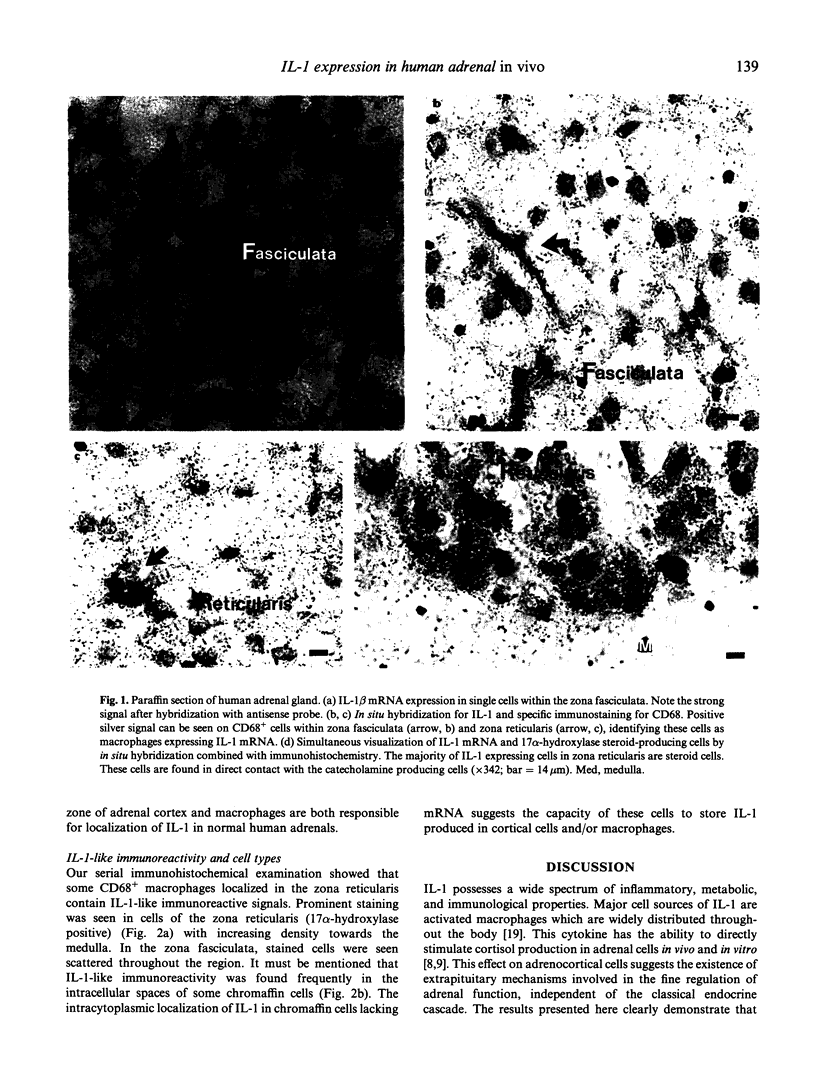
Abstract
IL-1 is an important mediator in the dialogue between the immune system and the hypothalamo-pituitary-adrenal axis. A direct influence of IL-1 upon adrenal steroidogenesis has been demonstrated in experimental animals. We therefore designed a study to see if IL-1 is expressed within the normal human adrenal gland. The combination of in situ hybridization and specific immunostaining to IL-1 beta was eminently suited to demonstrate both mRNA and protein production. The specific immunostaining of the different cells combined with in situ hybridization (IL-1) allowed us to identify the exact cellular source of IL-1. IL-1 mRNA occurred in the zona reticularis in 17 alpha-hydroxylase positive steroid cells surrounding the adrenomedullary cells. Some CD68+ macrophages in this zona showed a positive signal. A weak signal was seen to IL-1 mRNA in few chromaffin cells, while IL-1-like immunoreactivity was more frequent. We conclude that in the normal situation in man IL-1 is mainly expressed in specialized cortical cells. The occurrence of the major glucocorticoid inducing factor in the normal human adrenal gland itself provides evidence for an autocrine or paracrine reaction under physiological conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alheim K., Andersson C., Tingsborg S., Ziolkowska M., Schultzberg M., Bartfai T. Interleukin 1 expression is inducible by nerve growth factor in PC12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9302–9306. doi: 10.1073/pnas.88.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C., Svenson S. B., Van Deventer S., Cerami A., Bartfai T. Interleukin-1 alpha expression is inducible by cholinergic stimulation in the rat adrenal gland. Neuroscience. 1992;47(2):481–485. doi: 10.1016/0306-4522(92)90262-z. [DOI] [PubMed] [Google Scholar]
- Andreis P. G., Neri G., Belloni A. S., Mazzocchi G., Kasprzak A., Nussdorfer G. G. Interleukin-1 beta enhances corticosterone secretion by acting directly on the rat adrenal gland. Endocrinology. 1991 Jul;129(1):53–57. doi: 10.1210/endo-129-1-53. [DOI] [PubMed] [Google Scholar]
- Bernardini R., Kamilaris T. C., Calogero A. E., Johnson E. O., Gomez M. T., Gold P. W., Chrousos G. P. Interactions between tumor necrosis factor-alpha, hypothalamic corticotropin-releasing hormone, and adrenocorticotropin secretion in the rat. Endocrinology. 1990 Jun;126(6):2876–2881. doi: 10.1210/endo-126-6-2876. [DOI] [PubMed] [Google Scholar]
- Bornstein S. R., Gonzalez-Hernandez J. A., Ehrhart-Bornstein M., Adler G., Scherbaum W. A. Intimate contact of chromaffin and cortical cells within the human adrenal gland forms the cellular basis for important intraadrenal interactions. J Clin Endocrinol Metab. 1994 Jan;78(1):225–232. doi: 10.1210/jcem.78.1.7507122. [DOI] [PubMed] [Google Scholar]
- Breder C. D., Dinarello C. A., Saper C. B. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988 Apr 15;240(4850):321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Darling G., Goldstein D. S., Stull R., Gorschboth C. M., Norton J. A. Tumor necrosis factor: immune endocrine interaction. Surgery. 1989 Dec;106(6):1155–1160. [PubMed] [Google Scholar]
- Eskay R. L., Eiden L. E. Interleukin-1 alpha and tumor necrosis factor-alpha differentially regulate enkephalin, vasoactive intestinal polypeptide, neurotensin, and substance P biosynthesis in chromaffin cells. Endocrinology. 1992 Apr;130(4):2252–2258. doi: 10.1210/endo.130.4.1372239. [DOI] [PubMed] [Google Scholar]
- Fukata J., Usui T., Naitoh Y., Nakai Y., Imura H. Effects of recombinant human interleukin-1 alpha, -1 beta, 2 and 6 on ACTH synthesis and release in the mouse pituitary tumour cell line AtT-20. J Endocrinol. 1989 Jul;122(1):33–39. doi: 10.1677/joe.0.1220033. [DOI] [PubMed] [Google Scholar]
- Gwosdow A. R., O'Connell N. A., Spencer J. A., Kumar M. S., Agarwal R. K., Bode H. H., Abou-Samra A. B. Interleukin-1-induced corticosterone release occurs by an adrenergic mechanism from rat adrenal gland. Am J Physiol. 1992 Sep;263(3 Pt 1):E461–E466. doi: 10.1152/ajpendo.1992.263.3.E461. [DOI] [PubMed] [Google Scholar]
- Imura H., Fukata J. Endocrine-paracrine interaction in communication between the immune and endocrine systems. Activation of the hypothalamic-pituitary-adrenal axis in inflammation. Eur J Endocrinol. 1994 Jan;130(1):32–37. doi: 10.1530/eje.0.1300032. [DOI] [PubMed] [Google Scholar]
- Keller S. E., Weiss J. M., Schleifer S. J., Miller N. E., Stein M. Stress-induced suppression of immunity in adrenalectomized rats. Science. 1983 Sep 23;221(4617):1301–1304. doi: 10.1126/science.6612346. [DOI] [PubMed] [Google Scholar]
- Kennedy R. L., Jones T. H. Cytokines in endocrinology: their roles in health and in disease. J Endocrinol. 1991 May;129(2):167–178. doi: 10.1677/joe.0.1290167. [DOI] [PubMed] [Google Scholar]
- Koenig J. I., Snow K., Clark B. D., Toni R., Cannon J. G., Shaw A. R., Dinarello C. A., Reichlin S., Lee S. L., Lechan R. M. Intrinsic pituitary interleukin-1 beta is induced by bacterial lipopolysaccharide. Endocrinology. 1990 Jun;126(6):3053–3058. doi: 10.1210/endo-126-6-3053. [DOI] [PubMed] [Google Scholar]
- Matsuki Y., Yamamoto T., Hara K. Interleukin-1 mRNA-expressing macrophages in human chronically inflamed gingival tissues. Am J Pathol. 1991 Jun;138(6):1299–1305. [PMC free article] [PubMed] [Google Scholar]
- Murakami N., Fukata J., Usui T., Naito Y., Tominaga T., Nakai Y., Masui Y., Nakao K., Imura H. Effects of repetitive administration of recombinant human interleukin-1 beta, an analog or corticotropin-releasing hormone combined with lysine vasopressin on rats with glucocorticoid-induced secondary adrenocortical insufficiency. J Pharmacol Exp Ther. 1992 Mar;260(3):1344–1348. [PubMed] [Google Scholar]
- Rebuffat P., Malendowicz L. K., Andreis P. G., Meneghelli V., Kasprzak A., Nussdorfer G. G. Morphology and functional responses of isolated inner adrenocortical cells of rats infused with interleukin-beta. Histol Histopathol. 1992 Apr;7(2):183–188. [PubMed] [Google Scholar]
- Reichlin S. Neuroendocrine-immune interactions. N Engl J Med. 1993 Oct 21;329(17):1246–1253. doi: 10.1056/NEJM199310213291708. [DOI] [PubMed] [Google Scholar]
- Saperstein A., Brand H., Audhya T., Nabriski D., Hutchinson B., Rosenzweig S., Hollander C. S. Interleukin 1 beta mediates stress-induced immunosuppression via corticotropin-releasing factor. Endocrinology. 1992 Jan;130(1):152–158. doi: 10.1210/endo.130.1.1309324. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Andersson C., Undén A., Troye-Blomberg M., Svenson S. B., Bartfai T. Interleukin-1 in adrenal chromaffin cells. Neuroscience. 1989;30(3):805–810. doi: 10.1016/0306-4522(89)90171-1. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Fukata J., Naito Y., Usui T., Murakami N., Fukushima M., Nakai Y., Hirai Y., Imura H. Prostaglandin-dependent in vitro stimulation of adrenocortical steroidogenesis by interleukins. Endocrinology. 1991 Jan;128(1):526–531. doi: 10.1210/endo-128-1-526. [DOI] [PubMed] [Google Scholar]
- Uehara A., Gottschall P. E., Dahl R. R., Arimura A. Interleukin-1 stimulates ACTH release by an indirect action which requires endogenous corticotropin releasing factor. Endocrinology. 1987 Oct;121(4):1580–1582. doi: 10.1210/endo-121-4-1580. [DOI] [PubMed] [Google Scholar]
- Wick G., Hu Y., Schwarz S., Kroemer G. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr Rev. 1993 Oct;14(5):539–563. doi: 10.1210/edrv-14-5-539. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Ströbel M., Kieper D., Fingerle G., Schlunck T., Petersmann I., Ellwart J., Blumenstein M., Haas J. G. Differential expression of cytokines in human blood monocyte subpopulations. Blood. 1992 Jan 15;79(2):503–511. [PubMed] [Google Scholar]