Abstract
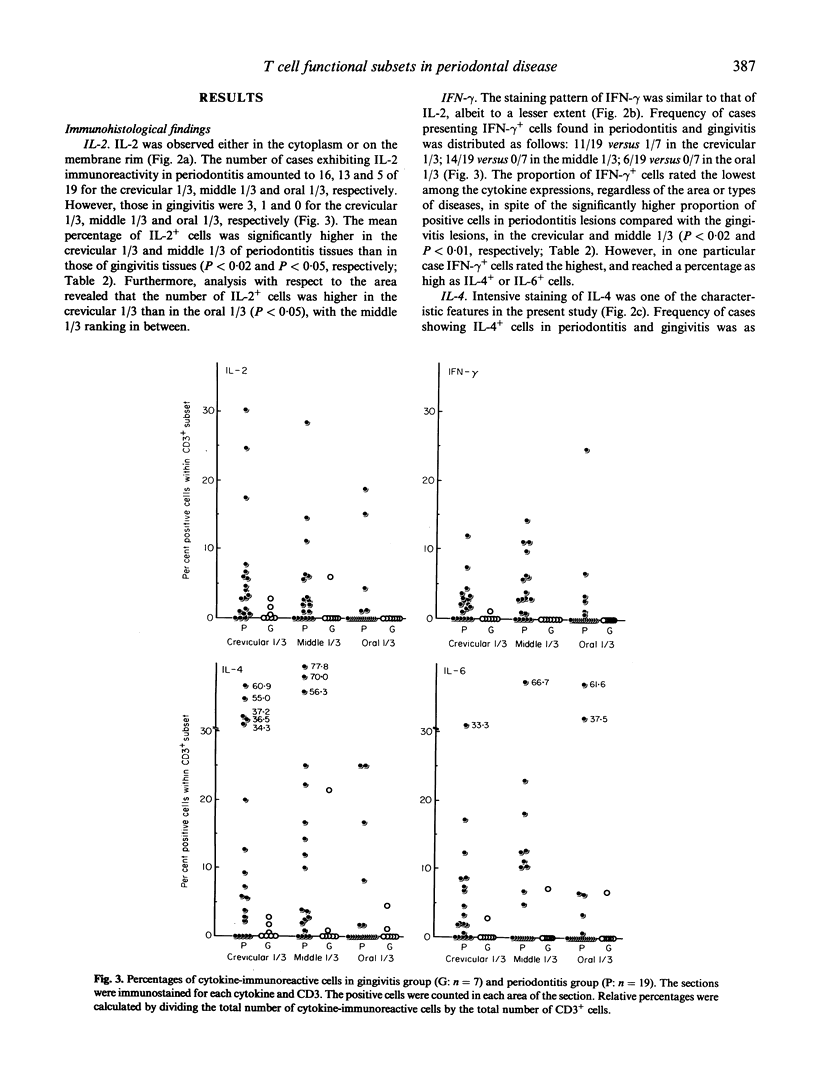
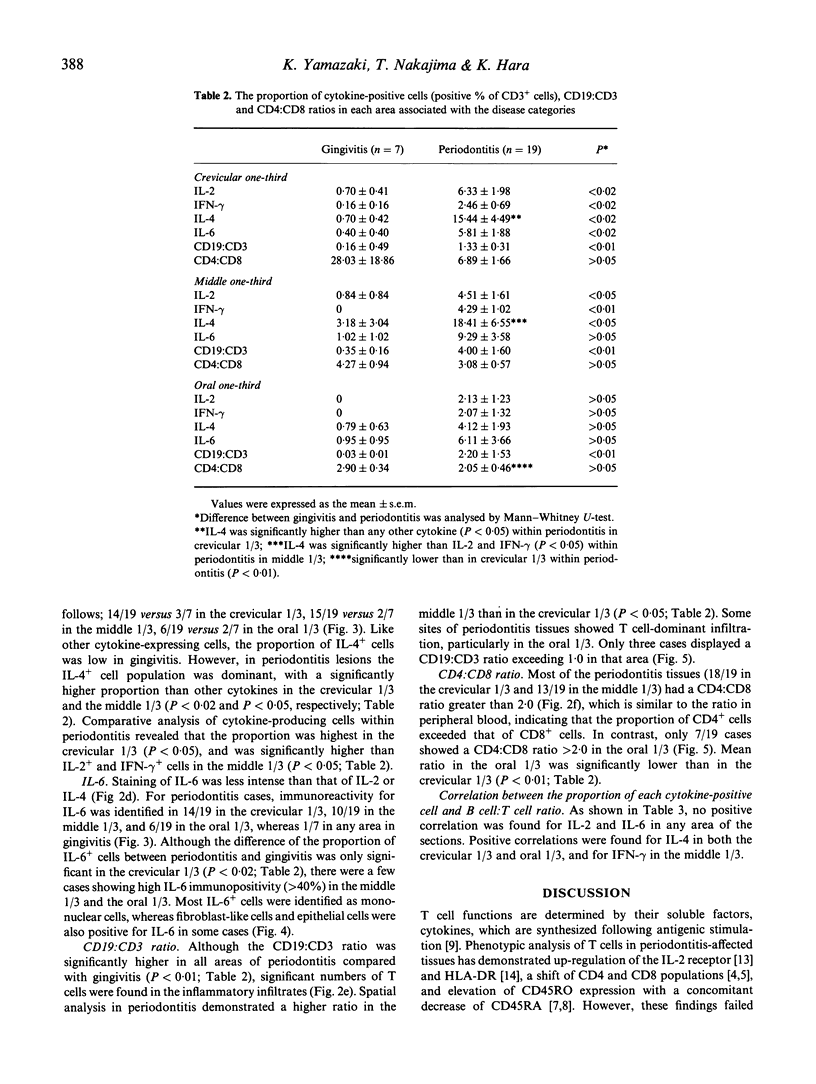
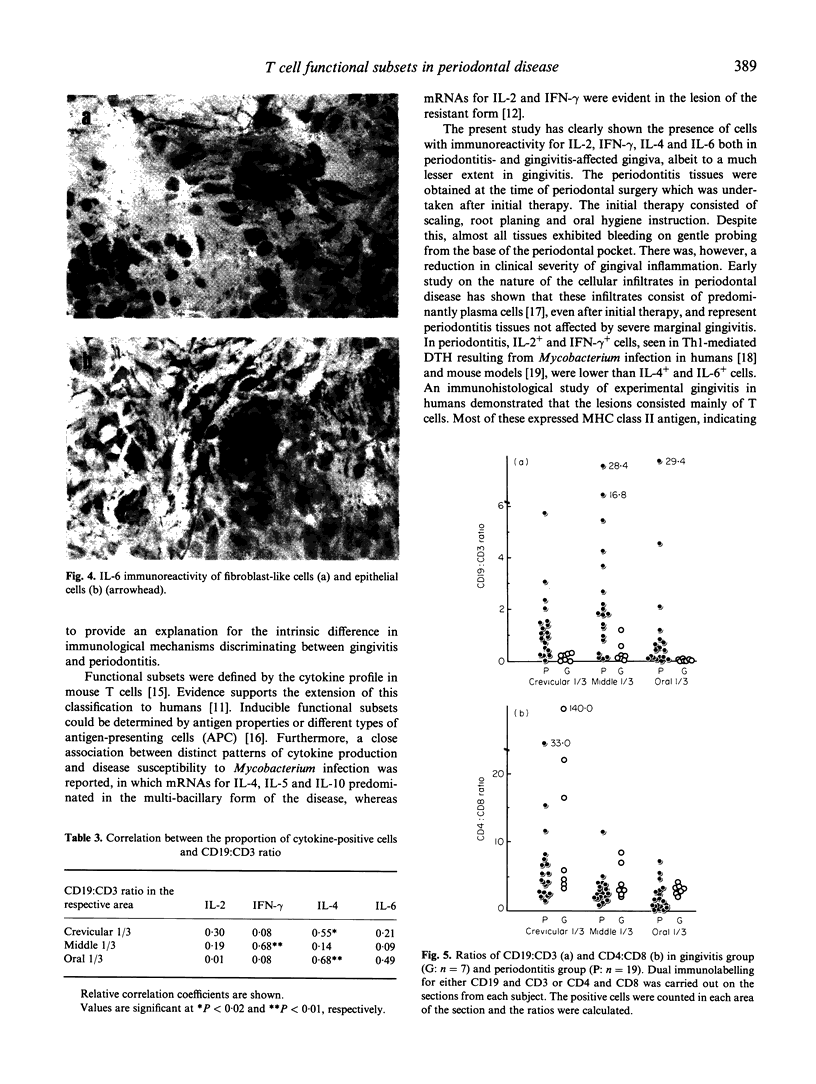
IL-2, interferon-gamma (IFN-gamma), IL-4 and IL-6 producing T cells in periodontitis and gingivitis-affected human tissues were investigated by immunohistochemistry to clarify the relationship between T cell functional subsets and disease entity. Using alkaline-phosphatase anti-alkaline-phosphatase technique, the relative proportions of each cytokine-producing T cell were calculated in the crevicular 1/3, middle 1/3 and oral 1/3 areas selected in the connective tissue of sections. CD19:CD3 and CD4:CD8 ratios were determined on the serial sections. Compared with gingivitis tissues, the proportion of cytokine-producing cells in periodontitis-affected samples was higher overall in the crevicular 1/3 (P < 0.02). The middle 1/3 exhibited a higher percentage of cytokine-producing cells, except for IL-6-producing cells. Frequencies of cytokine-producing cells in the oral 1/3 did not differ. IL-4 was the prominent cytokine in periodontitis-affected tissues, with the highest proportion detected in the crevicular 1/3. The CD19:CD3 ratio was higher in periodontitis tissues irrespective of the location, indicating a B cell dominance in periodontitis lesions. Furthermore, a significant positive correlation between the proportion of IL-4-producing cells and the CD19:CD3 ratio was noted. The CD4:CD8 ratio consistently exceeded 2.0 in both periodontitis and gingivitis. These results suggest that immunoregulation of both periodontitis and gingivitis are T cell-dependent, but in periodontitis type 2 helper T cells predominate and thereby control B cell activation.
Full text
PDF
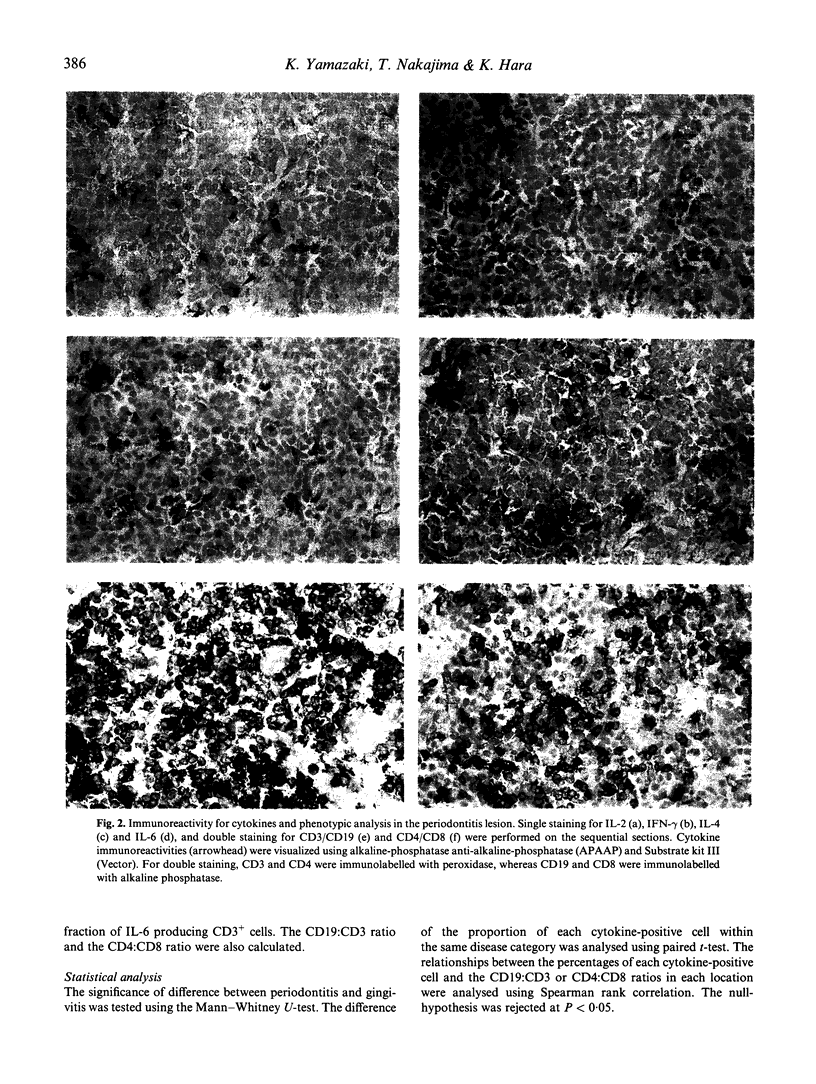


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson U., Andersson J., Lindfors A., Wagner K., Möller G., Heusser C. H. Simultaneous production of interleukin 2, interleukin 4 and interferon-gamma by activated human blood lymphocytes. Eur J Immunol. 1990 Jul;20(7):1591–1596. doi: 10.1002/eji.1830200727. [DOI] [PubMed] [Google Scholar]
- Angelopoulos A. P. Studies of mast cells in the human gingiva. II. Topographical distribution. J Periodontal Res. 1973;8(5):314–322. doi: 10.1111/j.1600-0765.1973.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Abrams J. S., Lu S., Sieling P. A., Rea T. H., Modlin R. L. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993 Jan;61(1):197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartold P. M., Haynes D. R. Interleukin-6 production by human gingival fibroblasts. J Periodontal Res. 1991 Jul;26(4):339–345. doi: 10.1111/j.1600-0765.1991.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Bradding P., Feather I. H., Wilson S., Bardin P. G., Heusser C. H., Holgate S. T., Howarth P. H. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993 Oct 1;151(7):3853–3865. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cole K. L., Seymour G. J., Powell R. N. Phenotypic and functional analysis of T cells extracted from chronically inflamed human periodontal tissues. J Periodontol. 1987 Aug;58(8):569–573. doi: 10.1902/jop.1987.58.8.569. [DOI] [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Ricci M., Romagnani S. Helper activity for immunoglobulin synthesis of T helper type 1 (Th1) and Th2 human T cell clones: the help of Th1 clones is limited by their cytolytic capacity. J Exp Med. 1991 Oct 1;174(4):809–813. doi: 10.1084/jem.174.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihashi K., Beagley K. W., Kono Y., Aicher W. K., Yamamoto M., DiFabio S., Xu-Amano J., McGhee J. R., Kiyono H. Gingival mononuclear cells from chronic inflammatory periodontal tissues produce interleukin (IL)-5 and IL-6 but not IL-2 and IL-4. Am J Pathol. 1993 Apr;142(4):1239–1250. [PMC free article] [PubMed] [Google Scholar]
- Gemmell E., Feldner B., Seymour G. J. CD45RA and CD45RO positive CD4 cells in human peripheral blood and periodontal disease tissue before and after stimulation with periodontopathic bacteria. Oral Microbiol Immunol. 1992 Apr;7(2):84–88. doi: 10.1111/j.1399-302x.1992.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Johannessen A. C., Nilsen R., Knudsen G. E., Kristoffersen T. In situ characterization of mononuclear cells in human chronic marginal periodontitis using monoclonal antibodies. J Periodontal Res. 1986 Mar;21(2):113–127. doi: 10.1111/j.1600-0765.1986.tb01444.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. The biology of interleukin-6. Blood. 1989 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Kristensson K., Borrebaeck C. A., Carlsson R. Human CD4+ T cells expressing CD45RA acquire the lymphokine gene expression of CD45RO+ T-helper cells after activation in vitro. Immunology. 1992 May;76(1):103–109. [PMC free article] [PubMed] [Google Scholar]
- Lewis D. B., Prickett K. S., Larsen A., Grabstein K., Weaver M., Wilson C. B. Restricted production of interleukin 4 by activated human T cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9743–9747. doi: 10.1073/pnas.85.24.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren M., Persson U., Larsson P., Magnusson C., Smith C. I., Hammarström L., Severinson E. Interleukin 4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989 Jul;19(7):1311–1315. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Noelle R. J., Snow E. C. Cognate interactions between helper T cells and B cells. Immunol Today. 1990 Oct;11(10):361–368. doi: 10.1016/0167-5699(90)90142-v. [DOI] [PubMed] [Google Scholar]
- Offenbacher S., Heasman P. A., Collins J. G. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993 May;64(5 Suppl):432–444. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- Reinhardt R. A., McDonald T. L., Bolton R. W., DuBois L. M., Feely D. E., Kaldahl W. B. In situ activated T lymphocytes in active versus stable periodontal lesions. J Periodontal Res. 1988 Sep;23(5):295–302. doi: 10.1111/j.1600-0765.1988.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Reinhardt R. A., McDonald T. L., Bolton R. W., DuBois L. M., Kaldahl W. B. IgG subclasses in gingival crevicular fluid from active versus stable periodontal sites. J Periodontol. 1989 Jan;60(1):44–50. doi: 10.1902/jop.1989.60.1.44. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Rosenkoetter M., Reder A. T., Oger J. J., Antel J. P. T cell regulation of polyclonally induced immunoglobulin secretion in humans. J Immunol. 1984 Apr;132(4):1779–1783. [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Bacon P. A. Production of lymphokine mRNA by CD45R+ and CD45R- helper T cells from human peripheral blood and by human CD4+ T cell clones. J Immunol. 1989 Aug 1;143(3):907–912. [PubMed] [Google Scholar]
- Seymour G. J., Gemmell E., Reinhardt R. A., Eastcott J., Taubman M. A. Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodontal Res. 1993 Nov;28(6 Pt 2):478–486. doi: 10.1111/j.1600-0765.1993.tb02108.x. [DOI] [PubMed] [Google Scholar]
- Seymour G. J., Gemmell E., Walsh L. J., Powell R. N. Immunohistological analysis of experimental gingivitis in humans. Clin Exp Immunol. 1988 Jan;71(1):132–137. [PMC free article] [PubMed] [Google Scholar]
- Seymour G. J., Greenspan J. S. The phenotypic characterization of lymphocyte subpopulations in established human periodontal disease. J Periodontal Res. 1979 Jan;14(1):39–46. doi: 10.1111/j.1600-0765.1979.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Seymour G. J., Powell R. N., Davies W. I. Conversion of a stable T-cell lesion to a progressive B-cell lesion in the pathogenesis of chronic inflammatory periodontal disease: an hypothesis. J Clin Periodontol. 1979 Oct;6(5):267–277. doi: 10.1111/j.1600-051x.1979.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Snijdewint F. G., Kaliński P., Wierenga E. A., Bos J. D., Kapsenberg M. L. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993 Jun 15;150(12):5321–5329. [PubMed] [Google Scholar]
- Stoufi E. D., Taubman M. A., Ebersole J. L., Smith D. J., Stashenko P. P. Phenotypic analyses of mononuclear cells recovered from healthy and diseased human periodontal tissues. J Clin Immunol. 1987 May;7(3):235–245. doi: 10.1007/BF00915729. [DOI] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Yoshie H., Hara K. Expression of interleukin-2 receptor and HLA-DR on lymphocyte subsets of gingival crevicular fluid in patients with periodontitis. J Periodontal Res. 1991 Nov;26(6):502–510. doi: 10.1111/j.1600-0765.1991.tb01802.x. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Ikarashi F., Aoyagi T., Takahashi K., Nakajima T., Hara K., Seymour G. J. Direct and indirect effects of Porphyromonas gingivalis lipopolysaccharide on interleukin-6 production by human gingival fibroblasts. Oral Microbiol Immunol. 1992 Aug;7(4):218–224. doi: 10.1111/j.1399-302x.1992.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Nakajima T., Aoyagi T., Hara K. Immunohistological analysis of memory T lymphocytes and activated B lymphocytes in tissues with periodontal disease. J Periodontal Res. 1993 Sep;28(5):324–334. doi: 10.1111/j.1600-0765.1993.tb01076.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Nakajima T., Gemmell E., Polak B., Seymour G. J., Hara K. IL-4- and IL-6-producing cells in human periodontal disease tissue. J Oral Pathol Med. 1994 Sep;23(8):347–353. doi: 10.1111/j.1600-0714.1994.tb00074.x. [DOI] [PubMed] [Google Scholar]