Abstract
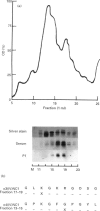
Goodpasture's disease is a rare form of glomerulonephritis characterized by the production of autoantibodies to the glomerular basement membrane (GBM). In order to understand the development of autoimmunity to the GBM, it is important to examine mechanisms underlying T cell responses to the autoantigen. A MoAb P1, with the same specificity as patients' autoantibodies, was used to affinity-purify the antigen from collagenase-digested human GBM. This material was enriched in the NC1 domain of the alpha 3 chain of type IV collagen (alpha 3(IV)NC1), known to be the principal target of anti-GBM antibodies, but also contained lower quantities of alpha 4(IV)NC1. In proliferation assays, T cells from 11/14 patients with Goodpasture's disease showed significant responses (SI > or = 2.0) to affinity-purified human GBM. Peak responses were demonstrated at 7 or 10 days at antigen concentrations of 10-30 micrograms/ml. As in other autoimmune disorders, the presence of autoantigen-reactive T cells was also demonstrated in 5/10 healthy volunteers. Tissue typing revealed that all patients possessed HLA-DR2 and/or -DR4 alleles, while normal individuals whose T cells responded possessed DR2 and/or DR7 alleles. The specificity of the T cell response in Goodpasture's disease was further investigated using monomeric components of human GBM purified by gel filtration and reverse phase high performance liquid chromatography (HPLC). Two antigenic monomer pools were obtained, which were shown by amino-terminal sequence analysis to contain alpha 3(IV)NC1 and alpha 4(IV)NC1, respectively. In all patients tested, significant T cell proliferation was observed in response to one or both of these alpha (IV)NC1 domains. These results demonstrate that patients with Goodpasture's disease possess T cells reactive with autoantigens known to be recognized by anti-GBM antibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asthana D., Fujii Y., Huston G. E., Lindstrom J. Regulation of antibody production by helper T cell clones in experimental autoimmune myasthenia gravis is mediated by IL-4 and antigen-specific T cell factors. Clin Immunol Immunopathol. 1993 Jun;67(3 Pt 1):240–248. doi: 10.1006/clin.1993.1071. [DOI] [PubMed] [Google Scholar]
- Bolton W. K., May W. J., Sturgill B. C. Proliferative autoimmune glomerulonephritis in rats: a model for autoimmune glomerulonephritis in humans. Kidney Int. 1993 Aug;44(2):294–306. doi: 10.1038/ki.1993.244. [DOI] [PubMed] [Google Scholar]
- Bowman C., Lockwood C. M. Clinical application of a radio-immunoassay for auto-antibodies to glomerular basement membrane. J Clin Lab Immunol. 1985 Aug;17(4):197–202. [PubMed] [Google Scholar]
- Campbell R. D., Milner C. M. MHC genes in autoimmunity. Curr Opin Immunol. 1993 Dec;5(6):887–893. doi: 10.1016/0952-7915(93)90101-w. [DOI] [PubMed] [Google Scholar]
- Dayan C. M., Londei M., Corcoran A. E., Grubeck-Loebenstein B., James R. F., Rapoport B., Feldmann M. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7415–7419. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry C. J., Dunn M. J., Rees A. J., Pusey C. D. Restricted specificity of the autoantibody response in Goodpasture's syndrome demonstrated by two-dimensional western blotting. Clin Exp Immunol. 1991 Dec;86(3):457–463. doi: 10.1111/j.1365-2249.1991.tb02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry C. J., Pickering M., Baker C., Pusey C. D. Identification of the Goodpasture antigen, alpha 3(IV) NC1, and four other NC1 domains of type IV collagen, by amino-terminal sequence analysis of human glomerular basement membrane separated by two-dimensional electrophoresis. Exp Nephrol. 1994 Jul-Aug;2(4):249–256. [PubMed] [Google Scholar]
- Derry C. J., Pusey C. D. Tissue-specific distribution of the Goodpasture antigen demonstrated by 2-D electrophoresis and western blotting. Nephrol Dial Transplant. 1994;9(4):355–361. [PubMed] [Google Scholar]
- Fukuma N., McLachlan S. M., Rapoport B., Goodacre J., Middleton S. L., Phillips D. I., Pegg C. A., Rees Smith B. Thyroid autoantigens and human T cell responses. Clin Exp Immunol. 1990 Nov;82(2):275–283. doi: 10.1111/j.1365-2249.1990.tb05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey B., McCormick K., Capper J., Ratliff C., Colombe B. W., Garovoy M. R., Wilson C. B. Associations of HLA-DR and HLA-DQ types with anti-GBM nephritis by sequence-specific oligonucleotide probe hybridization. Kidney Int. 1993 Aug;44(2):307–312. doi: 10.1038/ki.1993.245. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Identifying strategies for immune intervention. Science. 1993 May 14;260(5110):937–944. doi: 10.1126/science.8493532. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Glassock R. J., Dixon F. J. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967 Dec 1;126(6):989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liblau R., Tournier-Lasserve E., Maciazek J., Dumas G., Siffert O., Hashim G., Bach M. A. T cell response to myelin basic protein epitopes in multiple sclerosis patients and healthy subjects. Eur J Immunol. 1991 Jun;21(6):1391–1395. doi: 10.1002/eji.1830210610. [DOI] [PubMed] [Google Scholar]
- Mahieu P., Dardenne M., Bach J. F. Detection of humoral and cell-mediated immunity to kidney basement membranes in human renal diseases. Am J Med. 1972 Aug;53(2):185–192. doi: 10.1016/0002-9343(72)90128-3. [DOI] [PubMed] [Google Scholar]
- McGregor A. M. Autoimmunity in the thyroid--can the molecular revolution contribute to our understanding? Q J Med. 1992 Jan;82(297):1–13. [PubMed] [Google Scholar]
- Morrison K. E., Mariyama M., Yang-Feng T. L., Reeders S. T. Sequence and localization of a partial cDNA encoding the human alpha 3 chain of type IV collagen. Am J Hum Genet. 1991 Sep;49(3):545–554. [PMC free article] [PubMed] [Google Scholar]
- Moudgil K. D., Ametani A., Grewal I. S., Kumar V., Sercarz E. E. Processing of self-proteins and its impact on shaping the T cell repertoire, autoimmunity and immune regulation. Int Rev Immunol. 1993;10(4):365–377. doi: 10.3109/08830189309061711. [DOI] [PubMed] [Google Scholar]
- Newsom-Davis J., Harcourt G., Sommer N., Beeson D., Willcox N., Rothbard J. B. T-cell reactivity in myasthenia gravis. J Autoimmun. 1989 Jun;2 (Suppl):101–108. doi: 10.1016/0896-8411(89)90121-2. [DOI] [PubMed] [Google Scholar]
- Nolasco F. E., Cameron J. S., Hartley B., Coelho A., Hildreth G., Reuben R. Intraglomerular T cells and monocytes in nephritis: study with monoclonal antibodies. Kidney Int. 1987 May;31(5):1160–1166. doi: 10.1038/ki.1987.123. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Murphy K. T-cell subsets in autoimmunity. Curr Opin Immunol. 1993 Dec;5(6):880–886. doi: 10.1016/0952-7915(93)90100-7. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Pohjolainen E. R., Myers J. C. Complete primary structure of the triple-helical region and the carboxyl-terminal domain of a new type IV collagen chain, alpha 5(IV). J Biol Chem. 1990 Aug 15;265(23):13758–13766. [PubMed] [Google Scholar]
- Pusey C. D., Dash A., Kershaw M. J., Morgan A., Reilly A., Rees A. J., Lockwood C. M. A single autoantigen in Goodpasture's syndrome identified by a monoclonal antibody to human glomerular basement membrane. Lab Invest. 1987 Jan;56(1):23–31. [PubMed] [Google Scholar]
- Reynolds J., Pusey C. D. In vivo treatment with a monoclonal antibody to T helper cells in experimental autoimmune glomerulonephritis in the BN rat. Clin Exp Immunol. 1994 Jan;95(1):122–127. doi: 10.1111/j.1365-2249.1994.tb06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J., Sallie B. A., Syrganis C., Pusey C. D. The role of T-helper lymphocytes in priming for experimental autoimmune glomerulonephritis in the BN rat. J Autoimmun. 1993 Oct;6(5):571–585. doi: 10.1006/jaut.1993.1047. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Lewis E. J., David J. R. In vitro evidence for cellular hypersensitivity to glomerular-basement-membrane antigens in human glomerulonephritis. N Engl J Med. 1970 Sep 3;283(10):497–501. doi: 10.1056/NEJM197009032831001. [DOI] [PubMed] [Google Scholar]
- Saus J., Wieslander J., Langeveld J. P., Quinones S., Hudson B. G. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem. 1988 Sep 15;263(26):13374–13380. [PubMed] [Google Scholar]
- Savage C. O., Pusey C. D., Bowman C., Rees A. J., Lockwood C. M. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980-4. Br Med J (Clin Res Ed) 1986 Feb 1;292(6516):301–304. doi: 10.1136/bmj.292.6516.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer N., Willcox N., Harcourt G. C., Newsom-Davis J. Myasthenic thymus and thymoma are selectively enriched in acetylcholine receptor-reactive T cells. Ann Neurol. 1990 Sep;28(3):312–319. doi: 10.1002/ana.410280303. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Oohashi T., Yoshioka H., Matsuo N., Ninomiya Y. cDNA isolation and partial gene structure of the human alpha 4(IV) collagen chain. FEBS Lett. 1993 Sep 13;330(2):122–128. doi: 10.1016/0014-5793(93)80256-t. [DOI] [PubMed] [Google Scholar]
- Sun J. B., Harcourt G., Wang Z. Y., Hawke S., Olsson T., Fredrikson S., Link H. T cell responses to human recombinant acetylcholine receptor-alpha subunit in myasthenia gravis and controls. Eur J Immunol. 1992 Jun;22(6):1553–1559. doi: 10.1002/eji.1830220631. [DOI] [PubMed] [Google Scholar]
- Tandon N., Freeman M. A., Weetman A. P. T cell responses to synthetic TSH receptor peptides in Graves' disease. Clin Exp Immunol. 1992 Sep;89(3):468–473. doi: 10.1111/j.1365-2249.1992.tb06982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N., Weetman A. P. T cells and thyroid autoimmunity. J R Coll Physicians Lond. 1994 Jan-Feb;28(1):10–18. [PMC free article] [PubMed] [Google Scholar]
- Turner N., Forstová J., Rees A., Pusey C. D., Mason P. J. Production and characterization of recombinant Goodpasture antigen in insect cells. J Biol Chem. 1994 Jun 24;269(25):17141–17145. [PubMed] [Google Scholar]
- Turner N., Mason P. J., Brown R., Fox M., Povey S., Rees A., Pusey C. D. Molecular cloning of the human Goodpasture antigen demonstrates it to be the alpha 3 chain of type IV collagen. J Clin Invest. 1992 Feb;89(2):592–601. doi: 10.1172/JCI115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox N. Myasthenia gravis. Curr Opin Immunol. 1993 Dec;5(6):910–917. doi: 10.1016/0952-7915(93)90105-2. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Dixon F. J. Anti-glomerular basement membrane antibody-induced glomerulonephritis. Kidney Int. 1973 Feb;3(2):74–89. doi: 10.1038/ki.1973.14. [DOI] [PubMed] [Google Scholar]
- Zhang J., Markovic-Plese S., Lacet B., Raus J., Weiner H. L., Hafler D. A. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994 Mar 1;179(3):973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Mochizuki T., Smeets H., Antignac C., Laurila P., de Paepe A., Tryggvason K., Reeders S. T. Deletion of the paired alpha 5(IV) and alpha 6(IV) collagen genes in inherited smooth muscle tumors. Science. 1993 Aug 27;261(5125):1167–1169. doi: 10.1126/science.8356449. [DOI] [PubMed] [Google Scholar]