Abstract
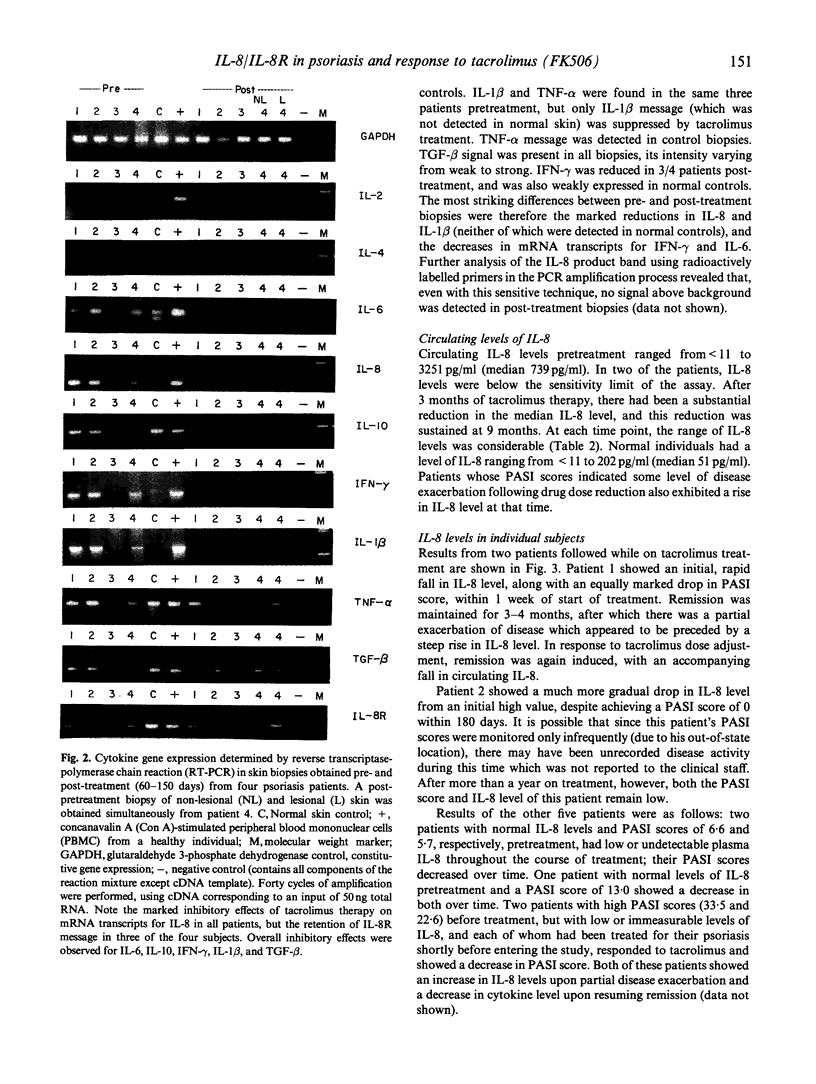
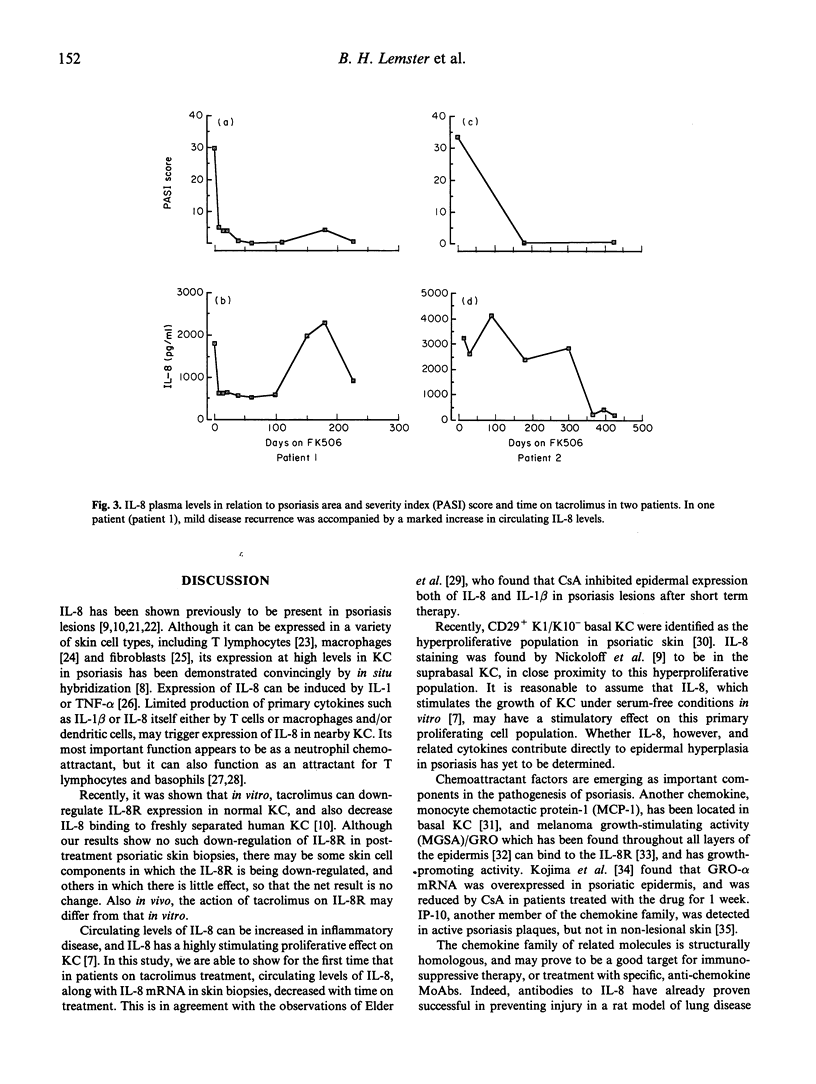
Recently, the keratinocyte IL-8/IL-8 receptor (IL-8R) pathway has been implicated in the pathogenesis of psoriasis, and there is evidence that the potent macrolide immune suppressant tacrolimus (formerly FK506) can inhibit this pathway in vitro. In this study, determination of the expression of cytokine mRNAs in lesional skin of patients with active disease by reverse transcriptase polymerase chain reaction revealed transcripts for IL-1 beta, tumour necrosis factor-alpha (TNF-alpha), IL-6, IL-8, IL-8R, IL-10, interferon-gamma (IFN-gamma), IL-2R and transforming growth factor-beta (TGF-beta), but not IL-2 or IL-4. IL-8 was the only cytokine expressed in affected skin of all patients but not in clinically normal skin of healthy subjects. In seven CD4+ T cell clones propagated from the lesional skin of an untreated psoriasis patient, IL-8 was expressed by the skin-derived T lymphocytes and not by feeder cells (irradiated autologous blood lymphocytes); IL-1 beta, IL-2, IL-6 and IL-10 were also expressed by some or all of the T cell clones. IL-8 mRNA was not detected in the skin of any patient after the start of systemic tacrolimus therapy; IL-1 beta, IL-6 and IFN-gamma transcripts were also reduced. By 12 weeks, the mean psoriasis area and severity index (PASI) had decreased from 18.8 to 3.8, a reduction of 80%. In the same post-treatment biopsies, however, message for IL-8R persisted. Estimation of circulating IL-8 levels by enzyme immunoassay showed that all patients with detectable IL-8 before treatment had decreased levels in response to treatment with tacrolimus; reductions in PASI scores were accompanied by decreases in IL-8 levels, that varied both in rate and extent. Partial relapse, which in a minority of patients followed the initial period of remission, and was precipitated by drug dose reduction, was accompanied by an increase in circulating IL-8. These findings add credence to the view that the IL-8/IL-8R autocrine/paracrine pathway may be important in the pathogenesis of psoriasis. They further suggest that interference with IL-8 production and/or that of other key chemokines may be an important mechanism underlying the therapeutic efficacy of tacrolimus, and other agents such as cyclosporin A, with similar molecular actions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. N., Mitra R. S., Griffiths C. E., Dixit V. M., Nickoloff B. J. Keratinocytes as initiators of inflammation. Lancet. 1991 Jan 26;337(8735):211–214. doi: 10.1016/0140-6736(91)92168-2. [DOI] [PubMed] [Google Scholar]
- Bata-Csorgo Z., Hammerberg C., Voorhees J. J., Cooper K. D. Flow cytometric identification of proliferative subpopulations within normal human epidermis and the localization of the primary hyperproliferative population in psoriasis. J Exp Med. 1993 Oct 1;178(4):1271–1281. doi: 10.1084/jem.178.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Hammerberg C., Cooper K. D., Kojima T., Nair R. P., Ellis C. N., Voorhees J. J. Cyclosporin A rapidly inhibits epidermal cytokine expression in psoriasis lesions, but not in cytokine-stimulated cultured keratinocytes. J Invest Dermatol. 1993 Dec;101(6):761–766. doi: 10.1111/1523-1747.ep12371691. [DOI] [PubMed] [Google Scholar]
- Ellis C. N., Gorsulowsky D. C., Hamilton T. A., Billings J. K., Brown M. D., Headington J. T., Cooper K. D., Baadsgaard O., Duell E. A., Annesley T. M. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986 Dec 12;256(22):3110–3116. [PubMed] [Google Scholar]
- Fredriksson T., Pettersson U. Severe psoriasis--oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- Gillitzer R., Berger R., Mielke V., Müller C., Wolff K., Stingl G. Upper keratinocytes of psoriatic skin lesions express high levels of NAP-1/IL-8 mRNA in situ. J Invest Dermatol. 1991 Jul;97(1):73–79. doi: 10.1111/1523-1747.ep12478128. [DOI] [PubMed] [Google Scholar]
- Gillitzer R., Wolff K., Tong D., Müller C., Yoshimura T., Hartmann A. A., Stingl G., Berger R. MCP-1 mRNA expression in basal keratinocytes of psoriatic lesions. J Invest Dermatol. 1993 Aug;101(2):127–131. doi: 10.1111/1523-1747.ep12363613. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Luster A. D., Posnett D. N., Carter D. M. Detection of a gamma interferon-induced protein IP-10 in psoriatic plaques. J Exp Med. 1988 Sep 1;168(3):941–948. doi: 10.1084/jem.168.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horroccks A., Ormerod A. D., Duncan J. I., Thomson A. W. Influence of systemic cyclosporin A on interleukin-2 and epidermal growth factor receptor expression in psoriatic skin lesions. Clin Exp Immunol. 1989 Nov;78(2):166–171. [PMC free article] [PubMed] [Google Scholar]
- Horrocks C., Duncan J. I., Oliver A. M., Thomson A. W. Adhesion molecule expression in psoriatic skin lesions and the influence of cyclosporin A. Clin Exp Immunol. 1991 Apr;84(1):157–162. doi: 10.1111/j.1365-2249.1991.tb08140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegasothy B. V., Ackerman C. D., Todo S., Fung J. J., Abu-Elmagd K., Starzl T. E. Tacrolimus (FK 506)--a new therapeutic agent for severe recalcitrant psoriasis. Arch Dermatol. 1992 Jun;128(6):781–785. [PMC free article] [PubMed] [Google Scholar]
- Kino T., Hatanaka H., Miyata S., Inamura N., Nishiyama M., Yajima T., Goto T., Okuhara M., Kohsaka M., Aoki H. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) 1987 Sep;40(9):1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- Kojima T., Cromie M. A., Fisher G. J., Voorhees J. J., Elder J. T. GRO-alpha mRNA is selectively overexpressed in psoriatic epidermis and is reduced by cyclosporin A in vivo, but not in cultured keratinocytes. J Invest Dermatol. 1993 Dec;101(6):767–772. doi: 10.1111/1523-1747.ep12371692. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Standiford T., Kasahara K., Strieter R. M. Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Exp Lung Res. 1991 Jan-Feb;17(1):17–23. doi: 10.3109/01902149109063278. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Oppenheim J. J., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989 Sep;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrowietz U., Sticherling M., Mielke V., Schröder J. M., Christophers E. Neutrophil-activating peptide 1/interleukin 8 mRNA expression and protein secretion by human monocytes: effect of cyclosporin A. Cytokine. 1991 Jul;3(4):322–326. doi: 10.1016/1043-4666(91)90500-d. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Jones M. L., Bolanowski M. A., Baganoff M. P., Deppeler C. L., Meyers D. M., Ryan U. S., Ward P. A. Inhibition of lung inflammatory reactions in rats by an anti-human IL-8 antibody. J Immunol. 1993 Jun 15;150(12):5585–5595. [PubMed] [Google Scholar]
- Nickoloff B. J., Griffiths C. E. T lymphocytes and monocytes bind to keratinocytes in frozen sections of biopsy specimens of normal skin treated with gamma interferon. J Am Acad Dermatol. 1989 May;20(5 Pt 1):736–743. doi: 10.1016/s0190-9622(89)70083-9. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Karabin G. D., Barker J. N., Griffiths C. E., Sarma V., Mitra R. S., Elder J. T., Kunkel S. L., Dixit V. M. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am J Pathol. 1991 Jan;138(1):129–140. [PMC free article] [PubMed] [Google Scholar]
- Nikaein A., Morris L., Phillips C., Soliman M., Ordonez G., Silverman A., Stone M. J., Menter A. Characterization of T-cell clones generated from skin of patients with psoriasis. J Am Acad Dermatol. 1993 Apr;28(4):551–557. doi: 10.1016/0190-9622(93)70073-3. [DOI] [PubMed] [Google Scholar]
- Nikaein A., Phillips C., Gilbert S. C., Savino D., Silverman A., Stone M. J., Menter A. Characterization of skin-infiltrating lymphocytes in patients with psoriasis. J Invest Dermatol. 1991 Jan;96(1):3–9. doi: 10.1111/1523-1747.ep12514646. [DOI] [PubMed] [Google Scholar]
- Paludan K., Thestrup-Pedersen K. Use of the polymerase chain reaction in quantification of interleukin 8 mRNA in minute epidermal samples. J Invest Dermatol. 1992 Dec;99(6):830–835. doi: 10.1111/1523-1747.ep12614794. [DOI] [PubMed] [Google Scholar]
- Schulz B. S., Michel G., Wagner S., Süss R., Beetz A., Peter R. U., Kemény L., Ruzicka T. Increased expression of epidermal IL-8 receptor in psoriasis. Down-regulation by FK-506 in vitro. J Immunol. 1993 Oct 15;151(8):4399–4406. [PubMed] [Google Scholar]
- Sekido N., Mukaida N., Harada A., Nakanishi I., Watanabe Y., Matsushima K. Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature. 1993 Oct 14;365(6447):654–657. doi: 10.1038/365654a0. [DOI] [PubMed] [Google Scholar]
- Sticherling M., Bornscheuer E., Schröder J. M., Christophers E. Localization of neutrophil-activating peptide-1/interleukin-8-immunoreactivity in normal and psoriatic skin. J Invest Dermatol. 1991 Jan;96(1):26–30. doi: 10.1111/1523-1747.ep12514689. [DOI] [PubMed] [Google Scholar]
- Tamura K., Kobayashi M., Hashimoto K., Kojima K., Nagase K., Iwasaki K., Kaizu T., Tanaka H., Niwa M. A highly sensitive method to assay FK-506 levels in plasma. Transplant Proc. 1987 Oct;19(5 Suppl 6):23–29. [PubMed] [Google Scholar]
- Tettelbach W., Nanney L., Ellis D., King L., Richmond A. Localization of MGSA/GRO protein in cutaneous lesions. J Cutan Pathol. 1993 Jun;20(3):259–266. doi: 10.1111/j.1600-0560.1993.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Thomson A. W. FK-506--how much potential? Immunol Today. 1989 Jan;10(1):6–9. doi: 10.1016/0167-5699(89)90057-1. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Nalesnik M. A., Rilo H. R., Woo J., Carroll P. B., Van Thiel D. H. ICAM-1 and E-selectin expression in lesional biopsies of psoriasis patients responding to systemic FK 506 therapy. Autoimmunity. 1993;15(3):215–223. doi: 10.3109/08916939309019930. [DOI] [PubMed] [Google Scholar]
- Tuschil A., Lam C., Haslberger A., Lindley I. Interleukin-8 stimulates calcium transients and promotes epidermal cell proliferation. J Invest Dermatol. 1992 Sep;99(3):294–298. doi: 10.1111/1523-1747.ep12616634. [DOI] [PubMed] [Google Scholar]
- Vita N., Lefort S., Brouillaud M. J., Magazin M., Guillemot J. C., Ferrara P. Functional linkage of the Gro beta and IL-8 receptors on the surface of human neutrophils. Eur Cytokine Netw. 1993 May-Jun;4(3):197–204. [PubMed] [Google Scholar]
- Zipfel P. F., Bialonski A., Skerka C. Induction of members of the IL-8/NAP-1 gene family in human T lymphocytes is suppressed by cyclosporin A. Biochem Biophys Res Commun. 1991 Nov 27;181(1):179–183. doi: 10.1016/s0006-291x(05)81398-1. [DOI] [PubMed] [Google Scholar]