Abstract
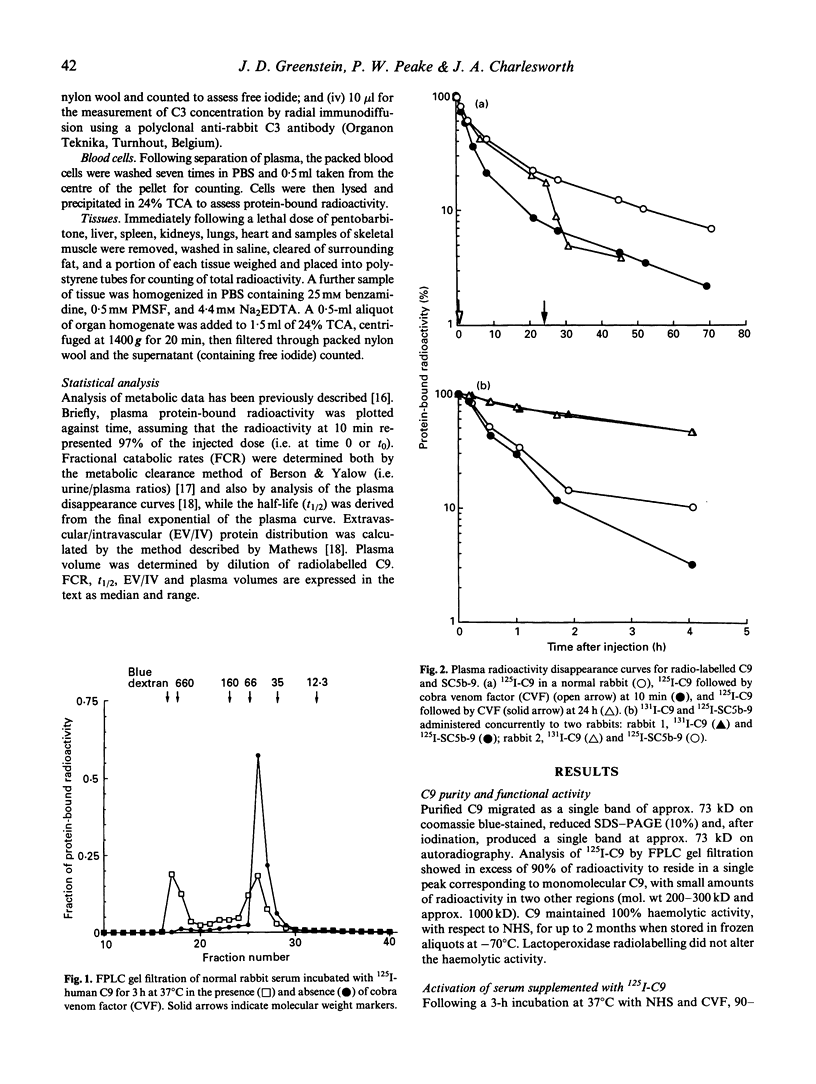
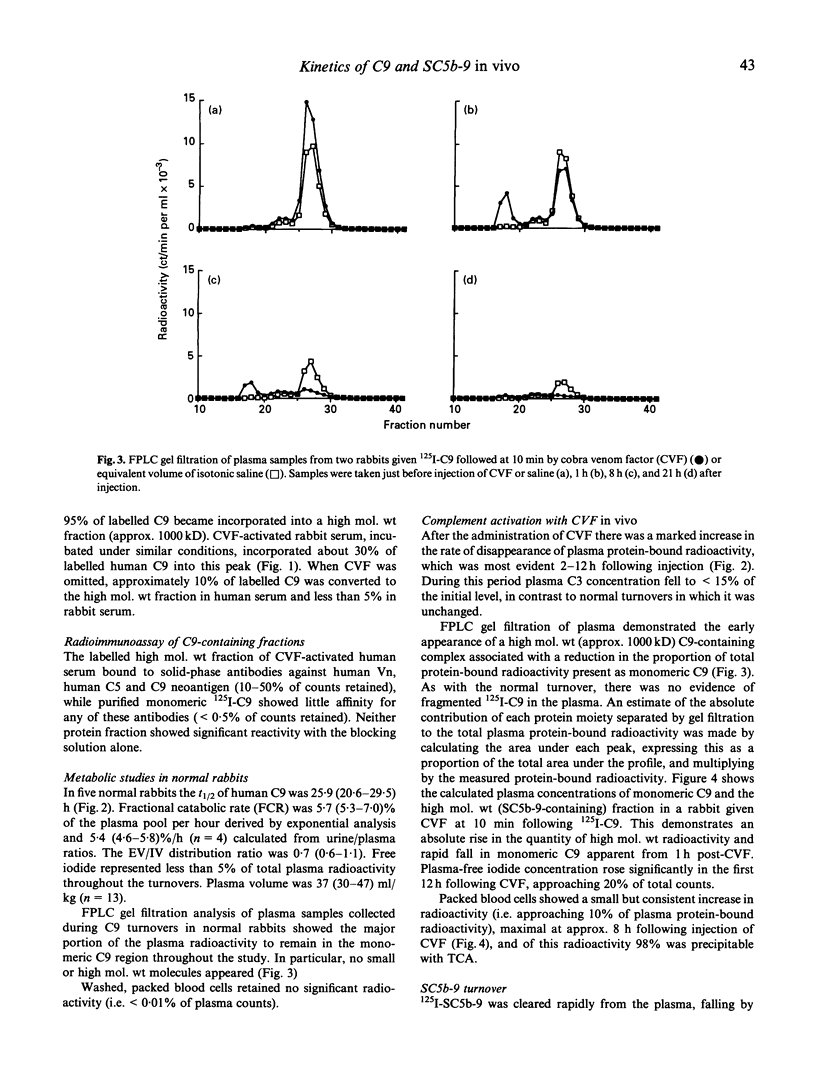
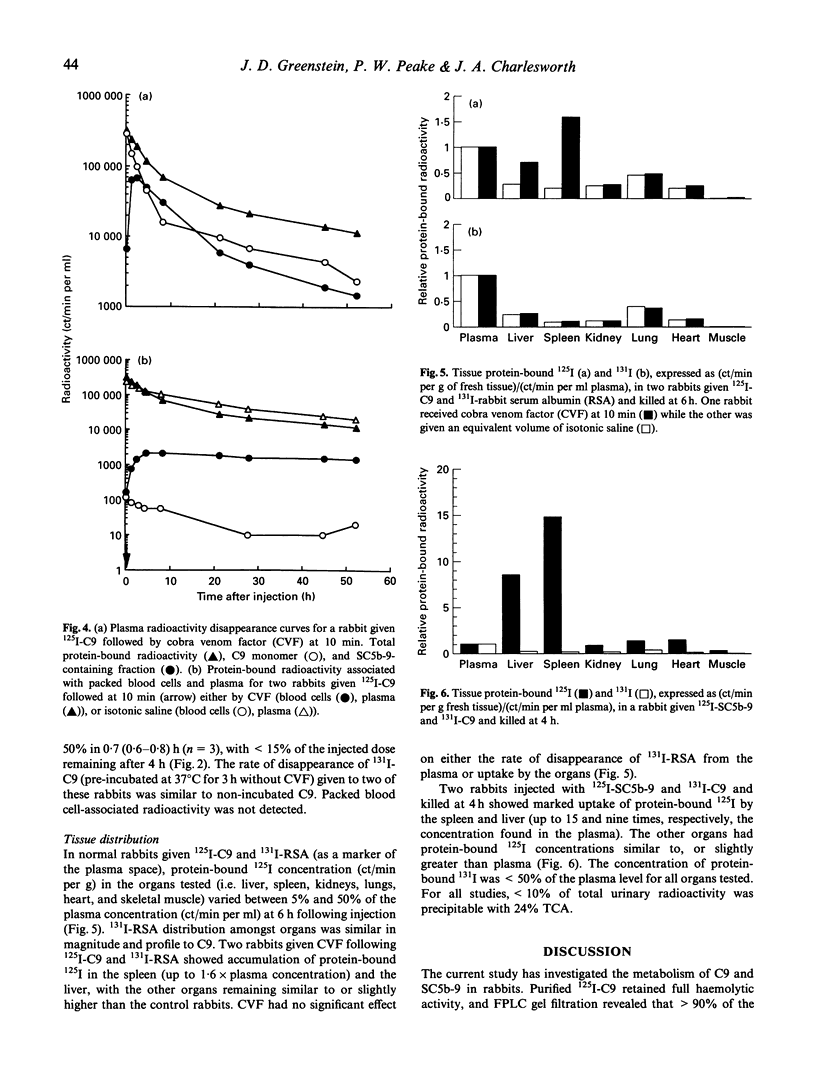
Many diseases associated with complement activation are characterized by tissue deposition of components of the terminal complement complex (TCC). The ninth component of complement (C9) plays an important role in the cytolytic effects, and may contribute to the non-lethal cell-regulating functions of the TCC. In this study we examined the behaviour of radiolabelled human C9 and its soluble complexed form SC5b-9 in vivo in order to determine the effects of complement activation on its turnover, distribution and molecular size. In normal rabbits the metabolic parameters of 125I-C9 (median and range) were: plasma half-life (t1/2) 25.9 (20.6-29.5) h, fractional catabolic rate (FCR) 5.7 (5.3-7.0)%/h, and extravascular/intravascular ratio (EV/IV) 0.7 (0.6-1.1). The distribution of radiolabelled C9 amongst body tissues was similar to that observed for rabbit serum albumin (RSA). Activation of the complement cascade with i.v. injection of cobra venom factor (CVF) resulted in rapid disappearance of C9 from the plasma and accumulation of protein-bound radiolabeled in the spleen (exceeding the plasma concentration) and the liver. RSA metabolism and distribution were unaffected by CVF. Fine performance liquid chromatography (FPLC) gel filtration of plasma samples suggested that monomeric C9 was the only major radiolabelled protein present during normal turnovers, whereas CVF administration was accompanied by the prompt appearance of a high mol. wt species consistent in size with SC5b-9. When injected directly, 125I-SC5b-9 disappeared rapidly from the plasma, falling by 50% in 0.7 (0.6-0.8) h, and less than 15% remaining after 4 h with accumulation of protein-bound label in the spleen and liver. These results demonstrate the complexity of C9 metabolism during complement activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERSON S. A., YALOW R. S. Distribution and metabolism of I131 labeled proteins in man. Fed Proc. 1957 Jul;16(2):suppl–18. [PubMed] [Google Scholar]
- Bhakdi S., Hugo F., Tranum-Jensen J. Functions and relevance of the terminal complement sequence. Blut. 1990 Jun;60(6):309–318. doi: 10.1007/BF01737843. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. C5b-9 assembly: average binding of one C9 molecule to C5b-8 without poly-C9 formation generates a stable transmembrane pore. J Immunol. 1986 Apr 15;136(8):2999–3005. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Molecular composition of the terminal membrane and fluid-phase C5b-9 complexes of rabbit complement. Absence of disulphide-bonded C9 dimers in the membrane complex. Biochem J. 1983 Mar 1;209(3):753–761. doi: 10.1042/bj2090753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Müller-Eberhard H. J. The ninth component of human complement: purification and physicochemical characterization. J Immunol. 1980 Mar;124(3):1291–1296. [PubMed] [Google Scholar]
- Charlesworth J. A., Scott D. M., Pussell B. A., Peters D. K. Metabolism of human beta 1H: studies in man and experimental animals. Clin Exp Immunol. 1979 Dec;38(3):397–404. [PMC free article] [PubMed] [Google Scholar]
- Charlesworth J. A., Williams D. G., Naish P., Lachmann P. J., Peters D. K. Metabolism of radio-labelled C3: effects of in vivo activation in rabbits. Clin Exp Immunol. 1974 Mar;16(3):445–452. [PMC free article] [PubMed] [Google Scholar]
- Couser W. G., Baker P. J., Adler S. Complement and the direct mediation of immune glomerular injury: a new perspective. Kidney Int. 1985 Dec;28(6):879–890. doi: 10.1038/ki.1985.214. [DOI] [PubMed] [Google Scholar]
- Dalmasso A. P., Falk R. J., Raij L. The pathobiology of the terminal complement complexes. Complement Inflamm. 1989;6(1):36–48. doi: 10.1159/000463070. [DOI] [PubMed] [Google Scholar]
- Deppisch R., Schmitt V., Bommer J., Hänsch G. M., Ritz E., Rauterberg E. W. Fluid phase generation of terminal complement complex as a novel index of bioincompatibility. Kidney Int. 1990 Feb;37(2):696–706. doi: 10.1038/ki.1990.36. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Podack E., Dalmasso A. P., Jennette J. C. Localization of S protein and its relationship to the membrane attack complex of complement in renal tissue. Am J Pathol. 1987 Apr;127(1):182–190. [PMC free article] [PubMed] [Google Scholar]
- French L. E., Tschopp J., Schifferli J. A. Clusterin in renal tissue: preferential localization with the terminal complement complex and immunoglobulin deposits in glomeruli. Clin Exp Immunol. 1992 Jun;88(3):389–393. doi: 10.1111/j.1365-2249.1992.tb06459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo F., Berstecher C., Krämer S., Fassbender W., Bhakdi S. In vivo clearance studies of the terminal fluid-phase complement complex in rabbits. Clin Exp Immunol. 1989 Jul;77(1):112–116. [PMC free article] [PubMed] [Google Scholar]
- Høgåsen K., Mollnes T. E., Harboe M. Heparin-binding properties of vitronectin are linked to complex formation as illustrated by in vitro polymerization and binding to the terminal complement complex. J Biol Chem. 1992 Nov 15;267(32):23076–23082. [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnes T. E. Early- and late-phase activation of complement evaluated by plasma levels of C3d,g and the terminal complement complex. Complement. 1985;2(2-3):156–164. doi: 10.1159/000467856. [DOI] [PubMed] [Google Scholar]
- Mollnes T. E., Lea T., Harboe M., Tschopp J. Monoclonal antibodies recognizing a neoantigen of poly(C9) detect the human terminal complement complex in tissue and plasma. Scand J Immunol. 1985 Aug;22(2):183–195. doi: 10.1111/j.1365-3083.1985.tb01870.x. [DOI] [PubMed] [Google Scholar]
- Morgan B. P. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989 Nov 15;264(1):1–14. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. A., Underwood P. A., Bean P. A., Sheehan M., Charlesworth J. A. Relative topography of biologically active domains of human vitronectin. Evidence from monoclonal antibody epitope and denaturation studies. J Biol Chem. 1994 Sep 23;269(38):23845–23852. [PubMed] [Google Scholar]
- O'Bryan M. K., Baker H. W., Saunders J. R., Kirszbaum L., Walker I. D., Hudson P., Liu D. Y., Glew M. D., d'Apice A. J., Murphy B. F. Human seminal clusterin (SP-40,40). Isolation and characterization. J Clin Invest. 1990 May;85(5):1477–1486. doi: 10.1172/JCI114594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkinson D. T., Baker P. J., Couser W. G., Johnson R. J., Adler S. Membrane attack complex deposition in experimental glomerular injury. Am J Pathol. 1985 Jul;120(1):121–128. [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Tschoop J., Müller-Eberhard H. J. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. doi: 10.1084/jem.156.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissner K. T. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]


