Abstract
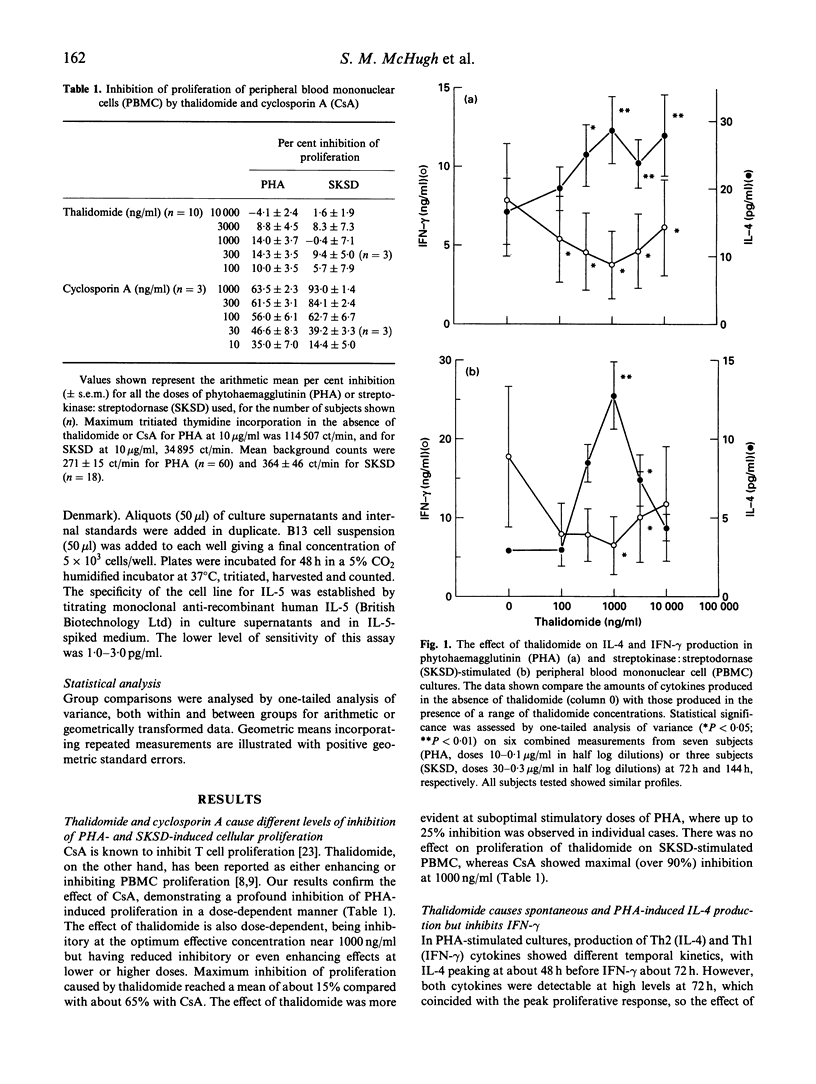
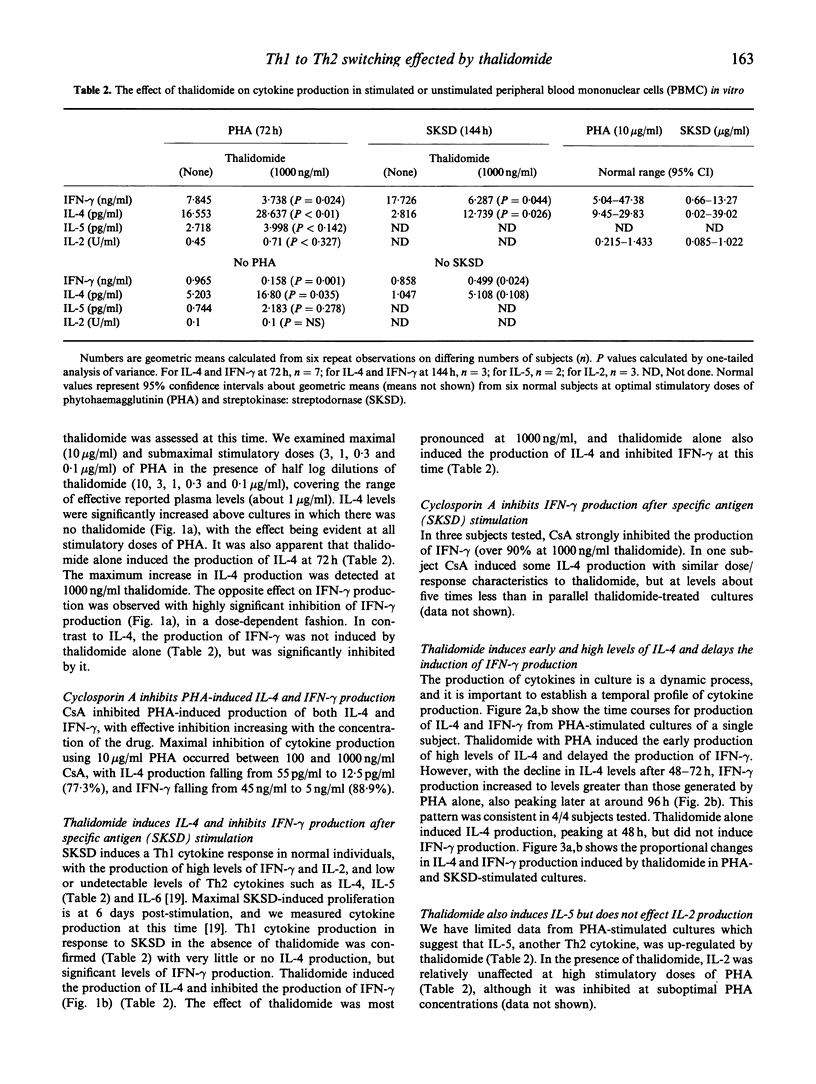
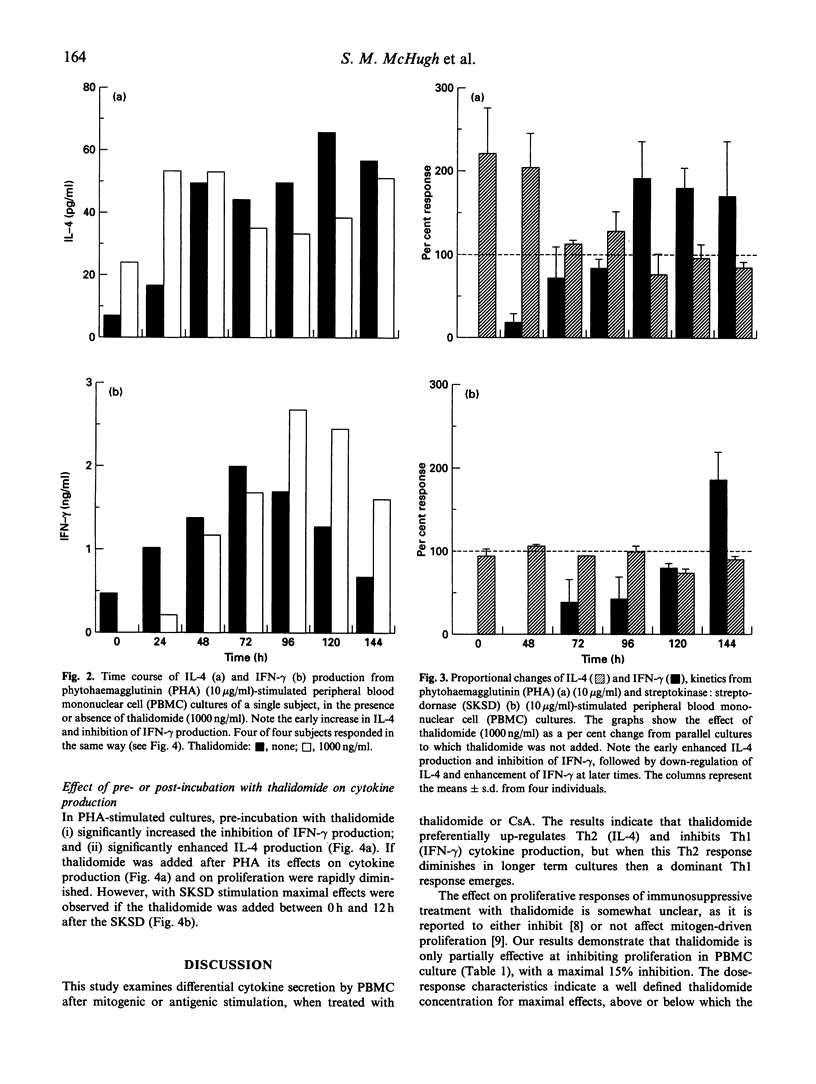
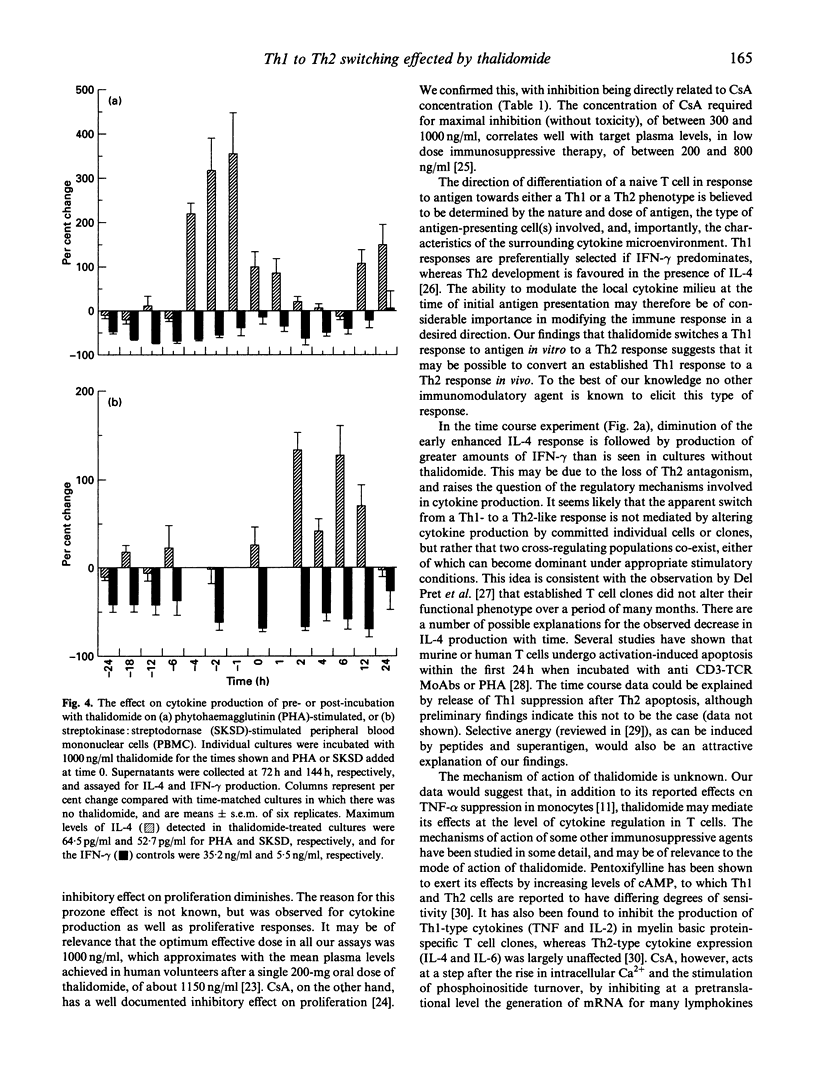
Thalidomide is an effective immunomodulatory drug in man, but its mechanism of action remains unclear. We hypothesized that, in addition to its reported inhibitory effects on production of monocyte-derived tumour necrosis factor-alpha (TNF-alpha), thalidomide might be effective at the level of Th immunoregulation. In a comparative study with the immunosuppressant cyclosporin A, we have demonstrated a potent and specific effect of thalidomide on cytokine production relating to the distinct Th1 and Th2 subsets. It induced and enhanced the production of IL-4 and IL-5 and, at the same dose (1000 ng/ml), significantly inhibited interferon-gamma (IFN-gamma) production in phytohaemagglutinin (PHA)-stimulated human peripheral blood mononuclear cell (PBMC) cultures. Stimulation of PBMC with recall antigen (streptokinase:streptodornase (SKSD)) at 144 h in the absence of thalidomide resulted in a predominantly Th1 response, with the production of IFN-gamma and IL-2. Thalidomide switched this response from a Th1 to a Th2 type. The effect was most pronounced at 1000 ng/ml thalidomide, where inhibition of IFN-gamma and enhancement of IL-4 production was maximal. In unstimulated cultures thalidomide alone induced IL-4 production. Cyclosporin A, in contrast, inhibited both Th1 and Th2 cytokine production by PHA-stimulated PBMC. Time course data from thalidomide-treated cultures revealed that the augmented IL-4 production diminished as the culture time increased, whereas IFN-gamma production was significantly increased. This response might be due to activation-induced apoptosis of Th2 cells or the induction of Th2 cell anergy, in the continued presence of stimulating agents, with the emergence of IFN-gamma-secreting Th1 cells when Th2 antagonism declines. The effects of thalidomide and related compounds may enhance our understanding of the mechanisms of T helper cell selection, offer the possibility of controlled therapeutic switching between Th1 and Th2 responses, and may lead to a rational approach for the treatment of some T cell-mediated immunological disorders.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhill R. L., Doll N. J., Millikan L. E., Hastings R. C. Studies on the anti-inflammatory properties of thalidomide: effects on polymorphonuclear leukocytes and monocytes. J Am Acad Dermatol. 1984 Nov;11(5 Pt 1):814–819. doi: 10.1016/s0190-9622(84)80458-2. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Vogelsang G. B., Petty B. G., Brundrett R. B., Noe D. A., Santos G. W., Colvin O. M. Plasma pharmacokinetics and urinary excretion of thalidomide after oral dosing in healthy male volunteers. Drug Metab Dispos. 1989 Jul-Aug;17(4):402–405. [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993 Mar;14(3):107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- Coulson A. S., Summers L. J., Lindahl-Kiessling K., Tucker D., Hellmann K. The effect of two soluble thalidomide derivatives on lymphocyte stimulation. Clin Exp Immunol. 1970 Aug;7(2):241–247. [PMC free article] [PubMed] [Google Scholar]
- Dearman R. J., Ramdin L. S., Basketter D. A., Kimber I. Inducible interleukin-4-secreting cells provoked in mice during chemical sensitization. Immunology. 1994 Apr;81(4):551–557. [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Mastromauro C., Biagiotti R., Macchia D., Falagiani P., Ricci M., Romagnani S. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest. 1991 Jul;88(1):346–350. doi: 10.1172/JCI115300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gutiérrez-Rodríguez O., Starusta-Bacal P., Gutiérrez-Montes O. Treatment of refractory rheumatoid arthritis--the thalidomide experience. J Rheumatol. 1989 Feb;16(2):158–163. [PubMed] [Google Scholar]
- Hatfill S. J., Fester E. D., de Beer D. P., Bohm L. Induction of morphological differentiation in the human leukemic cell line K562 by exposure to thalidomide metabolites. Leuk Res. 1991;15(2-3):129–136. doi: 10.1016/0145-2126(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Pohl T., Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993 Jul;14(7):338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- Keenan R. J., Eiras G., Burckart G. J., Stuart R. S., Hardesty R. L., Vogelsang G., Griffith B. P., Zeevi A. Immunosuppressive properties of thalidomide. Inhibition of in vitro lymphocyte proliferation alone and in combination with cyclosporine or FK506. Transplantation. 1991 Nov;52(5):908–910. [PubMed] [Google Scholar]
- Makonkawkeyoon S., Limson-Pobre R. N., Moreira A. L., Schauf V., Kaplan G. Thalidomide inhibits the replication of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5974–5978. doi: 10.1073/pnas.90.13.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh S. M., Wilson A. B., Deighton J., Lachmann P. J., Ewan P. W. The profiles of interleukin (IL)-2, IL-6, and interferon-gamma production by peripheral blood mononuclear cells from house-dust-mite-allergic patients: a role for IL-6 in allergic disease. Allergy. 1994 Oct;49(9):751–759. doi: 10.1111/j.1398-9995.1994.tb02098.x. [DOI] [PubMed] [Google Scholar]
- Moreira A. L., Sampaio E. P., Zmuidzinas A., Frindt P., Smith K. A., Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993 Jun 1;177(6):1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Murphy D. B. T cell mediated immunosuppression. Curr Opin Immunol. 1993 Jun;5(3):411–417. doi: 10.1016/0952-7915(93)90061-v. [DOI] [PubMed] [Google Scholar]
- Neubert R., Nogueira A. C., Neubert D. Thalidomide and the immune system. 2. Changes in receptors on blood cells of a healthy volunteer. Life Sci. 1992;51(26):2107–2116. doi: 10.1016/0024-3205(92)90162-i. [DOI] [PubMed] [Google Scholar]
- Pereira G. M., Miller J. F., Shevach E. M. Mechanism of action of cyclosporine A in vivo. II. T cell priming in vivo to alloantigen can be mediated by an IL-2-independent cyclosporine A-resistant pathway. J Immunol. 1990 Mar 15;144(6):2109–2116. [PubMed] [Google Scholar]
- Powrie F., Coffman R. L. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today. 1993 Jun;14(6):270–274. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- Rolink A. G., Melchers F., Palacios R. Monoclonal antibodies reactive with the mouse interleukin 5 receptor. J Exp Med. 1989 May 1;169(5):1693–1701. doi: 10.1084/jem.169.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott O., Cash E., Fleischer B. Phosphodiesterase inhibitor pentoxifylline, a selective suppressor of T helper type 1- but not type 2-associated lymphokine production, prevents induction of experimental autoimmune encephalomyelitis in Lewis rats. Eur J Immunol. 1993 Aug;23(8):1745–1751. doi: 10.1002/eji.1830230802. [DOI] [PubMed] [Google Scholar]
- SHESKIN J. THALIDOMIDE IN THE TREATMENT OF LEPRA REACTIONS. Clin Pharmacol Ther. 1965 May-Jun;6:303–306. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- Sampaio E. P., Kaplan G., Miranda A., Nery J. A., Miguel C. P., Viana S. M., Sarno E. N. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J Infect Dis. 1993 Aug;168(2):408–414. doi: 10.1093/infdis/168.2.408. [DOI] [PubMed] [Google Scholar]
- Sampaio E. P., Sarno E. N., Galilly R., Cohn Z. A., Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991 Mar 1;173(3):699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N. H., Lin C. S., Siekierka J. J. Inhibition of human T-cell activation by FK 506, rapamycin, and cyclosporine A. Transplant Proc. 1991 Apr;23(2 Suppl 2):1–5. [PubMed] [Google Scholar]
- Vogelsang G. B., Farmer E. R., Hess A. D., Altamonte V., Beschorner W. E., Jabs D. A., Corio R. L., Levin L. S., Colvin O. M., Wingard J. R. Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med. 1992 Apr 16;326(16):1055–1058. doi: 10.1056/NEJM199204163261604. [DOI] [PubMed] [Google Scholar]
- Vogelsang G. B., Hess A. D., Gordon G., Brundrette R., Santos G. W. Thalidomide induction of bone marrow transplantation tolerance. Transplant Proc. 1987 Feb;19(1 Pt 3):2658–2661. [PubMed] [Google Scholar]
- Wierenga E. A., Snoek M., de Groot C., Chrétien I., Bos J. D., Jansen H. M., Kapsenberg M. L. Evidence for compartmentalization of functional subsets of CD2+ T lymphocytes in atopic patients. J Immunol. 1990 Jun 15;144(12):4651–4656. [PubMed] [Google Scholar]
- Wilson A. B., McHugh S. M., Deighton J., Ewan P. W., Lachmann P. J. A competitive inhibition ELISA for the quantification of human interferon-gamma. J Immunol Methods. 1993 Jun 18;162(2):247–255. doi: 10.1016/0022-1759(93)90389-o. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Joost T., Stolz E., Heule F. Efficacy of low-dose cyclosporine in severe atopic skin disease. Arch Dermatol. 1987 Feb;123(2):166–167. doi: 10.1001/archderm.123.2.166. [DOI] [PubMed] [Google Scholar]


