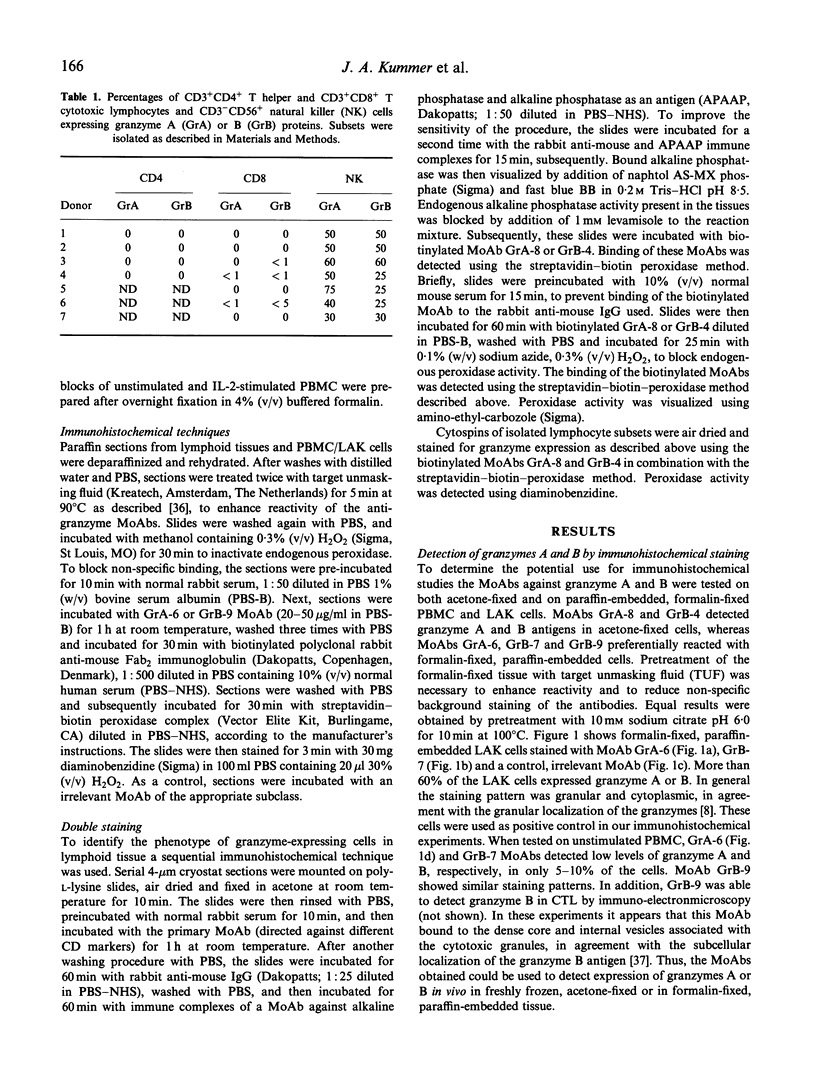
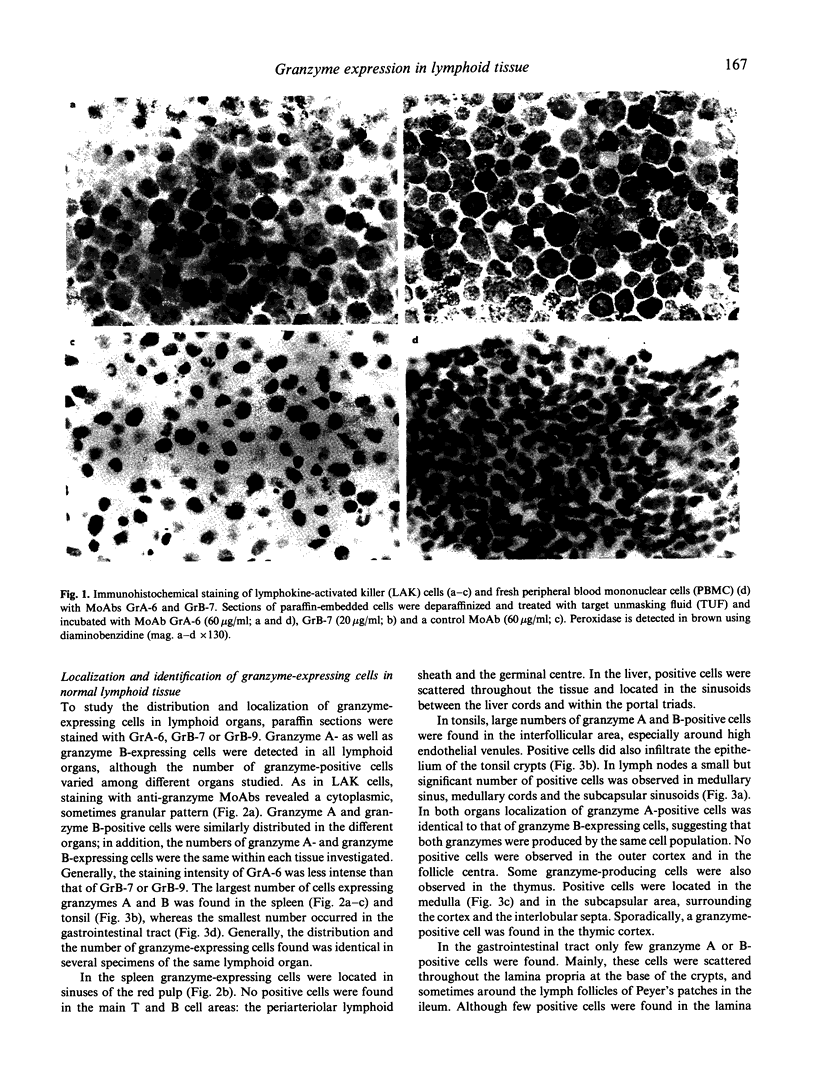
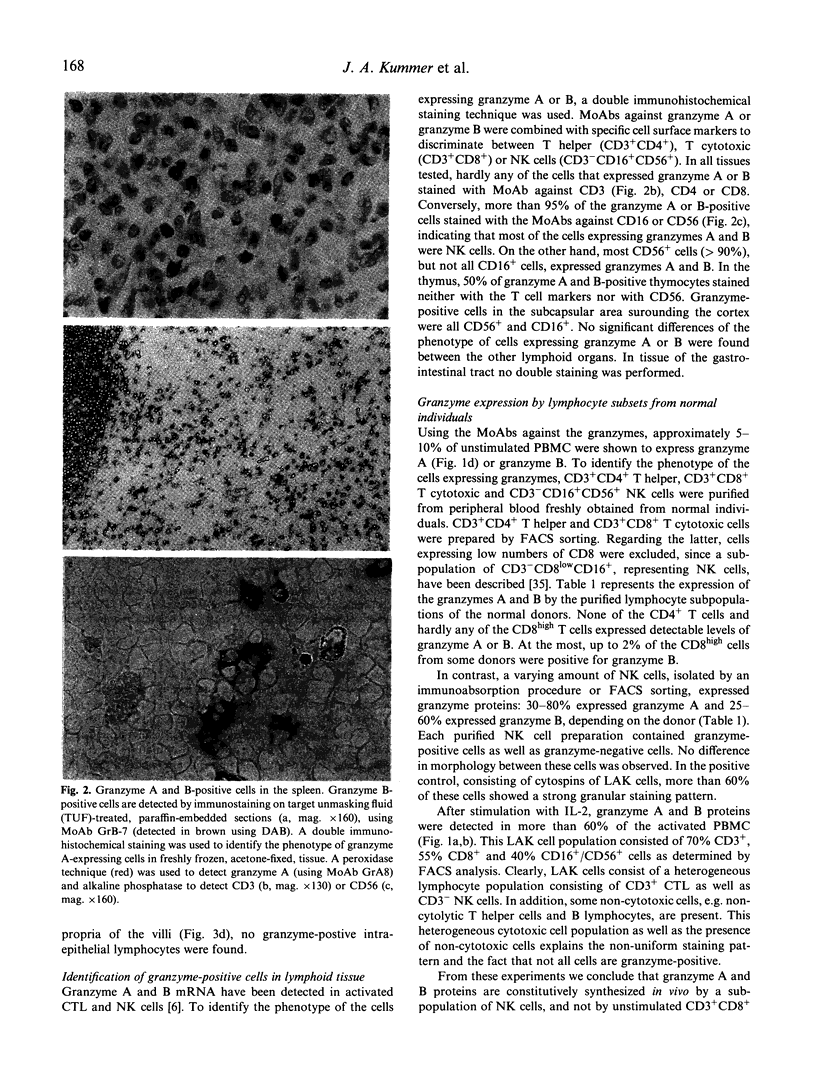
Abstract
Cytoplasmic granules from activated natural killer (NK) and cytotoxic T lymphocytes (CTL) contain a pore-forming protein, perforin, and several homologous serine proteinases called granzymes. Expression of these proteins correlates with the cytolytic potential of cytotoxic lymphocytes. Using a panel of MoAbs specific for human granzyme A and B, respectively, expression of these proteinases in non-pathological lymphoid tissue and peripheral blood lymphocyte (PBL) subpopulations was investigated. Using immunohistochemistry and double stainings, the phenotype of granzyme-expressing cells in lymphoid tissue was investigated. Granzyme-positive cells were detected in all lymphoid tissues tested. No large differences in the number and distribution between granzyme A- and granzyme B-positive cells were observed. The highest number of positive cells was located in the red pulp of the spleen. Significant numbers were detected in tonsil, lymph nodes, liver and thymus. Low numbers were present in the lamina propria of non-inflamed stomach, small intestine and colon. Phenotypic analysis and cell sorting showed that most of the granzyme-positive cells in lymphoid tissue and PBL consisted of CD3-CD16+CD56+ lymphocytes. Hardly any granzyme-positive CD3+CD8+ CTL were present in peripheral blood. The synthesis of granzyme A as well as B by both CD3+CD16+CD56+ and CD3+CD8+ cells in peripheral blood was increased upon IL-2 stimulation. These results indicate that in normal lymphoid tissue the predominant cytolytic cell population is formed by the NK cells, and activated CTL are rare.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunner G., Simon M. M., Kramer M. D. Activation of pro-urokinase by the human T cell-associated serine proteinase HuTSP-1. FEBS Lett. 1990 Jan 15;260(1):141–144. doi: 10.1016/0014-5793(90)80087-y. [DOI] [PubMed] [Google Scholar]
- Doherty P. C. Cell-mediated cytotoxicity. Cell. 1993 Nov 19;75(4):607–612. doi: 10.1016/0092-8674(93)90480-e. [DOI] [PubMed] [Google Scholar]
- Froelich C. J., Zhang X., Turbov J., Hudig D., Winkler U., Hanna W. L. Human granzyme B degrades aggrecan proteoglycan in matrix synthesized by chondrocytes. J Immunol. 1993 Dec 15;151(12):7161–7171. [PubMed] [Google Scholar]
- Garcia-Sanz J. A., MacDonald H. R., Jenne D. E., Tschopp J., Nabholz M. Cell specificity of granzyme gene expression. J Immunol. 1990 Nov 1;145(9):3111–3118. [PubMed] [Google Scholar]
- Garcia-Sanz J. A., Velotti F., MacDonald H. R., Masson D., Tschopp J., Nabholz M. Appearance of granule-associated molecules during activation of cytolytic T-lymphocyte precursors by defined stimuli. Immunology. 1988 May;64(1):129–134. [PMC free article] [PubMed] [Google Scholar]
- Garni-Wagner B. A., Witte P. L., Tutt M. M., Kuziel W. A., Tucker P. W., Bennett M., Kumar V. Natural killer cells in the thymus. Studies in mice with severe combined immune deficiency. J Immunol. 1990 Feb 1;144(3):796–803. [PubMed] [Google Scholar]
- Gershenfeld H. K., Hershberger R. J., Shows T. B., Weissman I. L. Cloning and chromosomal assignment of a human cDNA encoding a T cell- and natural killer cell-specific trypsin-like serine protease. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1184–1188. doi: 10.1073/pnas.85.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenfeld H. K., Weissman I. L. Cloning of a cDNA for a T cell-specific serine protease from a cytotoxic T lymphocyte. Science. 1986 May 16;232(4752):854–858. doi: 10.1126/science.2422755. [DOI] [PubMed] [Google Scholar]
- Griffiths G. M., Mueller C. Expression of perforin and granzymes in vivo: potential diagnostic markers for activated cytotoxic cells. Immunol Today. 1991 Nov;12(11):415–419. doi: 10.1016/0167-5699(91)90145-J. [DOI] [PubMed] [Google Scholar]
- Haddad P., Jenne D., Tschopp J., Clément M. V., Mathieu-Mahul D., Sasportes M. Structure and evolutionary origin of the human granzyme H gene. Int Immunol. 1991 Jan;3(1):57–66. doi: 10.1093/intimm/3.1.57. [DOI] [PubMed] [Google Scholar]
- Hameed A., Lowrey D. M., Lichtenheld M., Podack E. R. Characterization of three serine esterases isolated from human IL-2 activated killer cells. J Immunol. 1988 Nov 1;141(9):3142–3147. [PubMed] [Google Scholar]
- Hayes M. P., Berrebi G. A., Henkart P. A. Induction of target cell DNA release by the cytotoxic T lymphocyte granule protease granzyme A. J Exp Med. 1989 Sep 1;170(3):933–946. doi: 10.1084/jem.170.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W., MacDonald H. R., Mueller C. Expression of genes encoding cytotoxic cell-associated serine proteases in thymocytes. Int Immunol. 1990;2(1):57–62. doi: 10.1093/intimm/2.1.57. [DOI] [PubMed] [Google Scholar]
- Heusel J. W., Wesselschmidt R. L., Shresta S., Russell J. H., Ley T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994 Mar 25;76(6):977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- Hori T., Spits H. Clonal analysis of human CD4-CD8-CD3- thymocytes highly purified from postnatal thymus. J Immunol. 1991 Apr 1;146(7):2116–2121. [PubMed] [Google Scholar]
- Hudig D., Allison N. J., Kam C. M., Powers J. C. Selective isocoumarin serine protease inhibitors block RNK-16 lymphocyte granule-mediated cytolysis. Mol Immunol. 1989 Aug;26(8):793–798. doi: 10.1016/0161-5890(89)90040-0. [DOI] [PubMed] [Google Scholar]
- Hudig D., Allison N. J., Pickett T. M., Winkler U., Kam C. M., Powers J. C. The function of lymphocyte proteases. Inhibition and restoration of granule-mediated lysis with isocoumarin serine protease inhibitors. J Immunol. 1991 Aug 15;147(4):1360–1368. [PubMed] [Google Scholar]
- Hudig D., Haverty T., Fulcher C., Redelman D., Mendelsohn J. Inhibition of human natural cytotoxicity by macromolecular antiproteases. J Immunol. 1981 Apr;126(4):1569–1574. [PubMed] [Google Scholar]
- Jenne D. E., Masson D., Zimmer M., Haefliger J. A., Li W. H., Tschopp J. Isolation and complete structure of the lymphocyte serine protease granzyme G, a novel member of the granzyme multigene family in murine cytolytic T lymphocytes. Evolutionary origin of lymphocyte proteases. Biochemistry. 1989 Sep 19;28(19):7953–7961. doi: 10.1021/bi00445a060. [DOI] [PubMed] [Google Scholar]
- Jenne D. E., Tschopp J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol Rev. 1988 Mar;103:53–71. doi: 10.1111/j.1600-065x.1988.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Koizumi H., Liu C. C., Zheng L. M., Joag S. V., Bayne N. K., Holoshitz J., Young J. D. Expression of perforin and serine esterases by human gamma/delta T cells. J Exp Med. 1991 Feb 1;173(2):499–502. doi: 10.1084/jem.173.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krähenbühl O., Rey C., Jenne D., Lanzavecchia A., Groscurth P., Carrel S., Tschopp J. Characterization of granzymes A and B isolated from granules of cloned human cytotoxic T lymphocytes. J Immunol. 1988 Nov 15;141(10):3471–3477. [PubMed] [Google Scholar]
- Krähenbühl O., Tschopp J. Debate: the mechanism of lymphocyte-mediated killing. Perforin-induced pore formation. Immunol Today. 1991 Nov;12(11):399–403. doi: 10.1016/0167-5699(91)90139-k. [DOI] [PubMed] [Google Scholar]
- Kummer J. A., Kamp A. M., van Katwijk M., Brakenhoff J. P., Radosević K., van Leeuwen A. M., Borst J., Verweij C. L., Hack C. E. Production and characterization of monoclonal antibodies raised against recombinant human granzymes A and B and showing cross reactions with the natural proteins. J Immunol Methods. 1993 Jul 6;163(1):77–83. doi: 10.1016/0022-1759(93)90241-x. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lebow L. T., Bonavida B. Purification and characterization of cytolytic and noncytolytic human natural killer cell subsets. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6063–6067. doi: 10.1073/pnas.87.16.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenheld M. G., Olsen K. J., Lu P., Lowrey D. M., Hameed A., Hengartner H., Podack E. R. Structure and function of human perforin. Nature. 1988 Sep 29;335(6189):448–451. doi: 10.1038/335448a0. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Rafii S., Granelli-Piperno A., Trapani J. A., Young J. D. Perforin and serine esterase gene expression in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1989 Dec 1;170(6):2105–2118. doi: 10.1084/jem.170.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey D. M., Hameed A., Lichtenheld M., Podack E. R. Isolation and characterization of cytotoxic granules from human lymphokine (interleukin 2) activated killer cells. Cancer Res. 1988 Aug 15;48(16):4681–4688. [PubMed] [Google Scholar]
- Lu P., Garcia-Sanz J. A., Lichtenheld M. G., Podack E. R. Perforin expression in human peripheral blood mononuclear cells. Definition of an IL-2-independent pathway of perforin induction in CD8+ T cells. J Immunol. 1992 Jun 1;148(11):3354–3360. [PubMed] [Google Scholar]
- MacDermott R. P., Schmidt R. E., Caulfield J. P., Hein A., Bartley G. T., Ritz J., Schlossman S. F., Austen K. F., Stevens R. L. Proteoglycans in cell-mediated cytotoxicity. Identification, localization, and exocytosis of a chondroitin sulfate proteoglycan from human cloned natural killer cells during target cell lysis. J Exp Med. 1985 Dec 1;162(6):1771–1787. doi: 10.1084/jem.162.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M., Kwong P. C., Frégeau C. J., Atkinson E. A., Burrington M., Ehrman N., Sorensen O., Lin C. C., Wilkins J., Bleackley R. C. Cloning of a gene that encodes a new member of the human cytotoxic cell protease family. Biochemistry. 1990 May 1;29(17):4042–4049. doi: 10.1021/bi00469a003. [DOI] [PubMed] [Google Scholar]
- Michon J. M., Caligiuri M. A., Hazanow S. M., Levine H., Schlossman S. F., Ritz J. Induction of natural killer effectors from human thymus with recombinant IL-2. J Immunol. 1988 May 15;140(10):3660–3667. [PubMed] [Google Scholar]
- Nakata M., Smyth M. J., Norihisa Y., Kawasaki A., Shinkai Y., Okumura K., Yagita H. Constitutive expression of pore-forming protein in peripheral blood gamma/delta T cells: implication for their cytotoxic role in vivo. J Exp Med. 1990 Dec 1;172(6):1877–1880. doi: 10.1084/jem.172.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Peters P. J., Borst J., Oorschot V., Fukuda M., Krähenbühl O., Tschopp J., Slot J. W., Geuze H. J. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991 May 1;173(5):1099–1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell F. J., Golub S. H. Generation of lymphokine-activated killer cell activity from human thymocytes. J Immunol. 1987 Sep 1;139(5):1446–1453. [PubMed] [Google Scholar]
- Rolstad B., Herberman R. B., Reynolds C. W. Natural killer cell activity in the rat. V. The circulation patterns and tissue localization of peripheral blood large granular lymphocytes (LGL). J Immunol. 1986 Apr 15;136(8):2800–2808. [PubMed] [Google Scholar]
- Salcedo T. W., Azzoni L., Wolf S. F., Perussia B. Modulation of perforin and granzyme messenger RNA expression in human natural killer cells. J Immunol. 1993 Sep 1;151(5):2511–2520. [PubMed] [Google Scholar]
- Schmid J., Weissmann C. Induction of mRNA for a serine protease and a beta-thromboglobulin-like protein in mitogen-stimulated human leukocytes. J Immunol. 1987 Jul 1;139(1):250–256. [PubMed] [Google Scholar]
- Shiver J. W., Su L., Henkart P. A. Cytotoxicity with target DNA breakdown by rat basophilic leukemia cells expressing both cytolysin and granzyme A. Cell. 1992 Oct 16;71(2):315–322. doi: 10.1016/0092-8674(92)90359-k. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Simon H. G., Fruth U., Epplen J., Müller-Hermelink H. K., Kramer M. D. Cloned cytolytic T-effector cells and their malignant variants produce an extracellular matrix degrading trypsin-like serine proteinase. Immunology. 1987 Feb;60(2):219–230. [PMC free article] [PubMed] [Google Scholar]
- Smyth M. J., Strobl S. L., Young H. A., Ortaldo J. R., Ochoa A. C. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol. 1991 May 15;146(10):3289–3297. [PubMed] [Google Scholar]
- Trapani J. A., Klein J. L., White P. C., Dupont B. Molecular cloning of an inducible serine esterase gene from human cytotoxic lymphocytes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6924–6928. doi: 10.1073/pnas.85.18.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanguri P., Lee E., Henkart P., Shin M. L. Hydrolysis of myelin basic protein in myelin membranes by granzymes of large granular lymphocytes. J Immunol. 1993 Mar 15;150(6):2431–2439. [PubMed] [Google Scholar]
- Velotti F., Palmieri G., D'Ambrosio D., Piccoli M., Frati L., Santoni A. Differential expression of granzyme A and granzyme B proteases and their secretion by fresh rat natural killer cells (NK) and lymphokine-activated killer cells with NK phenotype (LAK-NK). Eur J Immunol. 1992 Apr;22(4):1049–1053. doi: 10.1002/eji.1830220426. [DOI] [PubMed] [Google Scholar]
- Vroom T. M., Scholte G., Ossendorp F., Borst J. Tissue distribution of human gamma delta T cells: no evidence for general epithelial tropism. J Clin Pathol. 1991 Dec;44(12):1012–1017. doi: 10.1136/jcp.44.12.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside T. L., Herberman R. B. The role of natural killer cells in human disease. Clin Immunol Immunopathol. 1989 Oct;53(1):1–23. doi: 10.1016/0090-1229(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Young L. H., Joag S. V., Lin P. Y., Luo S. F., Zheng L. M., Liu C. C., Young J. D. Expression of cytolytic mediators by synovial fluid lymphocytes in rheumatoid arthritis. Am J Pathol. 1992 May;140(5):1261–1268. [PMC free article] [PubMed] [Google Scholar]
- de Bruin P. C., Kummer J. A., van der Valk P., van Heerde P., Kluin P. M., Willemze R., Ossenkoppele G. J., Radaszkiewicz T., Meijer C. J. Granzyme B-expressing peripheral T-cell lymphomas: neoplastic equivalents of activated cytotoxic T cells with preference for mucosa-associated lymphoid tissue localization. Blood. 1994 Dec 1;84(11):3785–3791. [PubMed] [Google Scholar]
- van den Brink M. R., Palomba M. L., Basse P. H., Hiserodt J. C. In situ localization of 3.2.3+ natural killer cells in tissues from normal and tumor-bearing rats. Cancer Res. 1991 Sep 15;51(18):4931–4936. [PubMed] [Google Scholar]