662 [Google Scholar]
- Ehness R, Roitsch T (1997) Co-ordinated induction of RNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J 11: 539–548 [DOI] [PubMed] [Google Scholar]
- Etxeberria E, González P, Tomlinson P, Pozueta-Romero J (2005) Existence of two parallel mechanisms for glucose uptake in heterotrophic plant cells. J Exp Bot 56: 1905–1912 [DOI] [PubMed] [Google Scholar]
- Fillion L, Ageorges A, Picaud S, Coutos-Thevenot P, Lemoine R, Romieu C, Delrot S (1999) Cloning and expression of a hexose transporter gene expressed during the ripening of grape berry. Plant Physiol 120: 1083–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske CF, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66: 375–400 [Google Scholar]
- Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE (2003) The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atβfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol 132: 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Maurousset L, Lemoine R, Yoo SD, van Nocker S, Loescher W (2003) Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol 131: 1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI (2004) Sugar and phytohormone response pathways: navigating a signalling network. J Exp Bot 55: 253–264 [DOI] [PubMed] [Google Scholar]
- Gifford RM, Thorne JH, Hitz WD, Giaquinta RT (1984) Crop productivity and photoassimilate partitioning. Science 225: 801–808 [DOI] [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver CJ (1994) Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell 6: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak J, Wolf DH (1997) Catabolite inactivation of the galactose transporter in the yeast Saccharomyces cerevisiae: ubiquitination, endocytosis, and degradation in the vacuole. J Bacteriol 179: 1541–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SH, Hull R (1978) Replication of cauliflower mosaic virus and transcription of its genome in turnip leaf protoplasts. Virology 86: 468–481 [DOI] [PubMed] [Google Scholar]
- Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7: 235–246 [DOI] [PubMed] [Google Scholar]
- Komor E, Haass D, Tanner W (1972) Unusual features of the active hexose uptake system of Chlorella vulgaris. Biochim Biophys Acta 266: 649–660 [DOI] [PubMed] [Google Scholar]
- Krook J, Vreugdehil D, van der Plas LHW (2000) Uptake and phosphorylation of glucose and fructose in Daucus carota cell suspensions are differentially regulated. Plant Physiol Biochem 38: 603–612 [Google Scholar]
- Kühn C, Barker L, Burkle L, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298–1300 [DOI] [PubMed] [Google Scholar]
- Laemmli UK, Beguin F, Gujer-Kellenberger G (1970) A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol 47: 69–85 [DOI] [PubMed] [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM (1999) The dual function of sugar carriers: transport and sugar sensing. Plant Cell 11: 707–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55: 341–372 [DOI] [PubMed] [Google Scholar]
- Leterrier M, Atanassova R, Laquitaine L, Gaillard C, Coutos-Thevenot P, Delrot S (2003) Expression of a putative grapevine hexose transporter in tobacco alters morphogenesis and assimilate partitioning. J Exp Bot 54: 1193–1204 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AJ, Randall RJ (1951) Protein measurement with the Folin reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Ludewig U, Frommer WB (2002) Genes and proteins for solute transport and sensing. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD [DOI] [PMC free article] [PubMed]
- Matsukura C, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J (2000) Sugar uptake and transport in rice embryo: expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol 124: 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel K, Sakr S, Gerbe F, Guilliot A, Bonhomme M, Rageau R, Pétel G (2004) Sorbitol uptake is regulated by glucose through the hexokinase pathway in vegetative peach-tree buds. J Exp Bot 55: 879–888 [DOI] [PubMed] [Google Scholar]
- Milkowski C, Krampe S, Weirich J, Hasse V, Boles E, Breunig KD (2001) Feedback regulation of glucose transporter gene transcription in Kluyveromyces lactis by glucose uptake. J Bacteriol 183: 5223–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T, Sasakawa H, Sugiyama T (1987) Purification of H+-ATPase from the plasma membrane of maize roots and preparations of its antibody. Plant Cell Physiol 28: 1181–1186 [Google Scholar]
- Oliveira J, Tavares RM, Geros H (2002) Utilization and transport of glucose in Olea europaea cell suspensions. Plant Cell Physiol 43: 1510–1517 [DOI] [PubMed] [Google Scholar]
- Ollé N, Lozano YF, Brioullet JM (1996) Isolation and characterisation of soluble polysaccharides and insoluble cell wall material of the pulp from four mango (Mangifera indica L.) cultivars. J Agric Food Chem 44: 2658–2662 [Google Scholar]
- Patrick JW (1997) Sieve element unloading and post-sieve element transport. Annu Rev Plant Physiol Plant Mol Biol 48: 191–222 [DOI] [PubMed] [Google Scholar]
- Ransom-Hodgkins R, Vaughn MW, Bush DR (2003) Protein phosphorylation plays a key role in sucrose-mediated transcriptional regulation of a phloem-specific proton-sucrose symporter. Planta 217: 483–489 [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Ritte G, Rosenfeld J, Rohrig K, Raschke K (1999) Rates of sugar uptake by guard cell protoplasts of Pisum sativum L. related to the solute requirement for stomatal opening. Plant Physiol 121: 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin G, Sakr S, Bonmort J, Delrot S (1998) Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Lett 424: 165–168 [DOI] [PubMed] [Google Scholar]
- Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2: 198–206 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Tanner W (1994) Expression of a sugar-transporter gene family in a photoautotrophic suspension culture of Chenopodium rubrum L. Planta 193: 365–371 [DOI] [PubMed] [Google Scholar]
- Rolland F, Winderickx J, Thevelein JM (2001) Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem Sci 26: 310–317 [DOI] [PubMed] [Google Scholar]
- Rook F, Gerrits N, Kortstee A, van Kampen M, Borrias M, Weisbeek P, Smeekens S (1998) Sucrose-specific signalling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. Plant J 15: 253–263 [DOI] [PubMed] [Google Scholar]
- Rottenberg H (1979) The measurement of membrane potential and pH in cells, organelles and vesicles. Methods Enzymol 55: 547–569 [DOI] [PubMed] [Google Scholar]
- Sauer N, Friedlander K, Graml-Wicke U (1990) Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J 9: 3045–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R (1988) H+-ATPase from plasma membranes of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol 157: 533–544 [DOI] [PubMed] [Google Scholar]
- Shakya R, Sturm A (1998) Characterization of source- and sink-specific sucrose/H+ symporters from carrot. Plant Physiol 118: 1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Buttner M, Ache P, Hedrich R, Ivashikina N, Melzer M, Shearson SM, Smith SM, Sauer N (2003) Diurnal and light-regulated expression of AtSTP1 in guard cells of Arabidopsis. Plant Physiol 133: 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrier N, Glissant D, Grimplet J, Barrieu F, Abbal P, Couture C, Ageorges A, Atanassova R, Leon C, Renaudin JP, et al (2005) Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222: 832–847 [DOI] [PubMed] [Google Scholar]
- Truernit E, Schmid J, Epple P, Illig J, Sauer N (1996) The sink-specific and stress-regulated Arabidopsis STP4 gene: enhanced expression of a gene encoding a monosaccharide transporter by wounding, elicitors, and pathogen challenge. Plant Cell 8: 2169–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truernit E, Stadler R, Baier K, Sauer N (1999) A male gametophyte-specific monosaccharide transporter in Arabidopsis. Plant J 17: 191–201 [DOI] [PubMed] [Google Scholar]
- Vaughn MW, Harrington GN, Bush DR (2002) Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proc Natl Acad Sci USA 99: 10876–10880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstappen R, Ranostaj S, Rausch T (1991) The hexose transporters at the plasma membrane and the tonoplast of transformed plant cells: kinetic characterization of two distinct carriers. Biochim Biophys Acta 1073: 366–373 [DOI] [PubMed] [Google Scholar]
- Vignault C, Vachaud M, Cakir B, Glissant D, Dedaldechamp F, Buttner M, Atanassova R, Fleurat-Lessard P, Lemoine R, Delrot S (2005) VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. J Exp Bot 56: 1409–1418 [DOI] [PubMed] [Google Scholar]
- Watari J, Kobae Y, Yamaki S, Yamada K, Toyofuku K, Tabuchi T, Shiratake K (2004) Identification of sorbitol transporters expressed in the phloem of apple source leaves. Plant Cell Physiol 45: 1032–1041 [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Heim U, Sauer N, Wobus U (1997) A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell 9: 895–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell DL, Bisson LF (1994) Expression of high-affinity glucose transport protein Hxt2p of Saccharomyces cerevisiae is both repressed and induced by glucose and appears to be regulated posttranslationally. J Bacteriol 176: 3730–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese A, Elzinga N, Wobbes B, Smeekens S (2004) A conserved upstream open reading frame mediates sucrose-induced repression of translation. Plant Cell 16: 1717–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese A, Elzinga N, Wobbes B, Smeekens S (2005) Sucrose-induced translational repression of plant bZIP-type transcription factors. Biochem Soc Trans 33: 272–275 [DOI] [PubMed] [Google Scholar]
- Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants—a diversity of roles and complex regulation. Trends Plant Sci 5: 283–290 [DOI] [PubMed] [Google Scholar]
- Zhang LY, Peng YB, Pelleschi-Travier S, Fan Y, Lu YF, Lu YM, Gao XP, Shen YY, Delrot S, Zhang DP (2004) Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol 135: 574–586 [DOI] [PMC free article] [PubMed] [Google Scholar]