Abstract
Total and Ascaris-specific serum IgE levels were measured in a group of 98 Ascaris-infected children from a slum area of Caracas, Venezuela, in whom the infections were eliminated by regular treatment for 22 months with the anthelmint Oxantel/Pyrantel ('Quantrel'). The children were re-evaluated at the end of the treatment programme, and then 8 months later, at which time reinfection was assessed. Total IgE levels at the beginning of the study were significantly higher in the children who became reinfected after treatment, compared with those who did not. The anthelmint treatment caused a significant decrease in the total IgE levels in most of the children, and after a period of 8 months without treatment these continued to decrease in the non-reinfected group, but increased again in the reinfected children. The reverse pattern was found for Ascaris-specific IgE antibody levels, and in fact an inverse correlation was found between total and anti-Ascaris IgE levels. Striking associations were found between reinfection and high pretreatment values of total IgE, but low levels of specific IgE antibody. These data support the concept that specific IgE antibody may participate in the protection against helminthic infection, and suggest that the polyclonal stimulation of IgE synthesis caused by these parasites may reduce the effectiveness of such responses. The results also indicate that different individuals have varying propensities to respond polyclonally to the helminths, and this influences their resistance to infection.
Full text
PDF
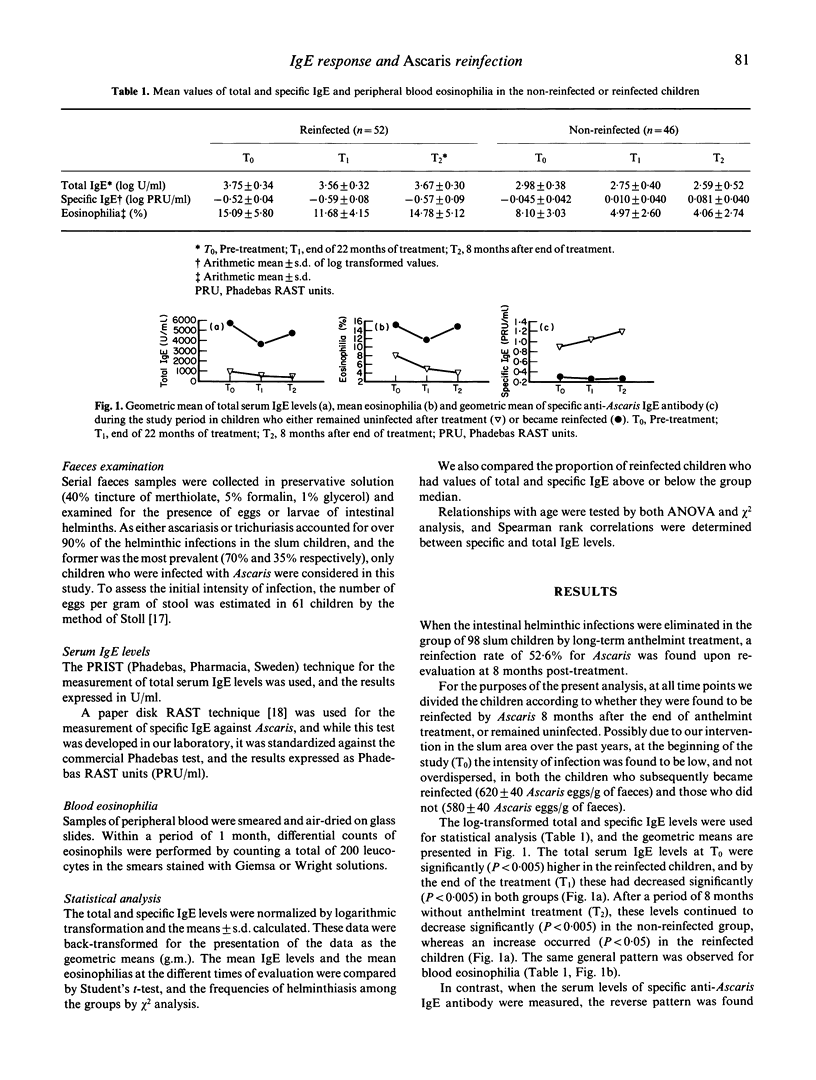
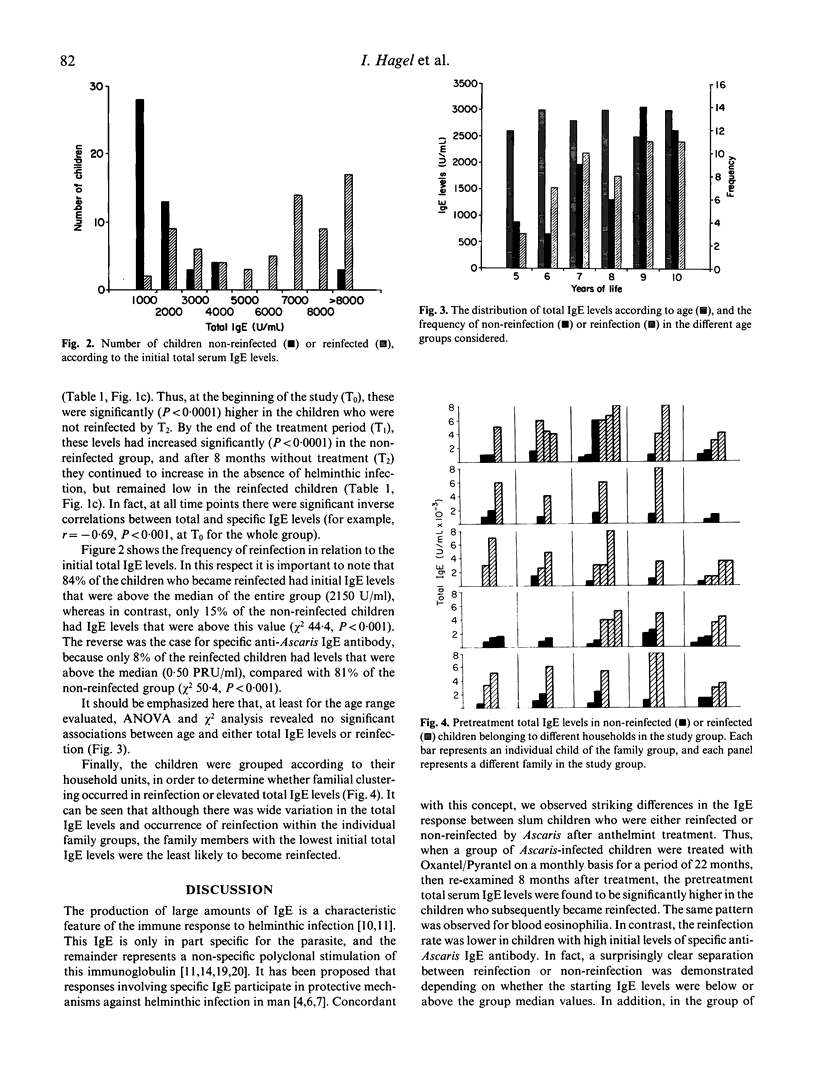

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. M., May R. M. Helminth infections of humans: mathematical models, population dynamics, and control. Adv Parasitol. 1985;24:1–101. doi: 10.1016/s0065-308x(08)60561-8. [DOI] [PubMed] [Google Scholar]
- Anderson R. M., Medley G. F. Community control of helminth infections of man by mass and selective chemotherapy. Parasitology. 1985 Apr;90(Pt 4):629–660. doi: 10.1017/s0031182000052288. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Orgel H. A., Hamburger R. N. The influence of serum IgE levels of selected recipients, including patients with allergy, helminthiasis and tuberculosis, on the apparent P-K titre of a reaginic serum. Clin Exp Immunol. 1973 May;14(1):117–125. [PMC free article] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Ouma J. H., Butterworth A. E. Immunity to schistosomes: progress toward vaccine. Science. 1987 Nov 20;238(4830):1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- Ceska M., Lundkvist U. A new and simple radioimmunoassay method for the determination of IgE. Immunochemistry. 1972 Oct;9(10):1021–1030. doi: 10.1016/0019-2791(72)90112-7. [DOI] [PubMed] [Google Scholar]
- Dunne D. W., Bickle Q. D., Butterworth A. E., Richardson B. A. The blocking of human antibody-dependent, eosinophil-mediated killing of Schistosoma mansoni schistosomula by monoclonal antibodies which cross-react with a polysaccharide-containing egg antigen. Parasitology. 1987 Apr;94(Pt 2):269–280. doi: 10.1017/s0031182000053944. [DOI] [PubMed] [Google Scholar]
- Dunne D. W., Butterworth A. E., Fulford A. J., Kariuki H. C., Langley J. G., Ouma J. H., Capron A., Pierce R. J., Sturrock R. F. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992 Jun;22(6):1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Pearce E. J., Urban J. F., Jr, Sher A. Regulation and biological function of helminth-induced cytokine responses. Immunol Today. 1991 Mar;12(3):A62–A66. doi: 10.1016/S0167-5699(05)80018-0. [DOI] [PubMed] [Google Scholar]
- Godfrey R. C., Gradidge C. F. Allergic sensitisation of human lung fragments prevented by saturation of IgE binding sites. Nature. 1976 Feb 12;259(5543):484–486. doi: 10.1038/259484a0. [DOI] [PubMed] [Google Scholar]
- Hagan P. Reinfection, exposure and immunity in human schistosomiasis. Parasitol Today. 1992 Jan;8(1):12–16. doi: 10.1016/0169-4758(92)90303-j. [DOI] [PubMed] [Google Scholar]
- Hagel I., Lynch N. R., Pérez M., Di Prisco M. C., López R., Rojas E. Modulation of the allergic reactivity of slum children by helminthic infection. Parasite Immunol. 1993 Jun;15(6):311–315. doi: 10.1111/j.1365-3024.1993.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Hagel I., Lynch N. R., Pérez M., Di Prisco M. C., López R., Rojas E. Relationship between the degree of poverty and the IgE response to Ascaris infection in slum children. Trans R Soc Trop Med Hyg. 1993 Jan-Feb;87(1):16–18. doi: 10.1016/0035-9203(93)90401-b. [DOI] [PubMed] [Google Scholar]
- Henry F. J. Reinfection with Ascaris lumbricoides after chemotherapy: a comparative study in three villages with varying sanitation. Trans R Soc Trop Med Hyg. 1988;82(3):460–464. doi: 10.1016/0035-9203(88)90162-9. [DOI] [PubMed] [Google Scholar]
- Hussain R., Hamilton R. G., Kumaraswami V., Adkinson N. F., Jr, Ottesen E. A. IgE responses in human filariasis. I. Quantitation of filaria-specific IgE. J Immunol. 1981 Oct;127(4):1623–1629. [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- Lynch N. R., Lopez R. I., Di Prisco-Fuenmayor M. C., Hagel I., Medouze L., Viana G., Ortega C., Prato G. Allergic reactivity and socio-economic level in a tropical environment. Clin Allergy. 1987 May;17(3):199–207. doi: 10.1111/j.1365-2222.1987.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Lynch N. R., López R., Istúriz G., Tenías-Salazar E. Allergic reactivity and helminthic infection in Amerindians of the Amazon Basin. Int Arch Allergy Appl Immunol. 1983;72(4):369–372. doi: 10.1159/000234899. [DOI] [PubMed] [Google Scholar]
- Moqbel R. Helminth-induced intestinal inflammation. Trans R Soc Trop Med Hyg. 1986;80(5):719–727. doi: 10.1016/0035-9203(86)90370-6. [DOI] [PubMed] [Google Scholar]
- Stephen J. M., Waterlow J. C. Effect of malnutrition on activity of two enzymes concerned with aminoacid metabolism in human liver. Lancet. 1968 Jan 20;1(7534):118–119. doi: 10.1016/s0140-6736(68)92724-4. [DOI] [PubMed] [Google Scholar]
- Turner K. J., Feddema L., Quinn E. H. Non-specific potentiation of IgE by parasitic infections in man. Int Arch Allergy Appl Immunol. 1979;58(2):232–236. doi: 10.1159/000232197. [DOI] [PubMed] [Google Scholar]


