Abstract
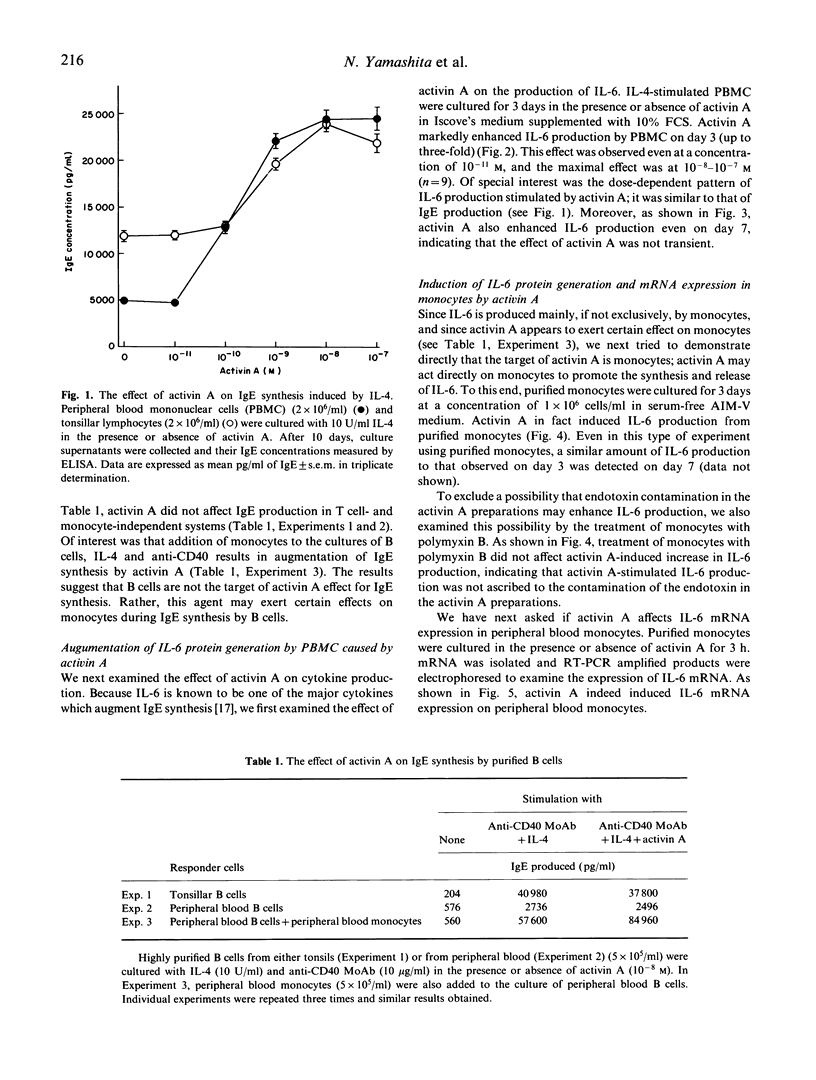
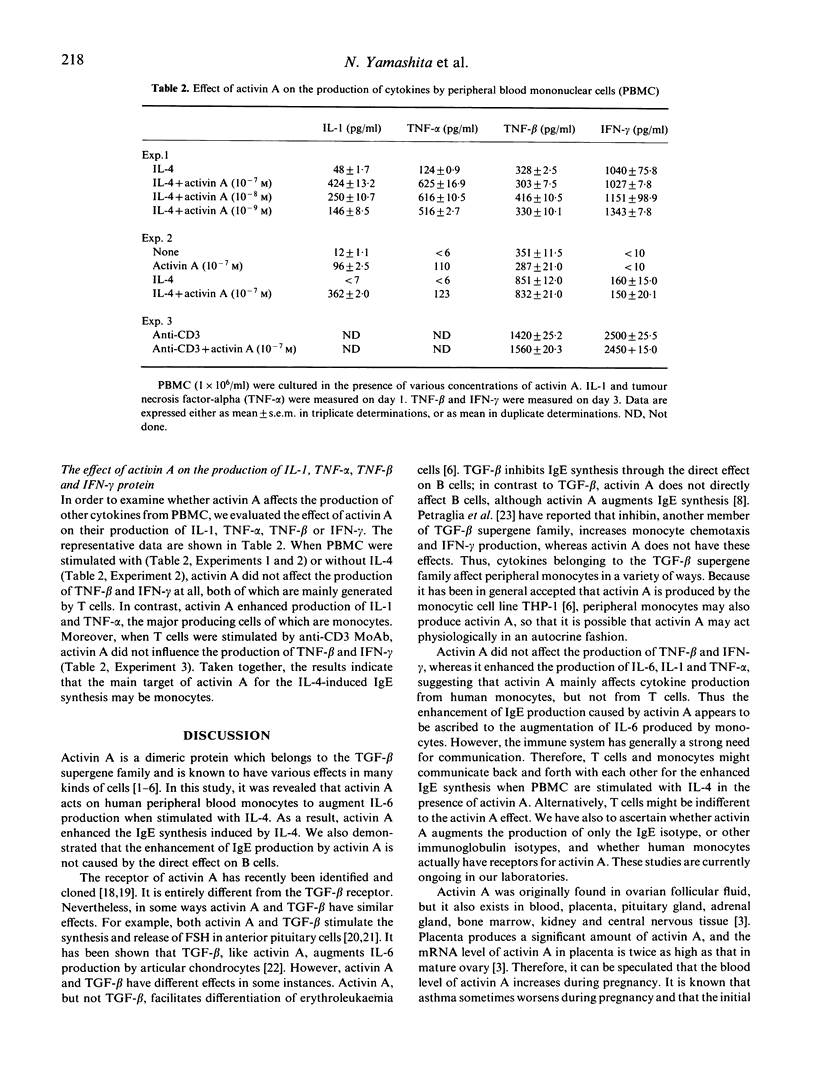
Activin A not only stimulates the synthesis and release of pituitary follicle-stimulating hormone, but exerts various effects on haematopoietic cells, embryos, and fibroblasts. In the present study we have examined effects of activin A on IgE synthesis and cytokine production by peripheral blood mononuclear cells (PBMC) in normal humans. When PBMC were cultured in the presence of IL-4, activin A significantly augmented IgE production induced by IL-4. Activin A did not affect, however, IgE production from highly purified B cells when they were stimulated with anti-CD40 MoAb and IL-4. The fact that in the latter condition IgE synthesis was T cell- and monocyte-independent indicated that activin A does not directly influence B cells for IgE synthesis. Rather, production as well as gene expression of IL-6, which is known to enhance IgE synthesis by purified monocytes, was induced by activin A alone. In addition, activin A induced other monokines such as IL-1 and tumour necrosis factor (TNF)-alpha from monocytes. In contrast, activin A neither induced nor augmented the production of TNF-beta or interferon-gamma (IFN-gamma), both of which are known to be exclusively generated by T cells. These data indicate that activin A plays a certain role in physiological functions for monocytes in normal humans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attisano L., Wrana J. L., Cheifetz S., Massagué J. Novel activin receptors: distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992 Jan 10;68(1):97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- Eto Y., Tsuji T., Takezawa M., Takano S., Yokogawa Y., Shibai H. Purification and characterization of erythroid differentiation factor (EDF) isolated from human leukemia cell line THP-1. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1095–1103. doi: 10.1016/0006-291x(87)91528-2. [DOI] [PubMed] [Google Scholar]
- Gauchat J. F., Aversa G., Gascan H., de Vries J. E. Modulation of IL-4 induced germline epsilon RNA synthesis in human B cells by tumor necrosis factor-alpha, anti-CD40 monoclonal antibodies or transforming growth factor-beta correlates with levels of IgE production. Int Immunol. 1992 Mar;4(3):397–406. doi: 10.1093/intimm/4.3.397. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Manchon C., Vale W. Activin-A, inhibin and transforming growth factor-beta modulate growth of two gonadal cell lines. Endocrinology. 1989 Sep;125(3):1666–1672. doi: 10.1210/endo-125-3-1666. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Carson D. A., Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990 Jan 15;144(2):499–505. [PubMed] [Google Scholar]
- Jabara H. H., Fu S. M., Geha R. S., Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990 Dec 1;172(6):1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Ogata E. Dual effect of activin A on cell growth in Balb/c 3T3 cells. Biochem Biophys Res Commun. 1989 Mar 31;159(3):1107–1113. doi: 10.1016/0006-291x(89)92223-7. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Graham D., Toy K., Lin L. S., Senyk G., Fendly B. M. Recombinant tumor necrosis factor causes activation of human granulocytes. Blood. 1987 Feb;69(2):640–644. [PubMed] [Google Scholar]
- Macy E., Kemeny M., Saxon A. Enhanced ELISA: how to measure less than 10 picograms of a specific protein (immunoglobulin) in less than 8 hours. FASEB J. 1988 Nov;2(14):3003–3009. doi: 10.1096/fasebj.2.14.3263291. [DOI] [PubMed] [Google Scholar]
- Maggi E., Del Prete G. F., Parronchi P., Tiri A., Macchia D., Biswas P., Simonelli C., Ricci M., Romagnani S. Role for T cells, IL-2 and IL-6 in the IL-4-dependent in vitro human IgE synthesis. Immunology. 1989 Nov;68(3):300–306. [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Meunier H., Rivier C., Evans R. M., Vale W. Gonadal and extragonadal expression of inhibin alpha, beta A, and beta B subunits in various tissues predicts diverse functions. Proc Natl Acad Sci U S A. 1988 Jan;85(1):247–251. doi: 10.1073/pnas.85.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Murata M., Eto Y., Shibai H., Sakai M., Muramatsu M. Erythroid differentiation factor is encoded by the same mRNA as that of the inhibin beta A chain. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2434–2438. doi: 10.1073/pnas.85.8.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Kitamura K., Yamashita N., Sakane T., Mizushima Y., Delespesse G., Lal R. B. Constitutive expression and production of tumor necrosis factor-beta in T-cell lines infected with HTLV-I and HTLV-II. Biochem Biophys Res Commun. 1993 Mar 15;191(2):371–377. doi: 10.1006/bbrc.1993.1227. [DOI] [PubMed] [Google Scholar]
- Petraglia F., Sacerdote P., Cossarizza A., Angioni S., Genazzani A. D., Franceschi C., Muscettola M., Grasso G. Inhibin and activin modulate human monocyte chemotaxis and human lymphocyte interferon-gamma production. J Clin Endocrinol Metab. 1991 Feb;72(2):496–502. doi: 10.1210/jcem-72-2-496. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Sokol S., Melton D. A. Pre-existent pattern in Xenopus animal pole cells revealed by induction with activin. Nature. 1991 May 30;351(6325):409–411. doi: 10.1038/351409a0. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Vale W., Rivier J., Vaughan J., McClintock R., Corrigan A., Woo W., Karr D., Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986 Jun 19;321(6072):776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- Ying S. Y., Becker A., Baird A., Ling N., Ueno N., Esch F., Guillemin R. Type beta transforming growth factor (TGF-beta) is a potent stimulator of the basal secretion of follicle stimulating hormone (FSH) in a pituitary monolayer system. Biochem Biophys Res Commun. 1986 Mar 28;135(3):950–956. doi: 10.1016/0006-291x(86)91020-x. [DOI] [PubMed] [Google Scholar]
- Zhang K., Clark E. A., Saxon A. CD40 stimulation provides an IFN-gamma-independent and IL-4-dependent differentiation signal directly to human B cells for IgE production. J Immunol. 1991 Mar 15;146(6):1836–1842. [PubMed] [Google Scholar]


