Abstract
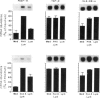
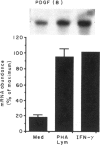
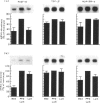
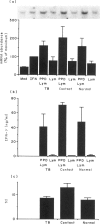
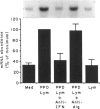
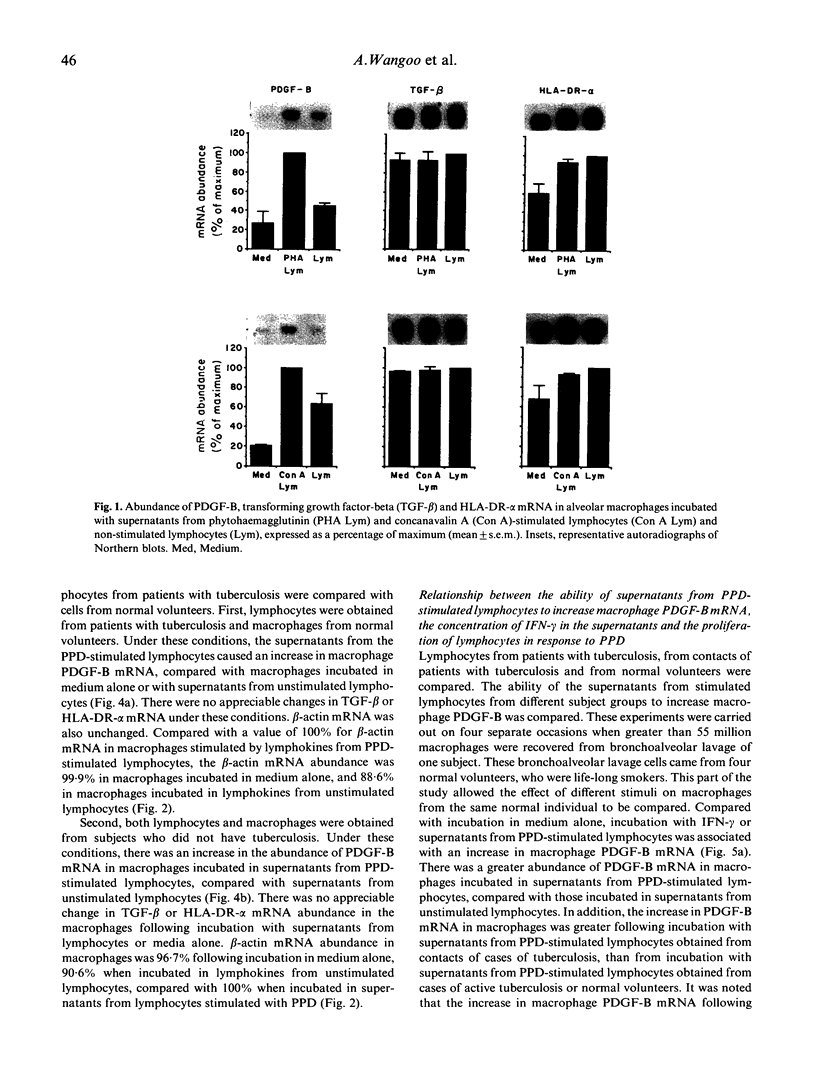
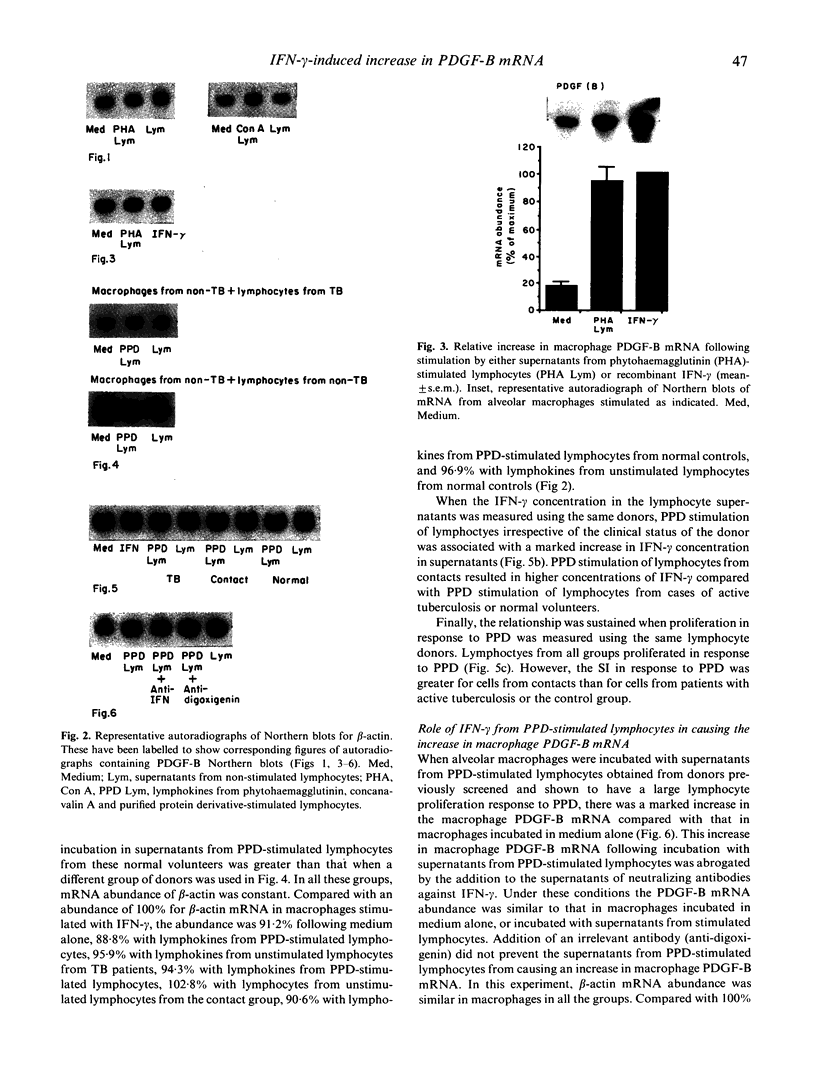
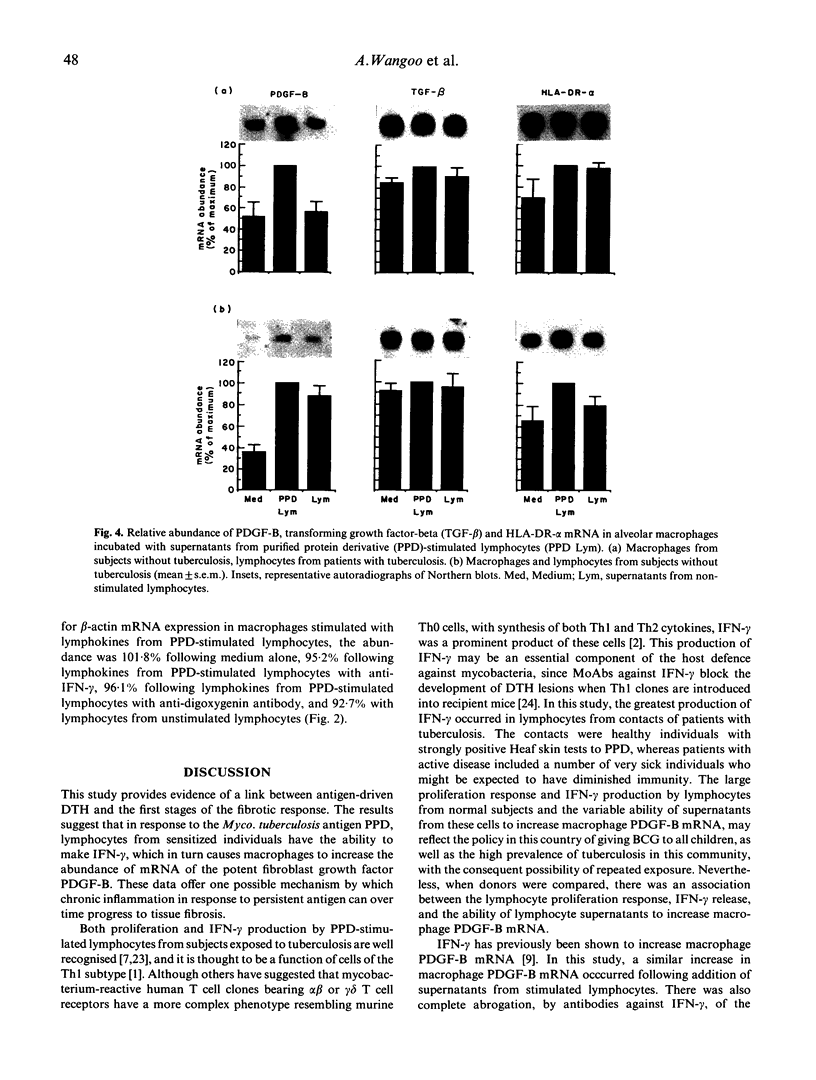
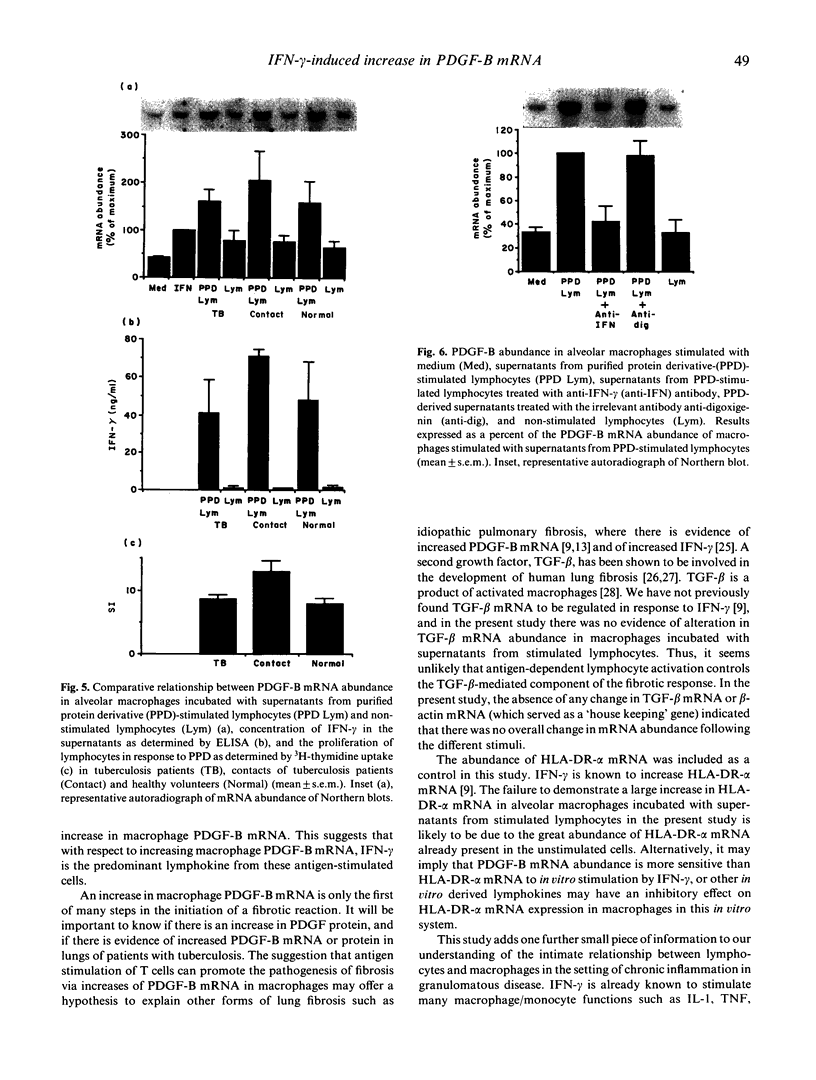
Macrophage production of PDGF-B is believed to be important in the pathogenesis of diseases where chronic lung inflammation develops into fibrosis. Since tuberculosis is characterized by chronic inflammation and tissue fibrosis, we asked if lymphokines from lymphocytes stimulated by the Mycobacterium tuberculosis antigen PPD, contained factors capable of increasing human alveolar macrophage PDGF-B mRNA. Supernatants from both phytohaemagglutinin (PHA)- and purified protein derivative (PPD)-stimulated lymphocytes, when added to macrophages, induced an increase in the mRNA of PDGF-B, but not transforming growth factor-beta (TGF-beta). When lymphocytes from contacts of patients with tuberculosis, patients with tuberculosis, and normal subjects were compared following PPD stimulation, the lymphocytes from the contacts had the greatest proliferation response, the greatest production of interferon-gamma (IFN-gamma), and their lymphokines induced the greatest increase in PDGF-B mRNA in macrophages. Recombinant human IFN-gamma reproduced this ability of lymphokines to increase macrophage PDGF-B mRNA. Finally, the increase in macrophage PDGF-B mRNA following incubation with supernatants from PPD-stimulated lymphocytes was shown to be due to IFN-gamma, when the increase in macrophage PDGF-B mRNA was prevented by addition of anti-human IFN-gamma antibody to the lymphocyte supernatant. This study indicated that antigen-stimulated lymphocytes released IFN-gamma, which in turn resulted in an increase in PDGF-B mRNA in alveolar macrophages. Such a mechanism provides a link between the DTH response and the first stages of a fibrotic reaction, and may offer an explanation for the progression of chronic inflammation to fibrosis, as occurs in the lungs of patients with untreated pulmonary tuberculosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Bravo M. A., Avila R. E., Galanopoulos T., Neville-Golden J., Maxwell M., Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990 Oct;86(4):1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Abrams J. S., Lu S., Sieling P. A., Rea T. H., Modlin R. L. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993 Jan;61(1):197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Beckmann M. P., Betsholtz C., Heldin C. H., Westermark B., Di Marco E., Di Fiore P. P., Robbins K. C., Aaronson S. A. Comparison of biological properties and transforming potential of human PDGF-A and PDGF-B chains. Science. 1988 Sep 9;241(4871):1346–1349. doi: 10.1126/science.2842868. [DOI] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Folkvord J. M., Hart C. E., Murray M. J., McPherson J. M. Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest. 1989 Sep;84(3):1036–1040. doi: 10.1172/JCI114227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989 Nov 1;143(9):2887–2893. [PubMed] [Google Scholar]
- Gay S., Jones R. E., Jr, Huang G. Q., Gay R. E. Immunohistologic demonstration of platelet-derived growth factor (PDGF) and sis-oncogene expression in scleroderma. J Invest Dermatol. 1989 Feb;92(2):301–303. doi: 10.1111/1523-1747.ep12276895. [DOI] [PubMed] [Google Scholar]
- Haynes A. R., Shaw R. J. Dexamethasone-induced increase in platelet-derived growth factor (B) mRNA in human alveolar macrophages and myelomonocytic HL60 macrophage-like cells. Am J Respir Cell Mol Biol. 1992 Aug;7(2):198–206. doi: 10.1165/ajrcmb/7.2.198. [DOI] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Khalil N., O'Connor R. N., Unruh H. W., Warren P. W., Flanders K. C., Kemp A., Bereznay O. H., Greenberg A. H. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991 Aug;5(2):155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- Kovacs E. J., Kelley J. Lymphokine regulation of macrophage-derived growth factor secretion following pulmonary injury. Am J Pathol. 1985 Nov;121(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Nagaoka I., Trapnell B. C., Crystal R. G. Upregulation of platelet-derived growth factor-A and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest. 1990 Jun;85(6):2023–2027. doi: 10.1172/JCI114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R. C. Effect of interferon on the induction of human monocyte secretion of interleukin-1 activity. Immunology. 1985 Nov;56(3):441–449. [PMC free article] [PubMed] [Google Scholar]
- Nistér M., Hammacher A., Mellström K., Siegbahn A., Rönnstrand L., Westermark B., Heldin C. H. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988 Mar 25;52(6):791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Olsen D. R., Uitto J. Differential expression of type IV procollagen and laminin genes by fetal vs adult skin fibroblasts in culture: determination of subunit mRNA steady-state levels. J Invest Dermatol. 1989 Jul;93(1):127–131. doi: 10.1111/1523-1747.ep12277381. [DOI] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Piacibello W., Lu L., Wachter M., Rubin B., Broxmeyer H. E. Release of granulocyte-macrophage colony stimulating factors from major histocompatibility complex class II antigen-positive monocytes is enhanced by human gamma interferon. Blood. 1985 Dec;66(6):1343–1351. [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribera E., Español T., Martinez-Vazquez J. M., Ocaña I., Encabo G. Lymphocyte proliferation and gamma-interferon production after "in vitro" stimulation with PPD. Differences between tuberculous and nontuberculous pleurisy in patients with positive tuberculin skin test. Chest. 1990 Jun;97(6):1381–1385. doi: 10.1378/chest.97.6.1381. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., Rose A. H. Pulmonary gamma interferon production in patients with fibrosing alveolitis. Thorax. 1990 Feb;45(2):105–108. doi: 10.1136/thx.45.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. J., Benedict S. H., Clark R. A., King T. E., Jr Pathogenesis of pulmonary fibrosis in interstitial lung disease. Alveolar macrophage PDGF(B) gene activation and up-regulation by interferon gamma. Am Rev Respir Dis. 1991 Jan;143(1):167–173. doi: 10.1164/ajrccm/143.1.167. [DOI] [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- Wangoo A., Haynes A. R., Sutcliffe S. P., Sorooshian M., Shaw R. J. Modulation of platelet-derived growth factor B mRNA abundance in macrophages by colchicine and dibutyryl-cAMP. Mol Pharmacol. 1992 Oct;42(4):584–589. [PubMed] [Google Scholar]