Abstract
Lipopolysaccharide (LPS) is abundant in the intestinal lumen. CD14 is the receptor for the LPS-LPS binding protein complex, and its presence on mononuclear phagocytes allows cell activation by pg/ml concentrations of LPS. We have shown that the recently recruited blood monocyte in inflammatory bowel disease mucosa is CD14+. This study examined the expression of CD14 on macrophages in inflamed (n = 13) and uninflamed (n = 7) intestine by immunohistochemistry, and on disaggregated lamina propria mononuclear cells (12 from inflamed, 17 from uninflamed intestine) and peripheral blood mononuclear cells (n = 26) by flow cytometry, using a panel of three MoAbs directed against CD14. Immunohistochemistry revealed that 3.7% of macrophages in uninflamed intestine were CD14+, while 25.1% of macrophages in active inflammatory bowel disease expressed CD14 (P < 0.02). Flow cytometry demonstrated that CD14 expression by macrophages from Crohn's disease and ulcerative colitis was augmented significantly (P = 0.02 and P = 0.01, respectively) compared with uninflamed intestine, with a discrete population of macrophages in inflammatory bowel disease, not present in normal intestine, which strongly expressed CD14. The characteristically high levels of CD14 on blood monocytes were unaffected by the presence of intestinal inflammation. Given the exposure of lamina propria cells to LPS present in the lumen of the terminal ileum and colon, the increased numbers of CD14+ macrophages in inflammatory bowel disease may result in greatly increased production of inflammatory mediators, thereby suggesting a mechanism for the perpetuation of mucosal inflammation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. C., Cornwall S., Poulter L. W., Dhillon A. P., Pounder R. E. Macrophage heterogeneity in normal colonic mucosa and in inflammatory bowel disease. Gut. 1988 Nov;29(11):1531–1538. doi: 10.1136/gut.29.11.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. C., Poulter L. W. Changes in phenotypically distinct mucosal macrophage populations may be a prerequisite for the development of inflammatory bowel disease. Clin Exp Immunol. 1991 Sep;85(3):504–509. doi: 10.1111/j.1365-2249.1991.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J Clin Invest. 1991 Nov;88(5):1445–1451. doi: 10.1172/JCI115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello M., Keshav S., Prince C., Jewell D. P., Gordon S. Detection of mRNAs for macrophage products in inflammatory bowel disease by in situ hybridisation. Gut. 1992 Sep;33(9):1214–1219. doi: 10.1136/gut.33.9.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentener M. A., Bazil V., Von Asmuth E. J., Ceska M., Buurman W. A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993 Apr 1;150(7):2885–2891. [PubMed] [Google Scholar]
- Doe W. F., Dorsman B. Chronic inflammatory bowel disease--increased plasminogen activator secretion by mononuclear phagocytes. Clin Exp Immunol. 1982 Apr;48(1):256–260. [PMC free article] [PubMed] [Google Scholar]
- Gallay P., Jongeneel C. V., Barras C., Burnier M., Baumgartner J. D., Glauser M. P., Heumann D. Short time exposure to lipopolysaccharide is sufficient to activate human monocytes. J Immunol. 1993 Jun 1;150(11):5086–5093. [PubMed] [Google Scholar]
- Golder J. P., Doe W. F. Isolation and preliminary characterization of human intestinal macrophages. Gastroenterology. 1983 Apr;84(4):795–802. [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Raetz C. R., Wright S. D. Human phagocytes have multiple lipid A-binding sites. Infect Immun. 1990 Dec;58(12):4069–4075. doi: 10.1128/iai.58.12.4069-4075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann D., Gallay P., Barras C., Zaech P., Ulevitch R. J., Tobias P. S., Glauser M. P., Baumgartner J. D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992 Jun 1;148(11):3505–3512. [PubMed] [Google Scholar]
- Hume D. A., Allan W., Hogan P. G., Doe W. F. Immunohistochemical characterisation of macrophages in human liver and gastrointestinal tract: expression of CD4, HLA-DR, OKM1, and the mature macrophage marker 25F9 in normal and diseased tissue. J Leukoc Biol. 1987 Nov;42(5):474–484. doi: 10.1002/jlb.42.5.474. [DOI] [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Landmann R., Wesp M., Obrecht J. P. Cytokine regulation of the myeloid glycoprotein CD14. Pathobiology. 1991;59(3):131–135. doi: 10.1159/000163630. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Soberman R. J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990 Sep 6;323(10):645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Lord P. C., Wilmoth L. M., Mizel S. B., McCall C. E. Expression of interleukin-1 alpha and beta genes by human blood polymorphonuclear leukocytes. J Clin Invest. 1991 Apr;87(4):1312–1321. doi: 10.1172/JCI115134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Patel S., Gionchetti P., Vaux D., Jewell D. P. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989 Jun;30(6):826–834. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
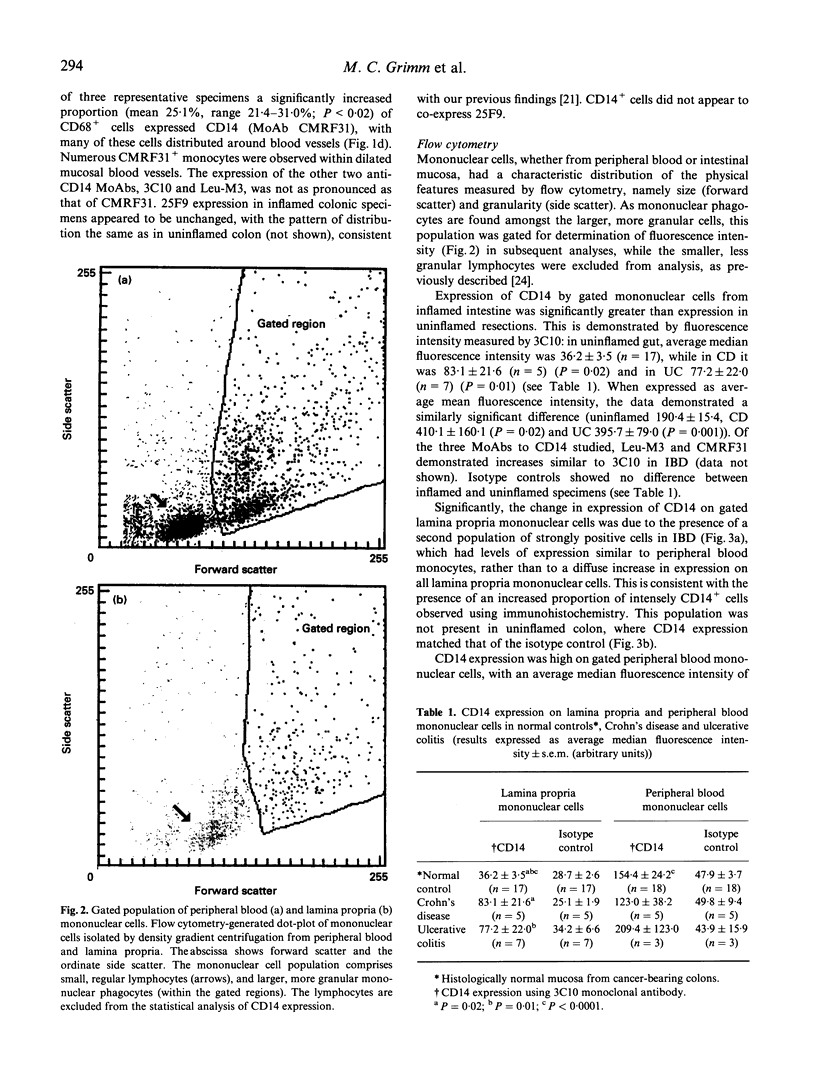
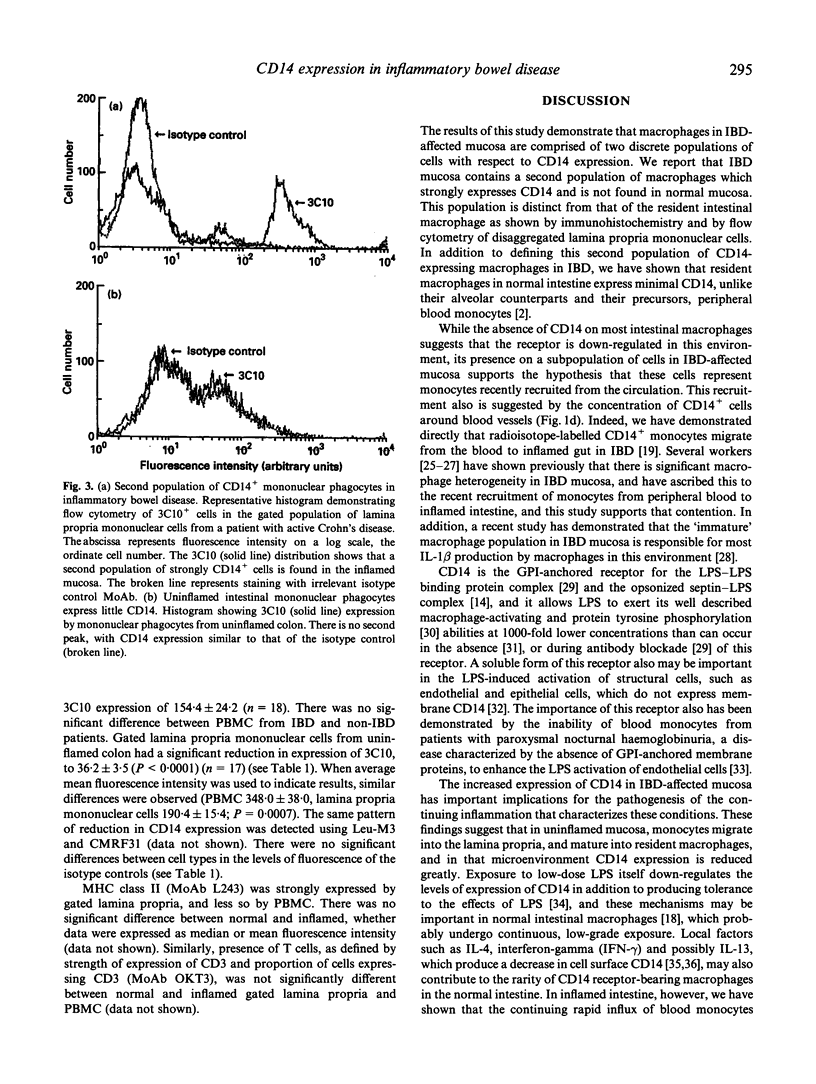
- Mahida Y. R., Wu K. C., Jewell D. P. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease. Gut. 1989 Oct;30(10):1362–1370. doi: 10.1136/gut.30.10.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. P., Secrist H., Botney M., Egan M., Peters M. G. Cytokine mRNA expression in intestine from normal and inflammatory bowel disease patients. Clin Immunol Immunopathol. 1993 Jan;66(1):52–58. doi: 10.1006/clin.1993.1007. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mengozzi M., Fantuzzi G., Sironi M., Bianchi M., Fratelli M., Peri G., Bernasconi S., Ghezzi P. Early down-regulation of TNF production by LPS tolerance in human monocytes: comparison with IL-1 beta, IL-6, and IL-8. Lymphokine Cytokine Res. 1993 Aug;12(4):231–236. [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. D., Ayass M., Chensue S. Tumor necrosis factor and IL-1 beta expression in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1993 Apr;16(3):241–246. doi: 10.1097/00005176-199304000-00003. [DOI] [PubMed] [Google Scholar]
- Pavli P., Hume D. A., Van De Pol E., Doe W. F. Dendritic cells, the major antigen-presenting cells of the human colonic lamina propria. Immunology. 1993 Jan;78(1):132–141. [PMC free article] [PubMed] [Google Scholar]
- Pugin J., Schürer-Maly C. C., Leturcq D., Moriarty A., Ulevitch R. J., Tobias P. S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin J., Ulevitch R. J., Tobias P. S. A critical role for monocytes and CD14 in endotoxin-induced endothelial cell activation. J Exp Med. 1993 Dec 1;178(6):2193–2200. doi: 10.1084/jem.178.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman W. E., Elsbury S., Kobayashi M., Hapel A. J., Doe W. F. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology. 1992 Feb;102(2):529–537. doi: 10.1016/0016-5085(92)90100-d. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Simmons D. L., Tan S., Tenen D. G., Nicholson-Weller A., Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989 Jan;73(1):284–289. [PubMed] [Google Scholar]
- Stephens R. W., Golder J. P., Fayle D. R., Hume D. A., Hapel A. J., Allan W., Fordham C. J., Doe W. F. Minactivin expression in human monocyte and macrophage populations. Blood. 1985 Aug;66(2):333–337. [PubMed] [Google Scholar]
- Surette M. E., Palmantier R., Gosselin J., Borgeat P. Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J Exp Med. 1993 Oct 1;178(4):1347–1355. doi: 10.1084/jem.178.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S. L., June C. H., DeFranco A. L. Lipopolysaccharide-induced protein tyrosine phosphorylation in human macrophages is mediated by CD14. J Immunol. 1993 Oct 1;151(7):3829–3838. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Patel M., Miller D. S. Septin: a factor in plasma that opsonizes lipopolysaccharide-bearing particles for recognition by CD14 on phagocytes. J Exp Med. 1992 Sep 1;176(3):719–727. doi: 10.1084/jem.176.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Youngman K. R., Simon P. L., West G. A., Cominelli F., Rachmilewitz D., Klein J. S., Fiocchi C. Localization of intestinal interleukin 1 activity and protein and gene expression to lamina propria cells. Gastroenterology. 1993 Mar;104(3):749–758. doi: 10.1016/0016-5085(93)91010-f. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Figdor C. G., Huijbens R., Mohan-Peterson S., Bennett B., Culpepper J., Dang W., Zurawski G., de Vries J. E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993 Dec 1;151(11):6370–6381. [PubMed] [Google Scholar]



