Abstract
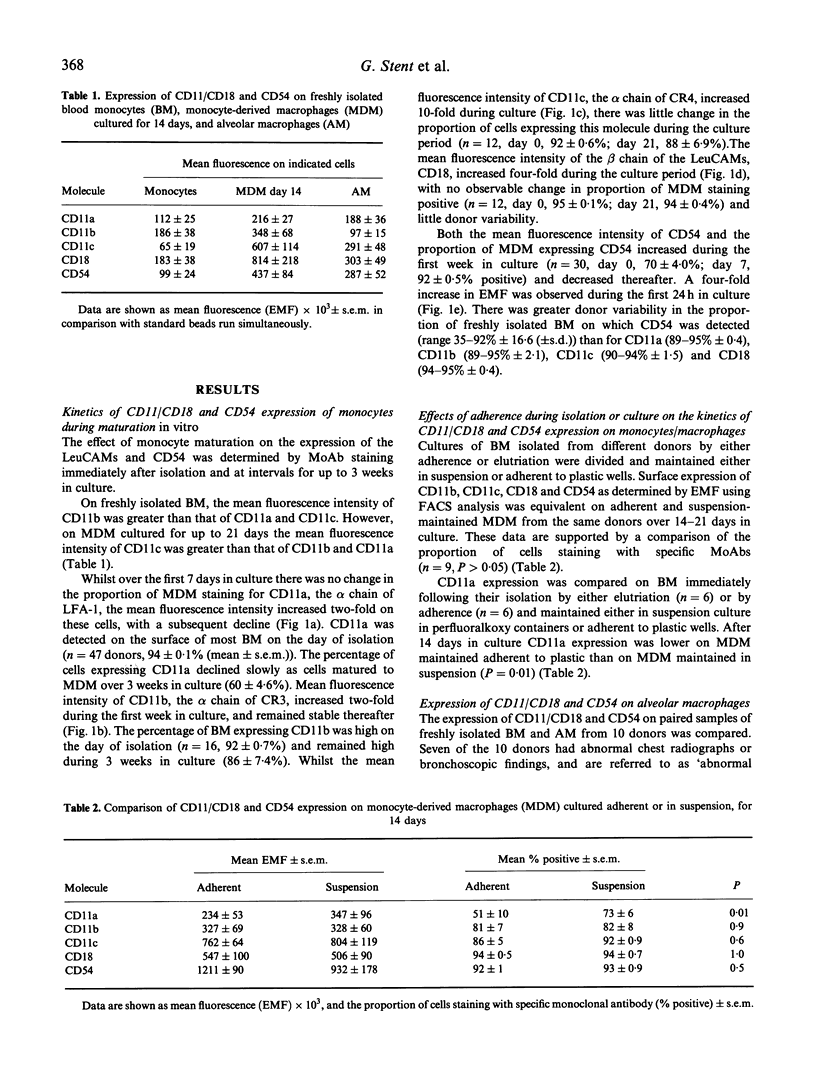
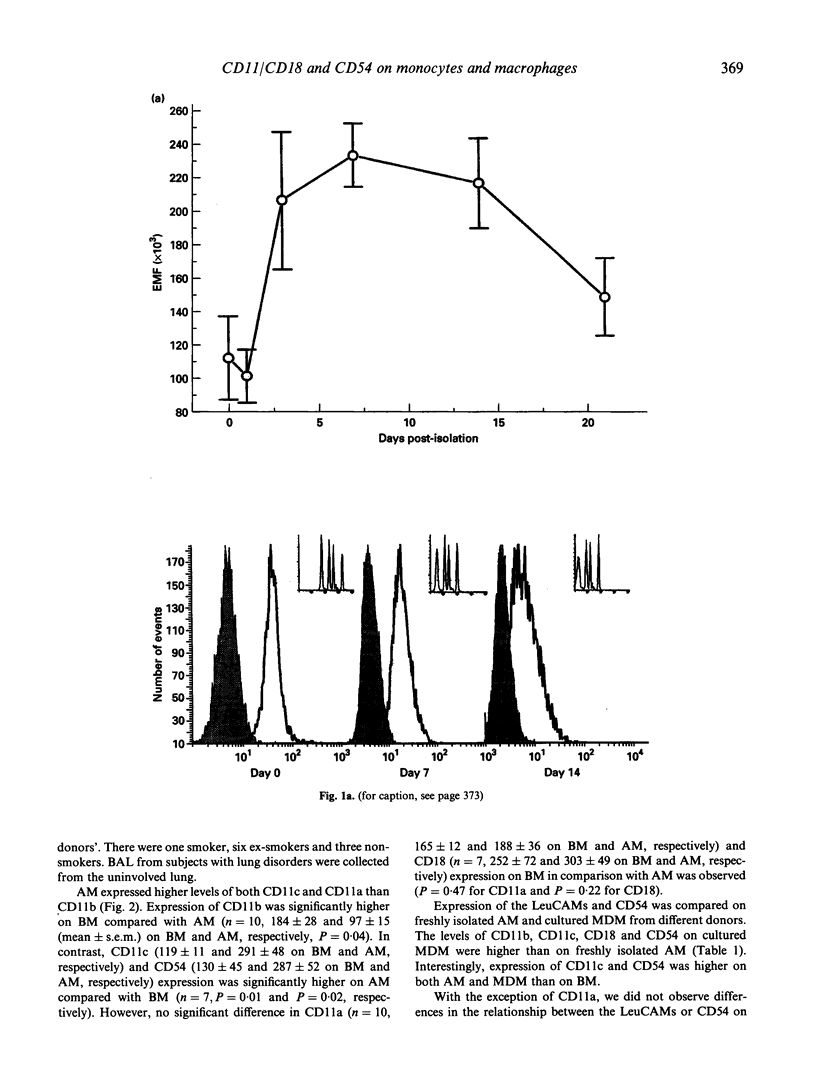
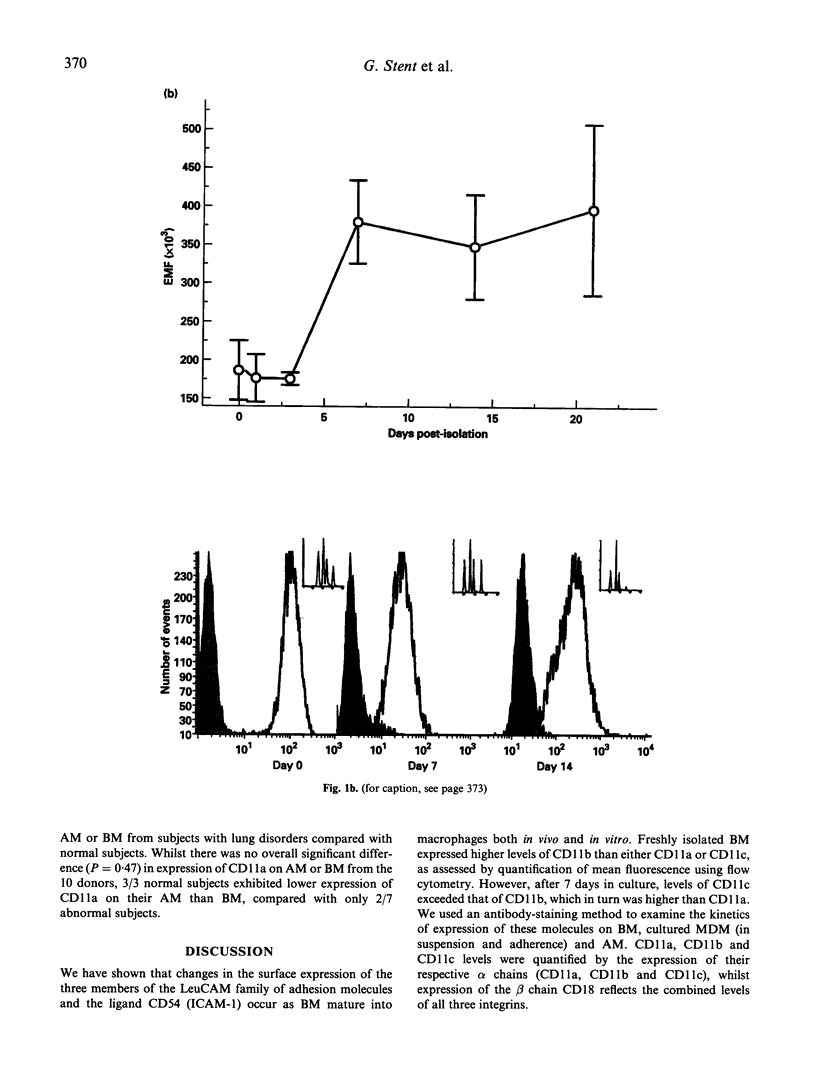
Cells of the macrophage lineage mediate extremely important normal functions of the immune system. Such functions are in part related to interactions between cell-bound LeuCAMs and their ligands. MoAb staining and flow cytometric analysis were used to follow changes in surface expression of LeuCAMs and the LFA-1 ligand CD54 during maturation of peripheral blood monocytes (BM) in vitro. Surface expression of these molecules increased on BM following isolation, the greatest increase being in CD54 and CD11c. Following an initial increase, there was a reduction in CD11a expression after 2 weeks in culture, this being greater on adherent compared with suspension-maintained cells. Expression of CD11b remained high throughout the culture period. LeuCAM and CD54 expression was further compared on freshly isolated alveolar macrophages (AM) and BM paired donors. A reciprocal relationship was observed between CD11c and CD11b on AM and BM, in that BM expressed higher levels of CD11b than CD11c, whilst the converse was true for AM. CD54 expression was also higher on AM than on BM, whilst there was no significant difference in expression of CD11a on these cells. These data suggest that consistent changes occur in the surface expression of the LeuCAMs and CD54 as monocytes mature into macrophages, which may reflect the specific functions of these cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostini C., Trentin L., Zambello R., Luca M., Masciarelli M., Cipriani A., Marcer G., Semenzato G. Pulmonary alveolar macrophages in patients with sarcoidosis and hypersensitivity pneumonitis: characterization by monoclonal antibodies. J Clin Immunol. 1987 Jan;7(1):64–70. doi: 10.1007/BF00915427. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Arkin S., Naprstek B., Guarini L., Ferrone S., Lipton J. M. Expression of intercellular adhesion molecule-1 (CD54) on hematopoietic progenitors. Blood. 1991 Mar 1;77(5):948–953. [PubMed] [Google Scholar]
- Arnaout M. A. Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990 Apr;114:145–180. doi: 10.1111/j.1600-065x.1990.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Buhl R., Jaffe H. A., Holroyd K. J., Borok Z., Roum J. H., Mastrangeli A., Wells F. B., Kirby M., Saltini C., Crystal R. G. Activation of alveolar macrophages in asymptomatic HIV-infected individuals. J Immunol. 1993 Feb 1;150(3):1019–1028. [PubMed] [Google Scholar]
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S., Mills J., McGrath M. S. Quantitative immunocytofluorographic analysis of CD4 surface antigen expression and HIV infection of human peripheral blood monocyte/macrophages. AIDS Res Hum Retroviruses. 1987 Summer;3(2):135–145. doi: 10.1089/aid.1987.3.135. [DOI] [PubMed] [Google Scholar]
- Denton M. D., Marsden P. A., Luscinskas F. W., Brenner B. M., Brady H. R. Cytokine-induced phagocyte adhesion to human mesangial cells: role of CD11/CD18 integrins and ICAM-1. Am J Physiol. 1991 Dec;261(6 Pt 2):F1071–F1079. doi: 10.1152/ajprenal.1991.261.6.F1071. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Duque R. E. To "B" (CD20) or not to "B". Cytometry. 1994 Mar 15;18(1):62–62. doi: 10.1002/cyto.990180113. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Ferro T. J., Kern J. A., Elias J. A., Kamoun M., Daniele R. P., Rossman M. D. Alveolar macrophages, blood monocytes, and density-fractionated alveolar macrophages differ in their ability to promote lymphocyte proliferation to mitogen and antigen. Am Rev Respir Dis. 1987 Mar;135(3):682–687. doi: 10.1164/arrd.1987.135.3.682. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Elliott M. J., Jaipargas E., Lopez A. F., Vadas M. A. Regulation of human monocyte adherence by granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7169–7173. doi: 10.1073/pnas.86.18.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Hibbs M. L., Wardlaw A. J., Stacker S. A., Anderson D. C., Lee A., Roberts T. M., Springer T. A. Transfection of cells from patients with leukocyte adhesion deficiency with an integrin beta subunit (CD18) restores lymphocyte function-associated antigen-1 expression and function. J Clin Invest. 1990 Mar;85(3):674–681. doi: 10.1172/JCI114491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickstein D. D., Smith A., Fisher W., Beatty P. G., Schwartz B. R., Harlan J. M., Root R. K., Locksley R. M. Expression of leukocyte adherence-related glycoproteins during differentiation of HL-60 promyelocytic leukemia cells. J Immunol. 1987 Jan 15;138(2):513–519. [PubMed] [Google Scholar]
- Hogg N., Takacs L., Palmer D. G., Selvendran Y., Allen C. The p150,95 molecule is a marker of human mononuclear phagocytes: comparison with expression of class II molecules. Eur J Immunol. 1986 Mar;16(3):240–248. doi: 10.1002/eji.1830160306. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Beller D. I., Frendl G., Graves D. T. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992 Apr 15;148(8):2423–2428. [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Te Velde A. A., Schwarting R., Figdor C. G., De Vries J. E. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987 Sep;17(9):1317–1322. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- Kent S. J., Stent G., Sonza S., Hunter S. D., Crowe S. M. HIV-1 infection of monocyte-derived macrophages reduces Fc and complement receptor expression. Clin Exp Immunol. 1994 Mar;95(3):450–454. doi: 10.1111/j.1365-2249.1994.tb07017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Faller D. V., Burakoff S. J. Interferon-gamma induction of LFA-1-mediated homotypic adhesion of human monocytes. J Immunol. 1986 Jul 1;137(1):108–113. [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Schwarting R., Springer T. A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986 Nov 1;137(9):2891–2900. [PubMed] [Google Scholar]
- Myones B. L., Dalzell J. G., Hogg N., Ross G. D. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988 Aug;82(2):640–651. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möst J., Schwaeble W., Drach J., Sommerauer A., Dierich M. P. Regulation of the expression of ICAM-1 on human monocytes and monocytic tumor cell lines. J Immunol. 1992 Mar 15;148(6):1635–1642. [PubMed] [Google Scholar]
- Newman S. L., Bucher C., Rhodes J., Bullock W. E. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J Clin Invest. 1990 Jan;85(1):223–230. doi: 10.1172/JCI114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg T., Rau M., Stephan H., Lode H. Increased number of alveolar macrophages expressing surface molecules of the CD11/CD18 family in sarcoidosis and idiopathic pulmonary fibrosis is related to the production of superoxide anions by these cells. Am Rev Respir Dis. 1993 Jun;147(6 Pt 1):1507–1513. doi: 10.1164/ajrccm/147.6_Pt_1.1507. [DOI] [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991 Sep 15;147(6):1983–1994. [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Sköld C. M., Eklund A., Halldén G., Hed J. Autofluorescence in human alveolar macrophages from smokers: relation to cell surface markers and phagocytosis. Exp Lung Res. 1989 Dec;15(6):823–835. doi: 10.3109/01902148909069629. [DOI] [PubMed] [Google Scholar]
- Sköld C. M., Eklund A., Halldén G., Hed J. Different cell surface and phagocytic properties in mononuclear phagocytes from blood and alveoli. A comparative study of blood monocytes and alveolar macrophages from human nonsmokers using flow cytofluorometry. APMIS. 1990 May;98(5):415–422. doi: 10.1111/j.1699-0463.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Miller L. J., Anderson D. C. p150,95, the third member of the Mac-1, LFA-1 human leukocyte adhesion glycoprotein family. J Immunol. 1986 Jan;136(1):240–245. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Schlossman S. F. Analysis of antigenic determinants on human monocytes and macrophages. Blood. 1982 Apr;59(4):775–786. [PubMed] [Google Scholar]
- Wasserman K., Subklewe M., Pothoff G., Banik N., Schell-Frederick E. Expression of surface markers on alveolar macrophages from symptomatic patients with HIV infection as detected by flow cytometry. Chest. 1994 May;105(5):1324–1334. doi: 10.1378/chest.105.5.1324. [DOI] [PubMed] [Google Scholar]
- te Velde A. A., Klomp J. P., Yard B. A., de Vries J. E., Figdor C. G. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. J Immunol. 1988 Mar 1;140(5):1548–1554. [PubMed] [Google Scholar]


