Abstract
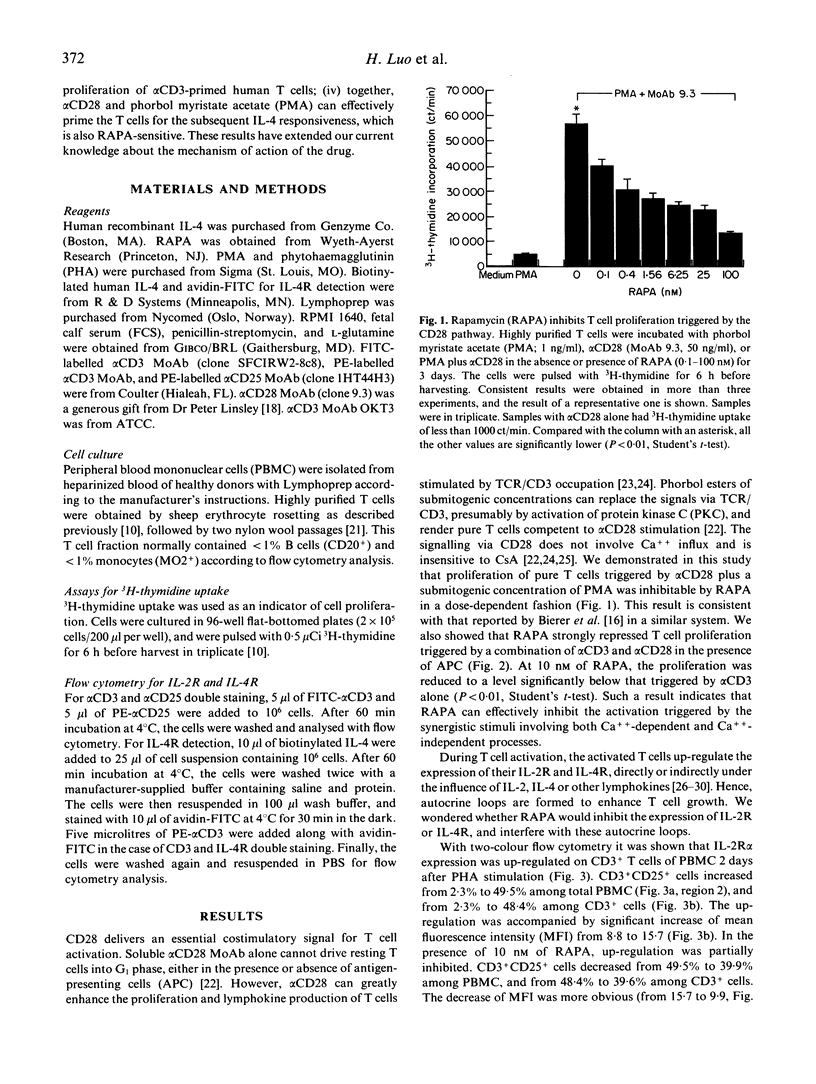
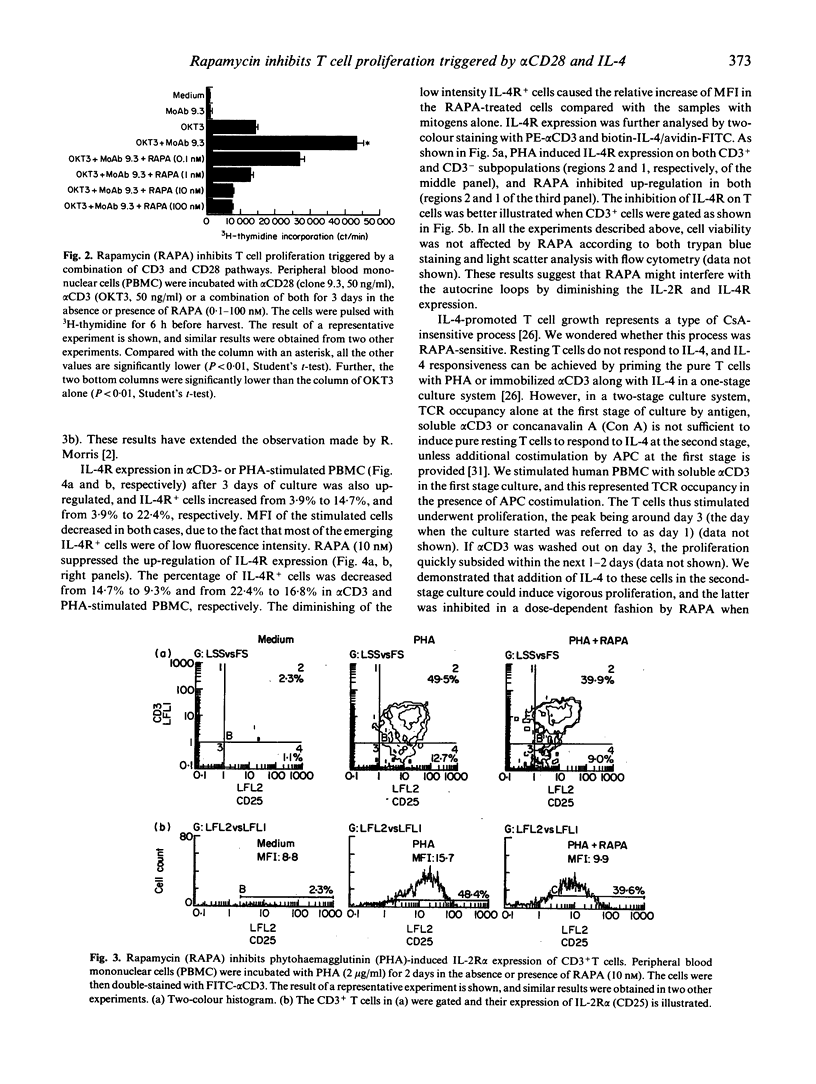
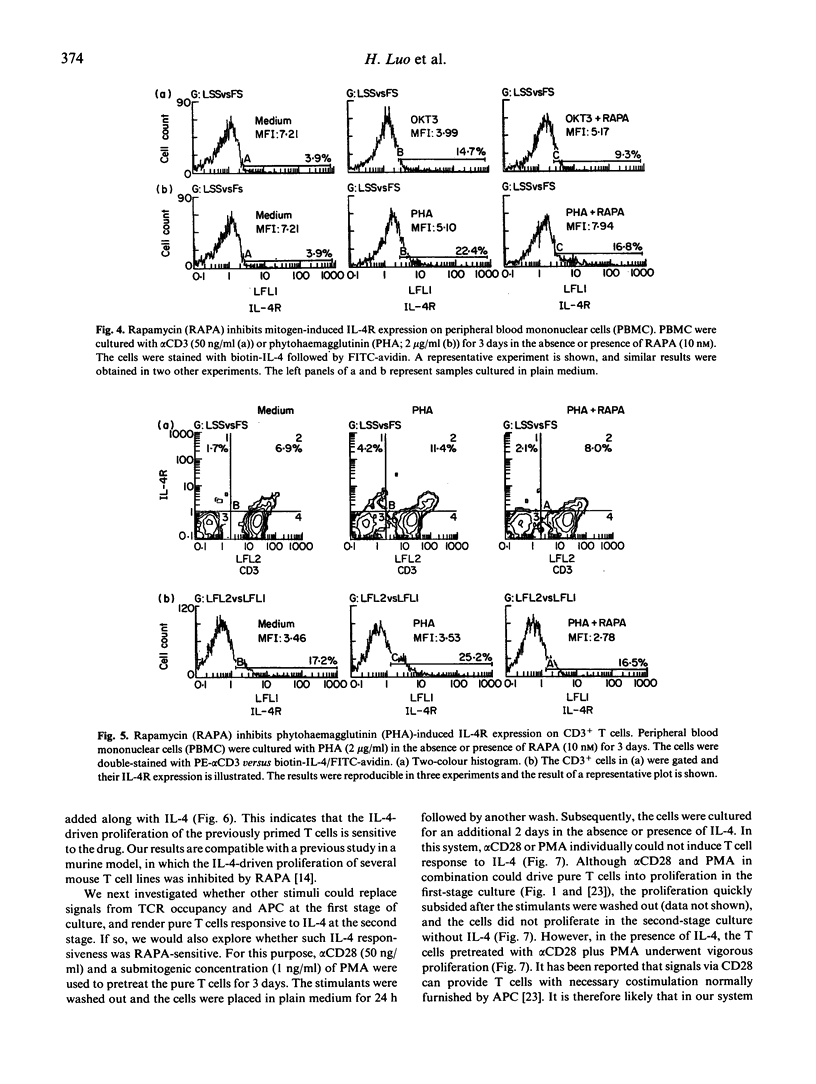
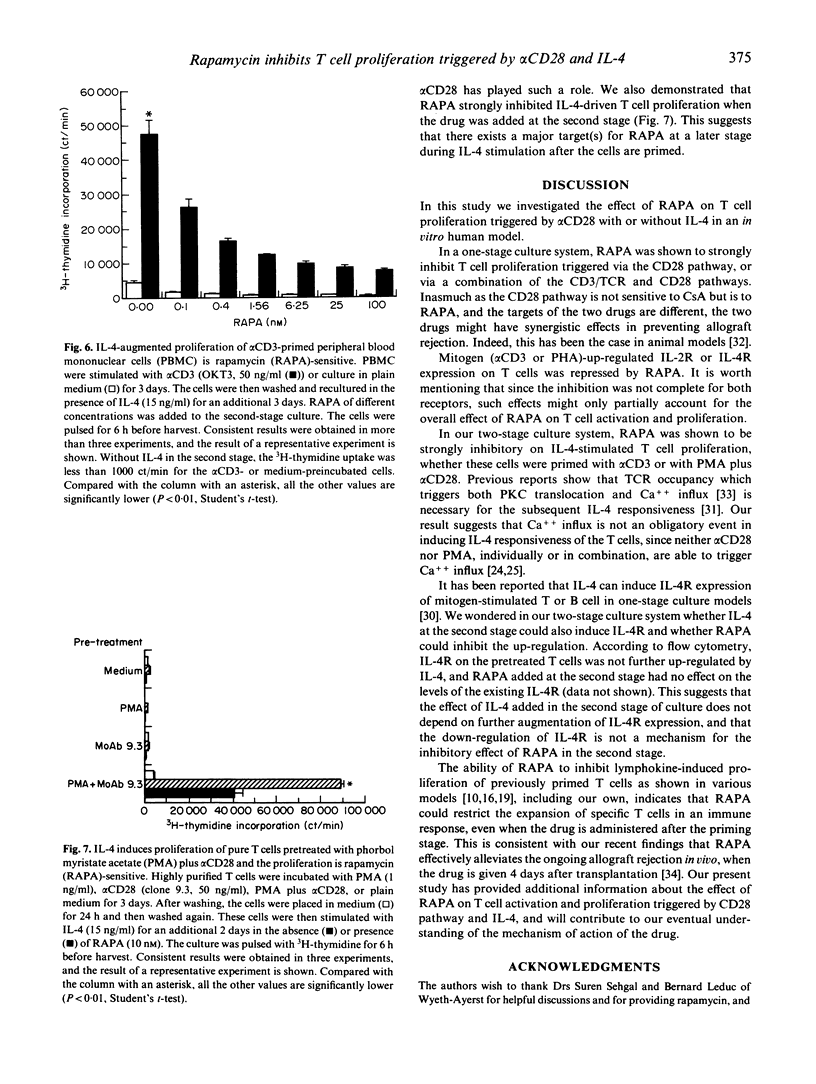
Rapamycin (RAPA) is a potent immunosuppressant. In this study we investigated the effect of RAPA on T cell proliferation triggered by various stimuli in an in vitro human model. The proliferation of T cells stimulated via an alternative pathway using phorbol myristate acetate (PMA) and anti-CD28 antibody (alpha CD28) in the absence of antigen-presenting cells (APC) was strongly inhibited by RAPA. T cell proliferation provoked via a combination of CD3/TCR and CD28 pathways using anti-CD3 antibody (alpha CD3) plus alpha CD28 was also inhibited by RAPA in the presence of APC. The mitogen (phytohaemagglutinin (PHA) or alpha CD3)-induced up-regulation of expression of the IL-2 receptor alpha chain (IL-2R alpha) and the IL-4 receptor (IL-4R) was sensitive to RAPA. This suggests that RAPA's interference with the IL-2 and IL-4 autocrine loops during T cell activation might contribute to RAPA's overall immunosuppressive effect. We have further demonstrated in a two-stage culture system that RAPA strongly inhibited IL-4-stimulated proliferation of T cells, the latter being either pretreated with alpha CD3 in the presence of APC, or with PMA plus alpha CD28 in the absence of APC. The result suggests that the Ca++ influx during the pretreatment is not obligatory for T cells to achieve IL-4 responsiveness. The results also indicate that RAPA's antiproliferative effect on IL-4-stimulated T cells is not contingent on the various mechanisms of cell priming. Therefore, RAPA's major target is probably at the second stage after the priming. Our study has extended current knowledge about the effect of RAPA on human T cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierer B. E., Schreiber S. L., Burakoff S. J. The effect of the immunosuppressant FK-506 on alternate pathways of T cell activation. Eur J Immunol. 1991 Feb;21(2):439–445. doi: 10.1002/eji.1830210228. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., Collier D. S., Lim S., Pollard S. G., Samaan A., White D. J., Thiru S. Rapamycin for immunosuppression in organ allografting. Lancet. 1989 Jul 22;2(8656):227–227. doi: 10.1016/s0140-6736(89)90417-0. [DOI] [PubMed] [Google Scholar]
- Chen H. F., Wu J. P., Luo H. Y., Daloze P. M. Reversal of ongoing rejection of allografts by rapamycin. Transplant Proc. 1991 Aug;23(4):2241–2242. [PubMed] [Google Scholar]
- Chen H. F., Wu J. P., Luo H. Y., Daloze P. M. The immunosuppressive effect of rapamycin on pancreaticoduodenal transplants in the rat. Transplant Proc. 1991 Aug;23(4):2239–2240. [PubMed] [Google Scholar]
- Chen H., Wu J., Xu D., Aboujaoude M., Stepkowski S., Kahan B., Daloze P. The effect of rapamycin on orthotopic small bowel transplantation in the rat. Transplant Proc. 1992 Jun;24(3):1157–1158. [PubMed] [Google Scholar]
- Chiodetti L., Schwartz R. H. Induction of competence to respond to IL-4 by CD4+ T helper type 1 cells requires costimulation. J Immunol. 1992 Aug 1;149(3):901–910. [PubMed] [Google Scholar]
- Dumont F. J., Staruch M. J., Koprak S. L., Melino M. R., Sigal N. H. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990 Jan 1;144(1):251–258. [PubMed] [Google Scholar]
- Freimuth W. W., Depper J. M., Nabel G. J. Regulation of the IL-2 receptor alpha-gene. Interaction of a kappa B binding protein with cell-specific transcription factors. J Immunol. 1989 Nov 1;143(9):3064–3068. [PubMed] [Google Scholar]
- Fryer J., Yatscoff R. W., Pascoe E. A., Thliveris J. The relationship of blood concentrations of rapamycin and cyclosporine to suppression of allograft rejection in a rabbit heterotopic heart transplant model. Transplantation. 1993 Feb;55(2):340–345. doi: 10.1097/00007890-199302000-00021. [DOI] [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J., Depper J. M. Growth of human T lymphocytes: an analysis of interleukin 2 and its cellular receptor. Prog Hematol. 1986;14:283–301. [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Taylor P. S., Norton S. D., Urdahl K. B. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991 Oct 15;147(8):2461–2466. [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987 Dec;7(12):4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan B. D., Chang J. Y., Sehgal S. N. Preclinical evaluation of a new potent immunosuppressive agent, rapamycin. Transplantation. 1991 Aug;52(2):185–191. doi: 10.1097/00007890-199108000-00001. [DOI] [PubMed] [Google Scholar]
- Kahan B. D., Gibbons S., Tejpal N., Stepkowski S. M., Chou T. C. Synergistic interactions of cyclosporine and rapamycin to inhibit immune performances of normal human peripheral blood lymphocytes in vitro. Transplantation. 1991 Jan;51(1):232–239. doi: 10.1097/00007890-199101000-00038. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Kromwel L., Doe S. E., Denyer M. Inhibition of T and B lymphocyte proliferation by rapamycin. Immunology. 1991 Apr;72(4):544–549. [PMC free article] [PubMed] [Google Scholar]
- Kimball P. M., Kerman R. H., Kahan B. D. Production of synergistic but nonidentical mechanisms of immunosuppression by rapamycin and cyclosporine. Transplantation. 1991 Feb;51(2):486–490. doi: 10.1097/00007890-199102000-00041. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Parsons M., Martin P. J., Hansen J. A., Rabinovitch P. S., June C. H. Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppression. J Immunol. 1986 Nov 15;137(10):3299–3305. [PubMed] [Google Scholar]
- Lin C. S., Boltz R. C., Siekierka J. J., Sigal N. H. FK-506 and cyclosporin A inhibit highly similar signal transduction pathways in human T lymphocytes. Cell Immunol. 1991 Apr 1;133(2):269–284. doi: 10.1016/0008-8749(91)90103-i. [DOI] [PubMed] [Google Scholar]
- Lu Y., Granelli-Piperno A., Bjorndahl J. M., Phillips C. A., Trevillyan J. M. CD28-induced T cell activation. Evidence for a protein-tyrosine kinase signal transduction pathway. J Immunol. 1992 Jul 1;149(1):24–29. [PubMed] [Google Scholar]
- Luo H. Y., Chen H. F., Daloze P., Chang J., St-Louis G., Wu J. P. In vitro IgE production by interleukin 4-stimulated human peripheral blood mononuclear cells is suppressed by rapamycin. Clin Immunol Immunopathol. 1991 Dec;61(3):410–420. doi: 10.1016/s0090-1229(05)80012-1. [DOI] [PubMed] [Google Scholar]
- Luo H., Chen H., Daloze P., Chang J. Y., St-Louis G., Wu J. Inhibition of in vitro immunoglobulin production by rapamycin. Transplantation. 1992 May;53(5):1071–1076. doi: 10.1097/00007890-199205000-00019. [DOI] [PubMed] [Google Scholar]
- Luo H., Chen H., Daloze P., Wu J. Effects of rapamycin on human HLA-unrestricted cell killing. Clin Immunol Immunopathol. 1992 Oct;65(1):60–64. doi: 10.1016/0090-1229(92)90248-m. [DOI] [PubMed] [Google Scholar]
- Mitchell L. C., Davis L. S., Lipsky P. E. Promotion of human T lymphocyte proliferation by IL-4. J Immunol. 1989 Mar 1;142(5):1548–1557. [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F. J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocci M. J., Matkovich D. A., Collier K. A., Kwok P., Dumont F., Lin S., Degudicibus S., Siekierka J. J., Chin J., Hutchinson N. I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989 Jul 15;143(2):718–726. [PubMed] [Google Scholar]
- Turka L. A., Linsley P. S., Paine R., 3rd, Schieven G. L., Thompson G. B., Ledbetter J. A. Signal transduction via CD4, CD8, and CD28 in mature and immature thymocytes. Implications for thymic selection. J Immunol. 1991 Mar 1;146(5):1428–1436. [PubMed] [Google Scholar]


