Abstract
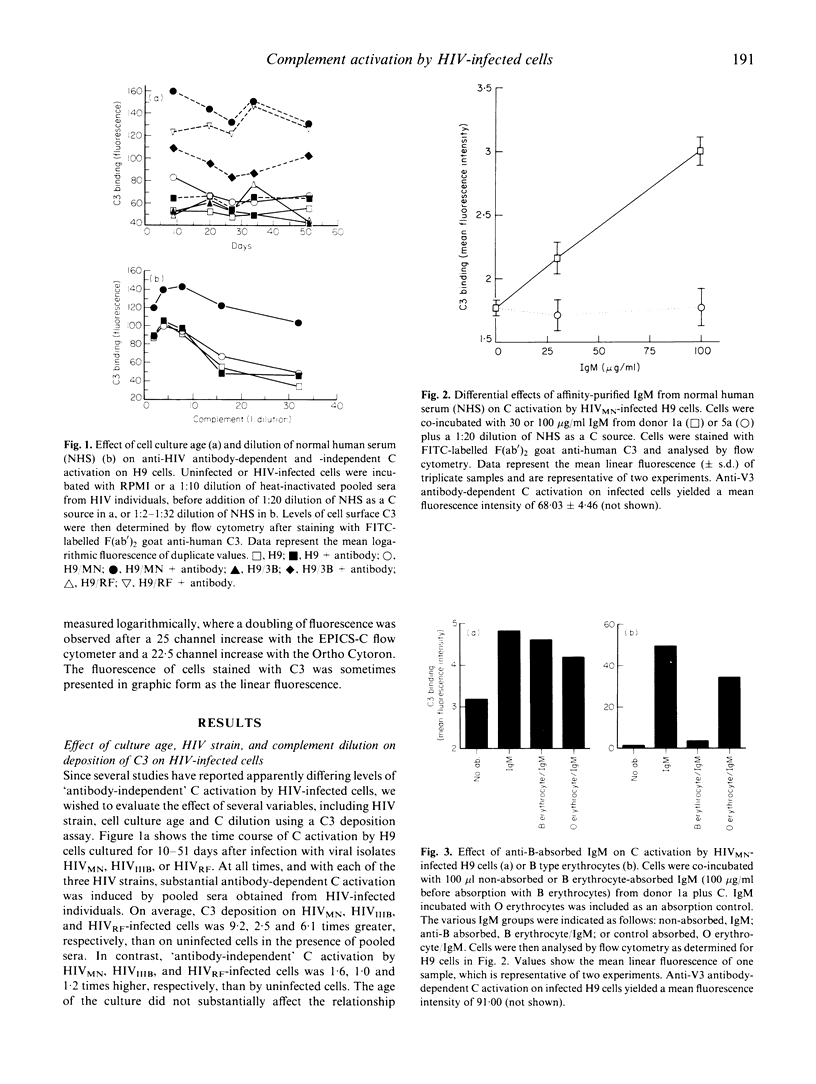
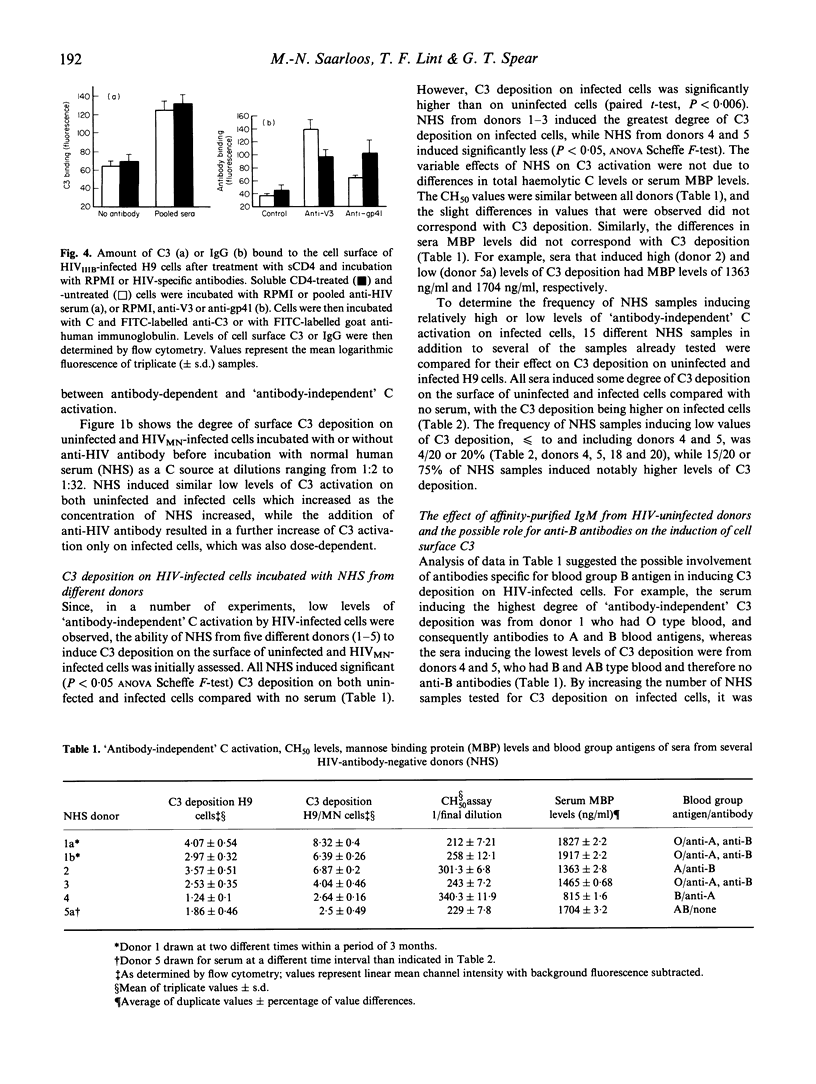
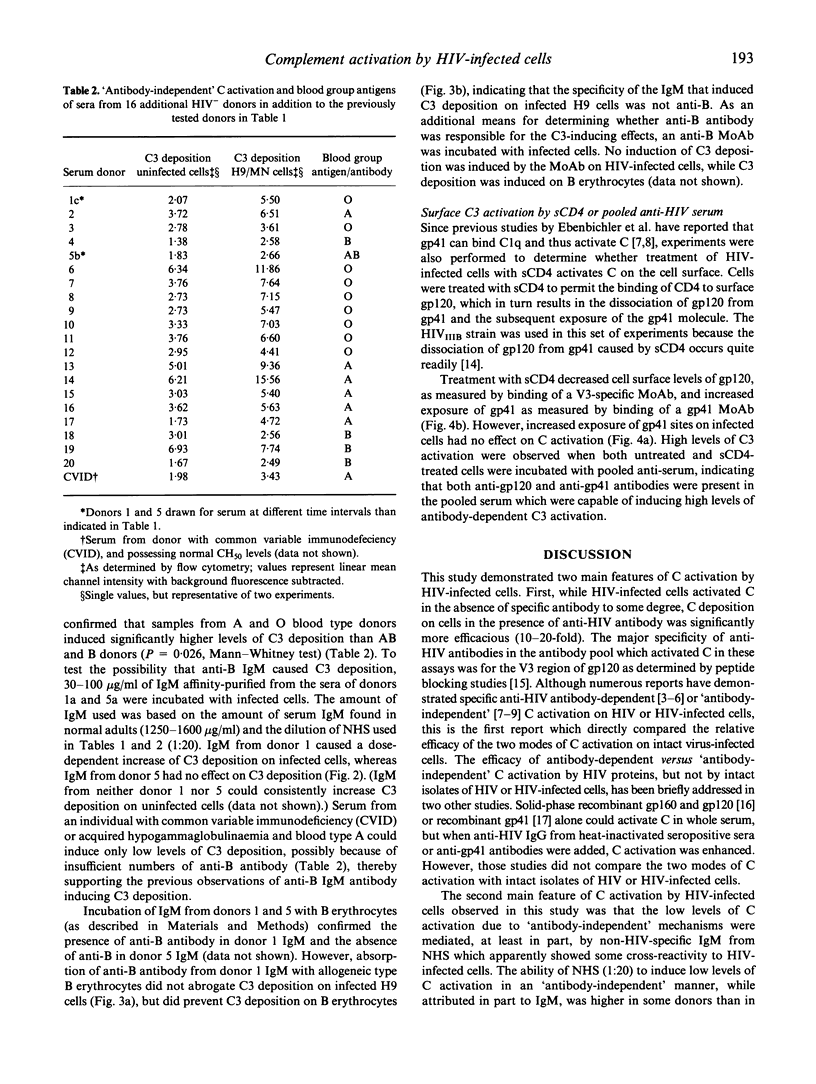
Previous studies in this laboratory have shown that efficient activation of complement (C) on HIV isolates and HIV-infected cells requires the binding of specific anti-HIV antibodies, while other investigators have observed 'antibody-independent' C activation. In an attempt to clarify these disparate findings, we investigated the effect of several variables on C activation by HIV-infected cells using flow cytometric analysis of C3 deposition. Antibody-mediated C activation using pooled sera from infected persons or human MoAbs directed against the V3 region of gp120 was always substantially higher than activation without antibody. Normal human serum (NHS) from a subset of HIV antibody-negative donors did, however, induce low levels of C3 deposition. Differences in C3 activation between the various NHS did not correlate with total haemolytic C levels or mannose-binding protein (MBP) levels. IgM isolated from NHS that induced high levels of C activation was at least partly responsible for the 'antibody-independent' C activation. Although there appeared to be a correlation between NHS that induced C activation and the presence of anti-blood type B IgM, absorption of anti-B did not abrogate the C3 deposition. Additionally, MoAb to the B antigen did not induce C3 deposition. These studies show that IgM in sera from HIV-uninfected donors can induce C3 deposition on HIV-infected cells, but that specific antibody-dependent C activation is substantially more efficient. Therefore, 'antibody-independent' C activation on HIV-infected cells may, in some cases, be more accurately described as HIV-cross-reactive antibody-dependent C activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendrup M., Hansen J. E., Clausen H., Nielsen C., Mathiesen L. R., Nielsen J. O. Antibody to histo-blood group A antigen neutralizes HIV produced by lymphocytes from blood group A donors but not from blood group B or O donors. AIDS. 1991 Apr;5(4):441–444. doi: 10.1097/00002030-199104000-00014. [DOI] [PubMed] [Google Scholar]
- Dierich M. P., Ebenbichler C. F., Marschang P., Füst G., Thielens N. M., Arlaud G. J. HIV and human complement: mechanisms of interaction and biological implication. Immunol Today. 1993 Sep;14(9):435–440. doi: 10.1016/0167-5699(93)90246-H. [DOI] [PubMed] [Google Scholar]
- Ebenbichler C. F., Thielens N. M., Vornhagen R., Marschang P., Arlaud G. J., Dierich M. P. Human immunodeficiency virus type 1 activates the classical pathway of complement by direct C1 binding through specific sites in the transmembrane glycoprotein gp41. J Exp Med. 1991 Dec 1;174(6):1417–1424. doi: 10.1084/jem.174.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Kuhlman M., Groopman J. E., Byrn R. A. A human serum mannose-binding protein inhibits in vitro infection by the human immunodeficiency virus. J Exp Med. 1989 Jan 1;169(1):185–196. doi: 10.1084/jem.169.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genesca J., Shih J. W., Jett B. W., Hewlett I. K., Epstein J. S., Alter H. J. What do western blot indeterminate patterns for human immunodeficiency virus mean in EIA-negative blood donors? Lancet. 1989 Oct 28;2(8670):1023–1025. doi: 10.1016/s0140-6736(89)91027-1. [DOI] [PubMed] [Google Scholar]
- Gregersen J. P., Mehdi S., Baur A., Hilfenhaus J. Antibody- and complement-mediated lysis of HIV-infected cells and inhibition of viral replication. J Med Virol. 1990 Apr;30(4):287–293. doi: 10.1002/jmv.1890300411. [DOI] [PubMed] [Google Scholar]
- Hidvégi T., Prohászka Z., Ujhelyi E., Thielens N. M., Dierich M. P., Hampl H., Arlaud G., Nagy K., Füst G. Studies on the mechanism of complement-mediated inhibition of antibody binding to HIV gp41. Clin Exp Immunol. 1993 Dec;94(3):490–493. doi: 10.1111/j.1365-2249.1993.tb08223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschang P., Gürtler L., Tötsch M., Thielens N. M., Arlaud G. J., Hittmair A., Katinger H., Dierich M. P. HIV-1 and HIV-2 isolates differ in their ability to activate the complement system on the surface of infected cells. AIDS. 1993 Jul;7(7):903–910. doi: 10.1097/00002030-199307000-00001. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Huang Y. X., Ashkenazi A., Ho D. D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992 Jan;66(1):235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Gewurz H., Osofsky S. G., Lint T. F. Inherited deficiency of the seventh component of complement associated with nephritis. Propensity to formation of C56 and related C7-consuming activity. J Clin Invest. 1978 Jun;61(6):1602–1610. doi: 10.1172/JCI109080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman M., Malde R., Contreras M. Comparison of IgM and IgG anti-A and anti-B levels in Asian, Caucasian and Negro donors in the North West Thames Region. Vox Sang. 1990;59(2):89–91. doi: 10.1111/j.1423-0410.1990.tb05016.x. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Gorny M. K., Xu J. Y., Mitchell W. M., Zolla-Pazner S. Two immunodominant domains of gp41 bind antibodies which enhance human immunodeficiency virus type 1 infection in vitro. J Virol. 1991 Aug;65(8):4169–4176. doi: 10.1128/jvi.65.8.4169-4176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. A role for natural antibody in the pathogenesis of leprosy: antibody in nonimmune serum mediates C3 fixation to the Mycobacterium leprae surface and hence phagocytosis by human mononuclear phagocytes. Infect Immun. 1994 Jan;62(1):280–289. doi: 10.1128/iai.62.1.280-289.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear G. T. Interaction of non-antibody factors with HIV in plasma. AIDS. 1993 Sep;7(9):1149–1157. doi: 10.1097/00002030-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Spear G. T. Interaction of non-antibody factors with HIV in plasma. AIDS. 1993 Sep;7(9):1149–1157. doi: 10.1097/00002030-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Spear G. T., Landay A. L., Sullivan B. L., Dittel B., Lint T. F. Activation of complement on the surface of cells infected by human immunodeficiency virus. J Immunol. 1990 Feb 15;144(4):1490–1496. [PubMed] [Google Scholar]
- Spear G. T., Takefman D. M., Sullivan B. L., Landay A. L., Zolla-Pazner S. Complement activation by human monoclonal antibodies to human immunodeficiency virus. J Virol. 1993 Jan;67(1):53–59. doi: 10.1128/jvi.67.1.53-59.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sölder B. M., Schulz T. F., Hengster P., Löwer J., Larcher C., Bitterlich G., Kurth R., Wachter H., Dierich M. P. HIV and HIV-infected cells differentially activate the human complement system independent of antibody. Immunol Lett. 1989 Aug;22(2):135–145. doi: 10.1016/0165-2478(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Thieblemont N., Haeffner-Cavaillon N., Weiss L., Maillet F., Kazatchkine M. D. Complement activation by gp160 glycoprotein of HIV-1. AIDS Res Hum Retroviruses. 1993 Mar;9(3):229–233. doi: 10.1089/aid.1993.9.229. [DOI] [PubMed] [Google Scholar]
- Thielens N. M., Bally I. M., Ebenbichler C. F., Dierich M. P., Arlaud G. J. Further characterization of the interaction between the C1q subcomponent of human C1 and the transmembrane envelope glycoprotein gp41 of HIV-1. J Immunol. 1993 Dec 1;151(11):6583–6592. [PubMed] [Google Scholar]
- Thornton B. P., Vetvicka V., Ross G. D. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes. J Immunol. 1994 Feb 15;152(4):1727–1737. [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S., Wood W. B., Jr Heat labile opsonins to Pneumococcus. 3. The participation of immunoglobulin and of the alternate pathway of C3 activation. J Immunol. 1972 Jun;108(6):1681–1689. [PubMed] [Google Scholar]
- Wrin T., Crawford L., Sawyer L., Weber P., Sheppard H. W., Hanson C. V. Neutralizing antibody responses to autologous and heterologous isolates of human immunodeficiency virus. J Acquir Immune Defic Syndr. 1994 Mar;7(3):211–219. [PubMed] [Google Scholar]


