Abstract
MRL-lpr/lpr mice spontaneously develop a severe autoimmune syndrome, characterized by massive generalized lymphadenopathy, arthritis, arteritis, dermatitis and immune complex-mediated glomerulonephritis. Bone marrow transplantation (BMT) from MHC-matched systemic lupus erythematosus (SLE)-resistant donors to susceptible recipients has proved effective in correcting autoimmune manifestations in autoimmune-prone mice. We investigated the effect of syngeneic BMT from MRL/lpr (donor) to immunocompromised MRL/lpr (recipient), after purging the bone marrow inoculum with MoAbs against mature T cells (anti-Thy 1.2). All the untreated mice developed lymphadenopathy and by the age of 36 weeks five of the eight were dead; in contrast, all the mice which underwent syngeneic BMT following acute immunosuppression with total body irradiation (900 cGy) (TBI) remained disease-free. In an additional experiment, it was found that conditioning with cyclophosphamide (CY) before BMT was more effective than TBI in inhibiting delayed-onset autoimmune manifestations (mean survival 350 days in the CY group and 305 days in the TBI group, versus 197 days in untreated controls). Under both immunosuppressive regimens T cell-depleted bone marrow grafts produced far better results than did unmanipulated BMT. Following syngeneic BMT the incidence of proteinuria and the level of serum anti-DNA (dd) antibodies were significantly reduced, compared with that of the age-matched untreated controls. CY was more effective than TBI in reducing the anti-DNA titres. Likewise, T depletion of bone marrow inocula before BMT induced a more drastic drop in autoantibodies, following both CY and TBI conditioning protocols. After syngeneic BMT (either CY or TBI) no signs of lymphadenopathy were observed even at an advanced age. Upon histopathological examination, the BMT-treated mice displayed normal glomeruli with occasional minimal signs of glomerulonephritis. Syngeneic T cell-depleted BMT following acute cytoreduction of anti-self immune lymphocytes may represent a new therapeutic approach for drug-resistant autoimmune diseases.
Full text
PDF

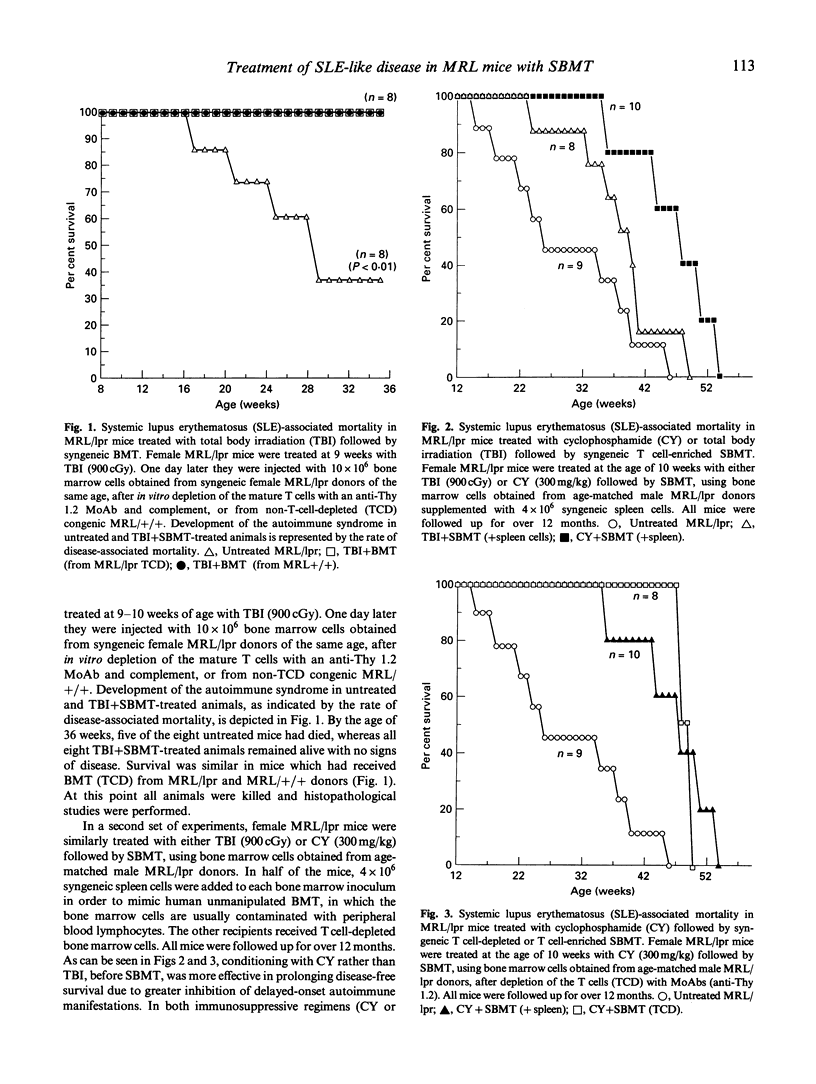
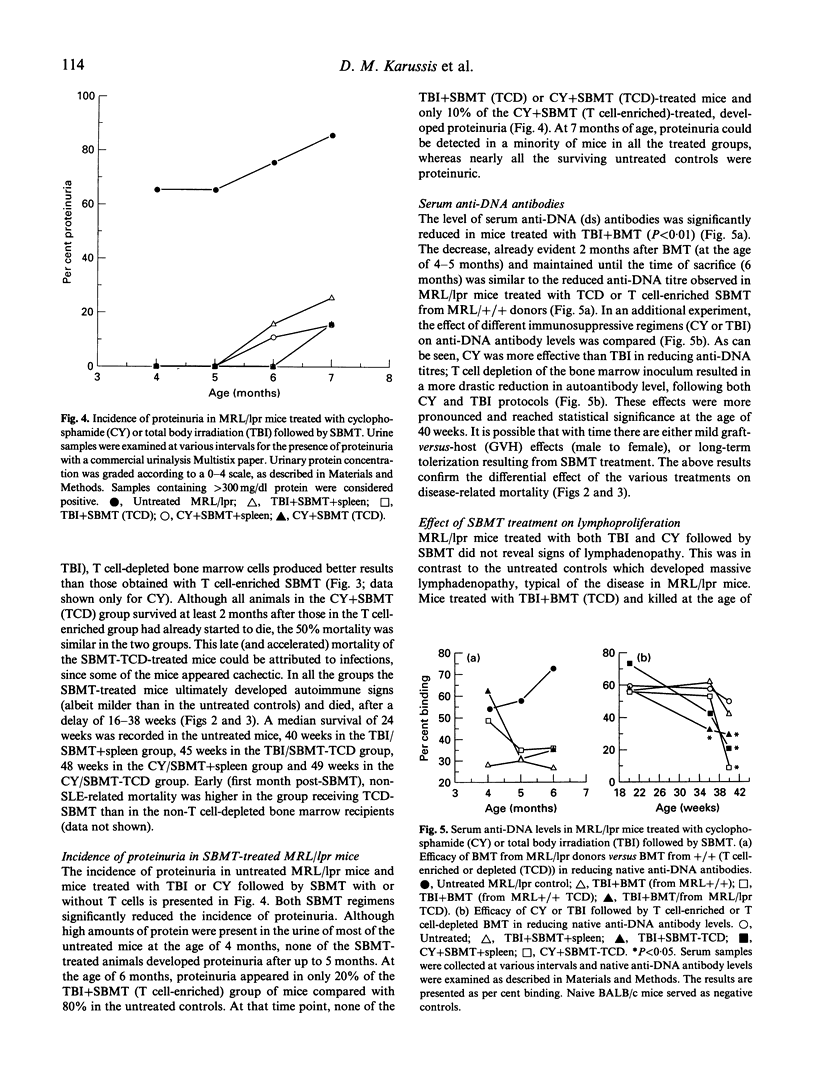
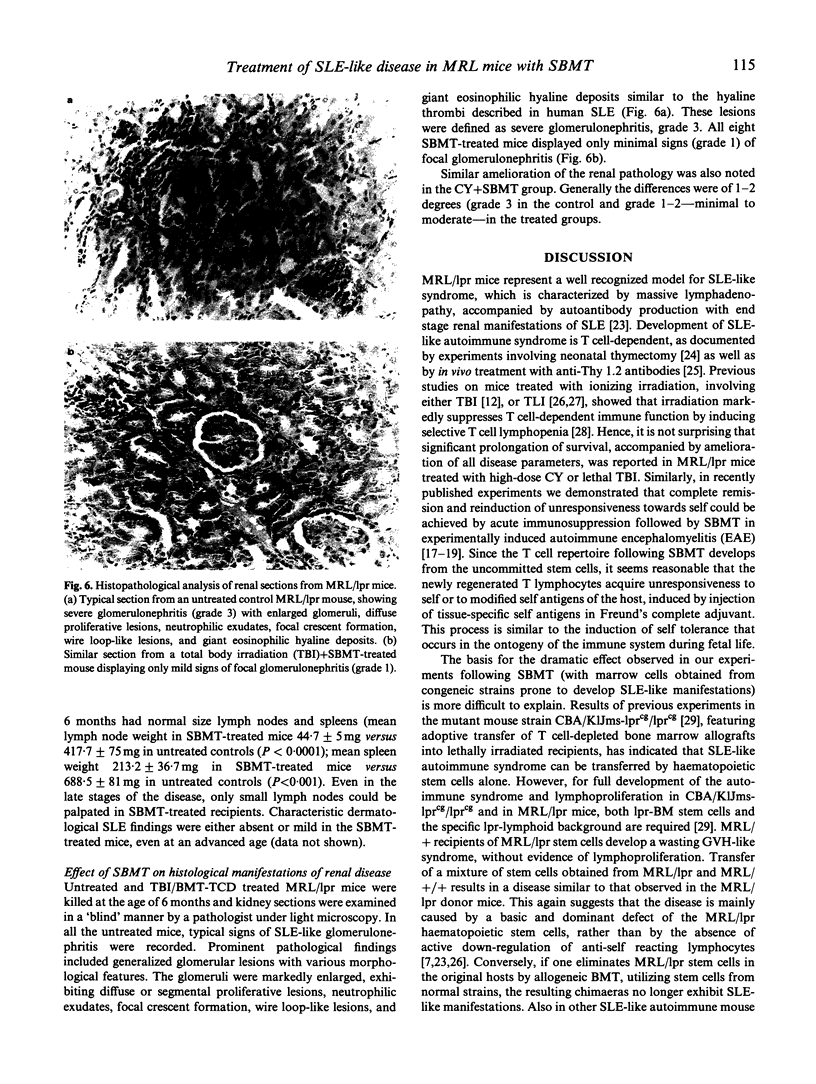


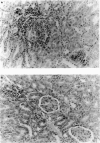
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balow J. E., Austin H. A., 3rd, Tsokos G. C., Antonovych T. T., Steinberg A. D., Klippel J. H. NIH conference. Lupus nephritis. Ann Intern Med. 1987 Jan;106(1):79–94. doi: 10.7326/0003-4819-106-1-79. [DOI] [PubMed] [Google Scholar]
- Cook S. D., Devereux C., Troiano R., Hafstein M. P., Zito G., Hernandez E., Lavenhar M., Vidaver R., Dowling P. C. Effect of total lymphoid irradiation in chronic progressive multiple sclerosis. Lancet. 1986 Jun 21;1(8495):1405–1409. doi: 10.1016/s0140-6736(86)91554-0. [DOI] [PubMed] [Google Scholar]
- Duwe A. K., Singhal S. K. The immunoregulatory role of bone marrow. II. Characterization of a suppressor cell inhibiting the in vitro antibody response. Cell Immunol. 1979 Mar 15;43(2):372–381. doi: 10.1016/0008-8749(79)90181-3. [DOI] [PubMed] [Google Scholar]
- Ginsberg B., Keiser H. A Millipore filter assay for antibodies to native DNA in sera of patients with systemic lupus erythematosus. Arthritis Rheum. 1973 Mar-Apr;16(2):199–207. doi: 10.1002/art.1780160210. [DOI] [PubMed] [Google Scholar]
- Ginzler E. M., Bollet A. J., Friedman E. A. The natural history and response to therapy of lupus nephritis. Annu Rev Med. 1980;31:463–487. doi: 10.1146/annurev.me.31.020180.002335. [DOI] [PubMed] [Google Scholar]
- Hang L., Theofilopoulos A. N., Balderas R. S., Francis S. J., Dixon F. J. The effect of thymectomy on lupus-prone mice. J Immunol. 1984 Apr;132(4):1809–1813. [PubMed] [Google Scholar]
- Hang L., Theofilopoulos A. N., Dixon F. J. A spontaneous rheumatoid arthritis-like disease in MRL/l mice. J Exp Med. 1982 Jun 1;155(6):1690–1701. doi: 10.1084/jem.155.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himeno K., Good R. A. Marrow transplantation from tolerant donors to treat and prevent autoimmune diseases in BXSB mice. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2235–2239. doi: 10.1073/pnas.85.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Yasumizu R., Inaba M., Izui S., Hayakawa K., Sekita K., Toki J., Sugiura K., Iwai H., Nakamura T. Long-term observations of autoimmune-prone mice treated for autoimmune disease by allogeneic bone marrow transplantation. Proc Natl Acad Sci U S A. 1989 May;86(9):3306–3310. doi: 10.1073/pnas.86.9.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya M., Sohen S., Yamane T., Tanaka S. Effective treatment of mice with type II collagen induced arthritis with lethal irradiation and bone marrow transplantation. J Rheumatol. 1993 Feb;20(2):225–230. [PubMed] [Google Scholar]
- Karussis D. M., Slavin S., Ben-Nun A., Ovadia H., Vourka-Karussis U., Lehmann D., Mizrachi-Kol R., Abramsky O. Chronic-relapsing experimental autoimmune encephalomyelitis (CR-EAE): treatment and induction of tolerance, with high dose cyclophosphamide followed by syngeneic bone marrow transplantation. J Neuroimmunol. 1992 Aug;39(3):201–210. doi: 10.1016/0165-5728(92)90254-i. [DOI] [PubMed] [Google Scholar]
- Karussis D. M., Slavin S., Lehmann D., Mizrachi-Koll R., Abramsky O., Ben-Nun A. Prevention of experimental autoimmune encephalomyelitis and induction of tolerance with acute immunosuppression followed by syngeneic bone marrow transplantation. J Immunol. 1992 Mar 15;148(6):1693–1698. [PubMed] [Google Scholar]
- Karussis D. M., Vourka-Karussis U., Lehmann D., Ovadia H., Mizrachi-Koll R., Ben-Nun A., Abramsky O., Slavin S. Prevention and reversal of adoptively transferred, chronic relapsing experimental autoimmune encephalomyelitis with a single high dose cytoreductive treatment followed by syngeneic bone marrow transplantation. J Clin Invest. 1993 Aug;92(2):765–772. doi: 10.1172/JCI116648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Katagiri T., Kikuchi Y., Shimada K., Nariuchi H., Wakabayashi T., Matsuzawa A. Role of bone marrow cells in autoantibody production and lymphoproliferation in the novel mutant strain of mice, CBA/KlJms-lprcg/lprcg. Eur J Immunol. 1991 Jan;21(1):63–69. doi: 10.1002/eji.1830210111. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Strober S. Reversal of nzb/nzw disease with total lymphoid irradiation. J Exp Med. 1979 Aug 1;150(2):371–378. doi: 10.1084/jem.150.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal R. M., Cohen M. L., Atkinson K., Biggs J. C. Apparent cure of rheumatoid arthritis by bone marrow transplantation. J Rheumatol. 1993 Jan;20(1):137–140. [PubMed] [Google Scholar]
- McIntosh K. R., Segre M., Segre D. Characterization of cyclophosphamide-induced suppressor cells. Immunopharmacology. 1982 Aug;4(4):279–289. doi: 10.1016/0162-3109(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Morton J. I., Siegel B. V. Transplantation of autoimmune potential. IV. Reversal of the NZB autoimmune syndrome by bone marrow transplantation. Transplantation. 1979 Feb;27(2):133–134. [PubMed] [Google Scholar]
- Moscovitch M., Rosenmann E., Neeman Z., Slavin S. Successful treatment of autoimmune manifestations in MRL/l and MRL/n mice using total lymphoid irradiation (TLI). Exp Mol Pathol. 1983 Feb;38(1):33–47. doi: 10.1016/0014-4800(83)90096-5. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Wofsy D., Greenspan J. S., Ledbetter J. A. Treatment of autoimmune MRL/Ipr mice with monoclonal antibody to Thy-1.2: a single injection has sustained effects on lymphoproliferation and renal disease. J Immunol. 1983 Apr;130(4):1713–1718. [PubMed] [Google Scholar]
- Slavin S. Successful treatment of autoimmune disease in (NZB/NZW)F1 female mice by using fractionated total lymphoid irradiation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5274–5276. doi: 10.1073/pnas.76.10.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin S. Treatment of life-threatening autoimmune diseases with myeloablative doses of immunosuppressive agents: experimental background and rationale for ABMT. Bone Marrow Transplant. 1993 Jul;12(1):85–88. [PubMed] [Google Scholar]
- Steinberg A. D., Steinberg S. C. Long-term preservation of renal function in patients with lupus nephritis receiving treatment that includes cyclophosphamide versus those treated with prednisone only. Arthritis Rheum. 1991 Aug;34(8):945–950. doi: 10.1002/art.1780340803. [DOI] [PubMed] [Google Scholar]
- Strober S., Kotzin B., Field E., Hoppe R., Myers B., Tanay A. Treatment of autoimmune disease with total lymphoid irradiation. Cellular and humoral mechanisms. Ann N Y Acad Sci. 1986;475:285–295. doi: 10.1111/j.1749-6632.1986.tb20877.x. [DOI] [PubMed] [Google Scholar]
- Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol. 1984;2:219–237. doi: 10.1146/annurev.iy.02.040184.001251. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Balderas R., Shawler D. L., Izui S., Kotzin B. L., Strober S., Dixon F. J. Inhibition of T cells proliferation and SLE-like syndrome of MRL/1 mice by whole body or total lymphoid irradiation. J Immunol. 1980 Nov;125(5):2137–2142. [PubMed] [Google Scholar]
- Theofilopoulos A. N. Role of the thymus in murine lupus and cellular transfer of the disease. Arthritis Rheum. 1982 Jul;25(7):726–733. doi: 10.1002/art.1780250703. [DOI] [PubMed] [Google Scholar]
- Tochner Z., Slavin S. Immune modulation by ionized irradiation. Curr Opin Immunol. 1988 Dec;1(2):261–268. doi: 10.1016/0952-7915(88)90012-x. [DOI] [PubMed] [Google Scholar]
- Yin J. A., Jowitt S. N. Resolution of immune-mediated diseases following allogeneic bone marrow transplantation for leukaemia. Bone Marrow Transplant. 1992 Jan;9(1):31–33. [PubMed] [Google Scholar]
- van Gelder M., Kinwel-Bohré E. P., van Bekkum D. W. Treatment of experimental allergic encephalomyelitis in rats with total body irradiation and syngeneic BMT. Bone Marrow Transplant. 1993 Mar;11(3):233–241. [PubMed] [Google Scholar]