Abstract
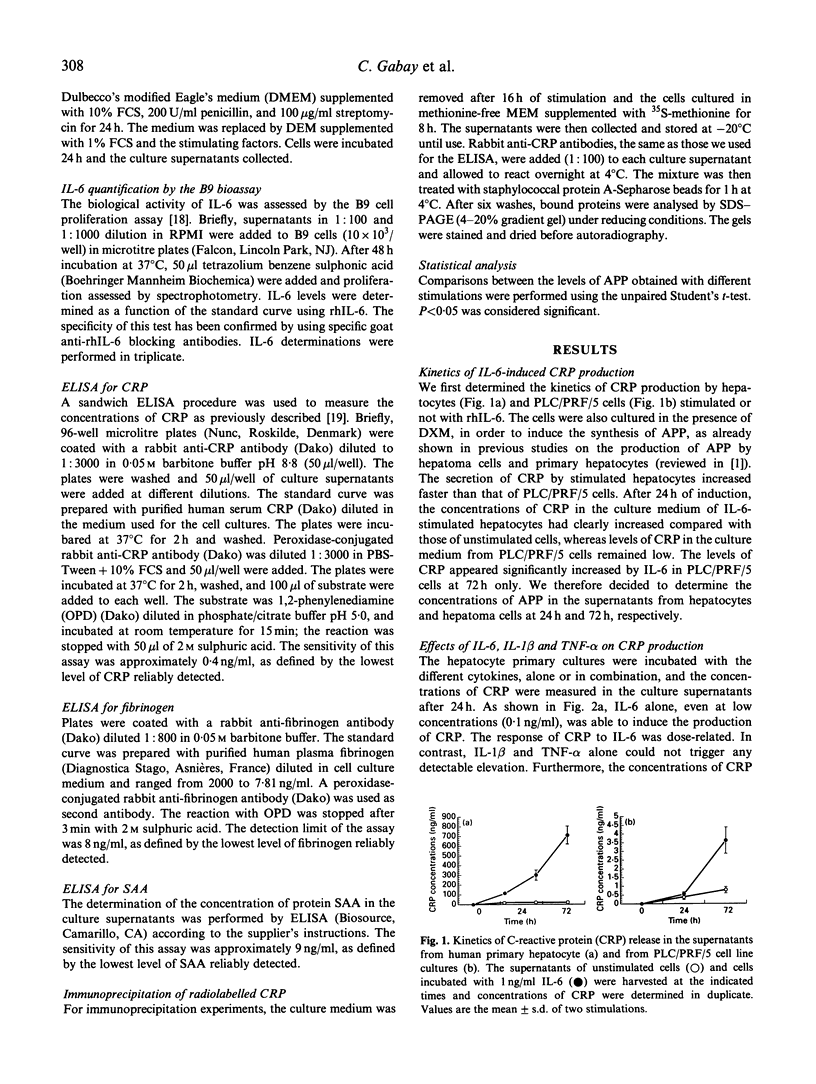
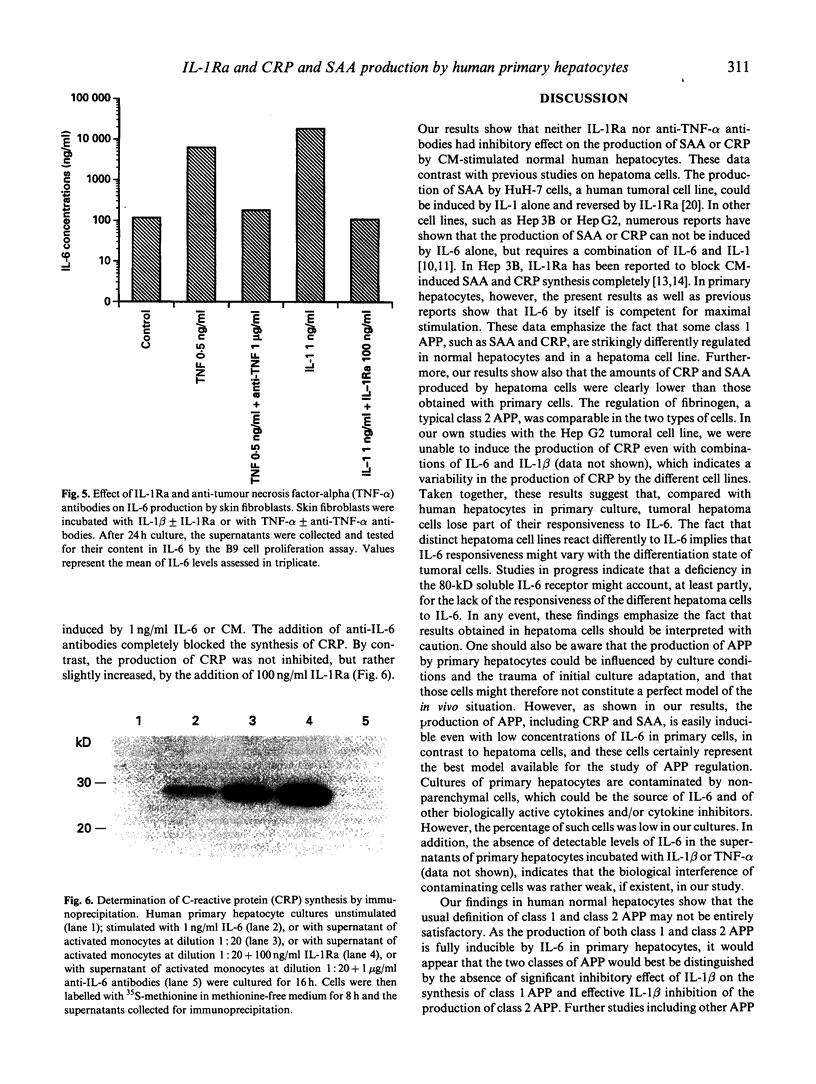
The synthesis of some class 1 acute-phase proteins (APP), including C-reactive protein (CRP) and serum amyloid A (SAA) protein is completely blocked by the IL-1 receptor antagonist (IL-1Ra), whereas the production of fibrinogen, a class 2 APP, is increased by IL-1Ra in hepatoma cells, but this has never been tested in human hepatocytes in primary culture. Since previous studies on the contributions of cytokine inhibitors in connective tissues diseases suggested that IL-1 and tumour necrosis factor-alpha (TNF-alpha) might play an important role in the regulation of CRP, we decided to examine in more detail the respective roles of IL-1 beta, IL-6, and TNF-alpha and their inhibitors in the production of APP by human primary hepatocytes versus the hepatoma cell line PLC/PRF/5. In the hepatoma cell line, IL-1 beta and/or TNF-alpha had synergistic effects with IL-6 on the production of CRP and SAA. In contrast, these cytokines were devoid of effect in normal hepatocytes. The production of fibrinogen was increased by IL-6 and decreased by IL-1 (and TNF-alpha) in both cell types. The secretion of CRP and SAA by primary hepatocytes incubated with a cytokine-rich mononuclear cell-conditioned medium was totally unaffected by IL-1Ra or anti-TNF-alpha antibodies. In contrast, the addition of IL-1Ra increased the production of fibrinogen by both hepatoma cells and primary hepatocytes incubated with the mononuclear cell-conditioned medium. We therefore conclude that IL-1 beta and TNF-alpha do not exert any significant effect on the synthesis of CRP and SAA by human primary hepatocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990 Apr;7(2):147–159. [PubMed] [Google Scholar]
- Baumann H., Prowse K. R., Marinković S., Won K. A., Jahreis G. P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci. 1989;557:280-95, discussion 295-6. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- Baumann H., Schendel P. Interleukin-11 regulates the hepatic expression of the same plasma protein genes as interleukin-6. J Biol Chem. 1991 Oct 25;266(30):20424–20427. [PubMed] [Google Scholar]
- Baumann H., Wong G. G. Hepatocyte-stimulating factor III shares structural and functional identity with leukemia-inhibitory factor. J Immunol. 1989 Aug 15;143(4):1163–1167. [PubMed] [Google Scholar]
- Bevan S., Raynes J. G. IL-1 receptor antagonist regulation of acute phase protein synthesis in human hepatoma cells. J Immunol. 1991 Oct 15;147(8):2574–2578. [PubMed] [Google Scholar]
- Castell J. V., Gómez-Lechón M. J., David M., Andus T., Geiger T., Trullenque R., Fabra R., Heinrich P. C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989 Jan 2;242(2):237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- Damtew B., Rzewnicki D., Lozanski G., Kushner I. IL-1 receptor antagonist affects the plasma protein response of Hep 3B cells to conditioned medium from lipopolysaccharide-stimulated monocytes. J Immunol. 1993 May 1;150(9):4001–4007. [PubMed] [Google Scholar]
- Gabay C., Gay-Croisier F., Roux-Lombard P., Meyer O., Maineti C., Guerne P. A., Vischer T., Dayer J. M. Elevated serum levels of interleukin-1 receptor antagonist in polymyositis/dermatomyositis. A biologic marker of disease activity with a possible role in the lack of acute-phase protein response. Arthritis Rheum. 1994 Dec;37(12):1744–1751. doi: 10.1002/art.1780371206. [DOI] [PubMed] [Google Scholar]
- Gabay C., Roux-Lombard P., de Moerloose P., Dayer J. M., Vischer T., Guerne P. A. Absence of correlation between interleukin 6 and C-reactive protein blood levels in systemic lupus erythematosus compared with rheumatoid arthritis. J Rheumatol. 1993 May;20(5):815–821. [PubMed] [Google Scholar]
- Ganapathi M. K., Rzewnicki D., Samols D., Jiang S. L., Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J Immunol. 1991 Aug 15;147(4):1261–1265. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P. C., Castell J. V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990 Feb 1;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton J., Hessian P. A solid-phase enzyme immunoassay for C-reactive protein: clinical value and the effect of rheumatoid factor. J Immunol Methods. 1984 Mar 30;68(1-2):185–192. doi: 10.1016/0022-1759(84)90149-2. [DOI] [PubMed] [Google Scholar]
- Houssiau F. A., Devogelaer J. P., Van Damme J., de Deuxchaisnes C. N., Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988 Jun;31(6):784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Magielska-Zero D., Bereta J., Czuba-Pelech B., Pajdak W., Gauldie J., Koj A. Inhibitory effect of human recombinant interferon gamma on synthesis of acute phase proteins in human hepatoma Hep G2 cells stimulated by leukocyte cytokines, TNF alpha and IFN-beta 2/BSF-2/IL-6. Biochem Int. 1988 Jul;17(1):17–23. [PubMed] [Google Scholar]
- Metsärinne K. P., Nordström D. C., Konttinen Y. T., Teppo A. M., Fyhrquist F. Y. Plasma interleukin-6 and renin substrate in reactive arthritis, rheumatoid arthritis, and systemic lupus erythematosus. Rheumatol Int. 1992;12(3):93–96. doi: 10.1007/BF00290261. [DOI] [PubMed] [Google Scholar]
- Moshage H. J., Roelofs H. M., van Pelt J. F., Hazenberg B. P., van Leeuwen M. A., Limburg P. C., Aarden L. A., Yap S. H. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem Biophys Res Commun. 1988 Aug 30;155(1):112–117. doi: 10.1016/s0006-291x(88)81056-8. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Lanham J. G., De Beer F. C. C-reactive protein in SLE. Clin Rheum Dis. 1982 Apr;8(1):91–103. [PubMed] [Google Scholar]
- Richards C. D., Brown T. J., Shoyab M., Baumann H., Gauldie J. Recombinant oncostatin M stimulates the production of acute phase proteins in HepG2 cells and rat primary hepatocytes in vitro. J Immunol. 1992 Mar 15;148(6):1731–1736. [PubMed] [Google Scholar]
- Rokita H., Loose L. D., Bartle L. M., Sipe J. D. Synergism of interleukin 1 and interleukin 6 induces serum amyloid A production while depressing fibrinogen: a quantitative analysis. J Rheumatol. 1994 Mar;21(3):400–405. [PubMed] [Google Scholar]
- Schooltink H., Stoyan T., Roeb E., Heinrich P. C., Rose-John S. Ciliary neurotrophic factor induces acute-phase protein expression in hepatocytes. FEBS Lett. 1992 Dec 21;314(3):280–284. doi: 10.1016/0014-5793(92)81489-9. [DOI] [PubMed] [Google Scholar]
- Smith J. W., McDonald T. L. Production of serum amyloid A and C-reactive protein by HepG2 cells stimulated with combinations of cytokines or monocyte conditioned media: the effects of prednisolone. Clin Exp Immunol. 1992 Nov;90(2):293–299. doi: 10.1111/j.1365-2249.1992.tb07945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. W., Ku N. O., Mortensen R. F. Regulation of cytokine-induced human C-reactive protein production by transforming growth factor-beta. J Immunol. 1990 Oct 15;145(8):2507–2513. [PubMed] [Google Scholar]
- Yap S. H., Moshage H. J., Hazenberg B. P., Roelofs H. M., Bijzet J., Limburg P. C., Aarden L. A., van Rijswijk M. H. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochim Biophys Acta. 1991 Feb 19;1091(3):405–408. doi: 10.1016/0167-4889(91)90207-e. [DOI] [PubMed] [Google Scholar]



