Abstract
Human monocytes and monocyte-derived macrophages were studied for their ability to phagocytose Pneumocystis carinii and produce superoxide (O2-) during the process. One x 10(6) freshly isolated monocytes, incubated with 0.1-3.75 x 10(6) P. carinii cysts, increased O2- production in a dose-related way. Antibodies were essential for the process since opsonized, but not unopsonized, pneumocysts induced O2- production significantly above the response obtained by lung tissue from rats (10.7 and 4.9 versus 3.0 fmol/cell per 90 min). The difference between pneumocysts opsonized in untreated versus complement-depleted serum was not significant (10.7 versus 12.6 fmol/cell per 90 min). Monocyte-derived macrophages also activated the respiratory burst when stimulated with pneumocysts, and this effect could be significantly increased, from 4.2 to 8.8 fmol/cell per 90 min, when cells were primed with interferon-gamma (IFN-gamma). Cells primed with IL-3 also increased O2- production, though to a lesser extent. In contrast, granulocyte-macrophage colony-stimulating factor (GM-CSF) had only a small effect on the respiratory burst in cells stimulated with P. carinii. Priming with IFN-gamma increased the rate of phagocytosis in macrophages. After incubation for 90 min or more, however, the percentage of cells with phagocytic vacuoles was only slightly higher in IFN-gamma-primed cells. When examined by electron microscopy (EM), most vacuoles contained partially or totally degraded pneumocysts. In conclusion, we have demonstrated the ability of monocytes and monocyte-derived macrophages to ingest and degrade pneumocysts, activating the respiratory burst during the process.
Full text
PDF

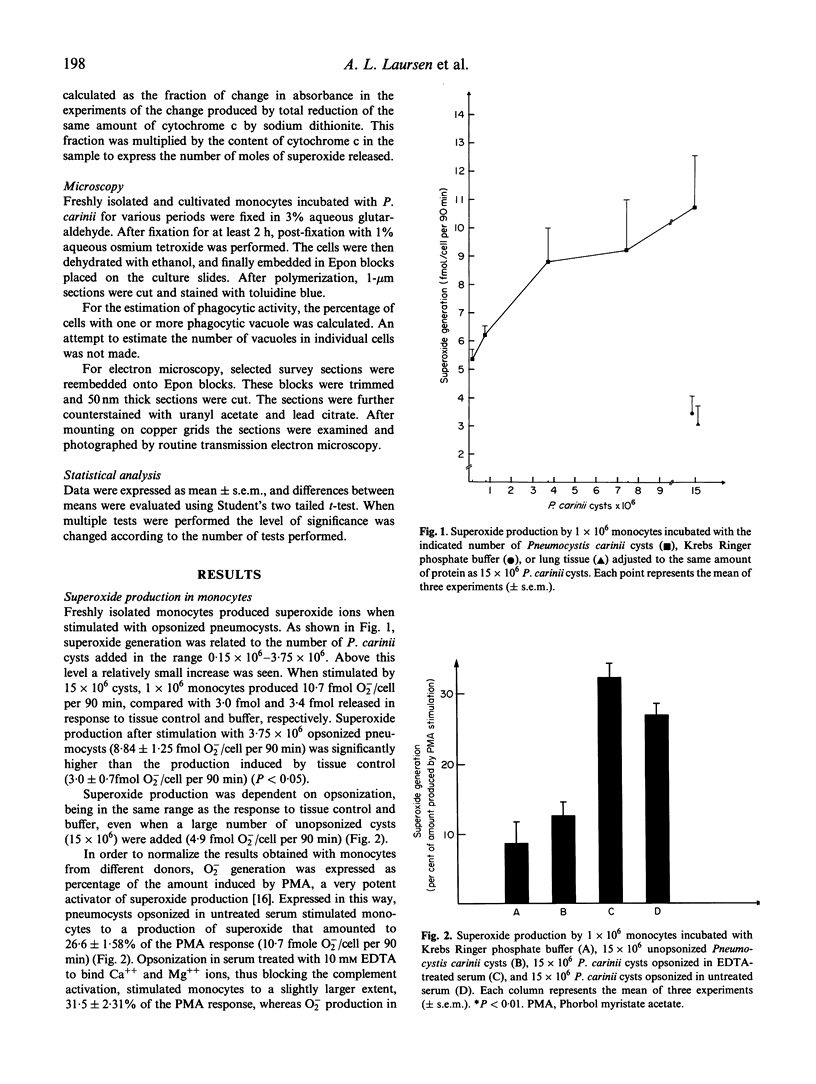
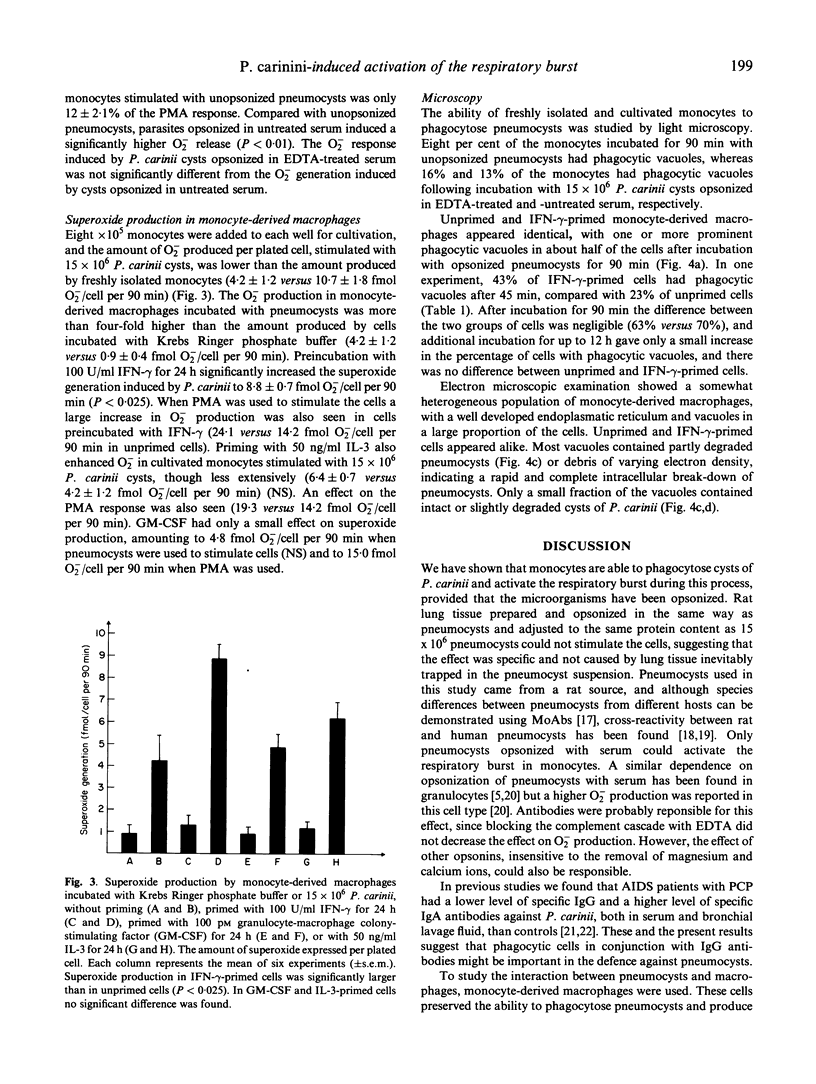
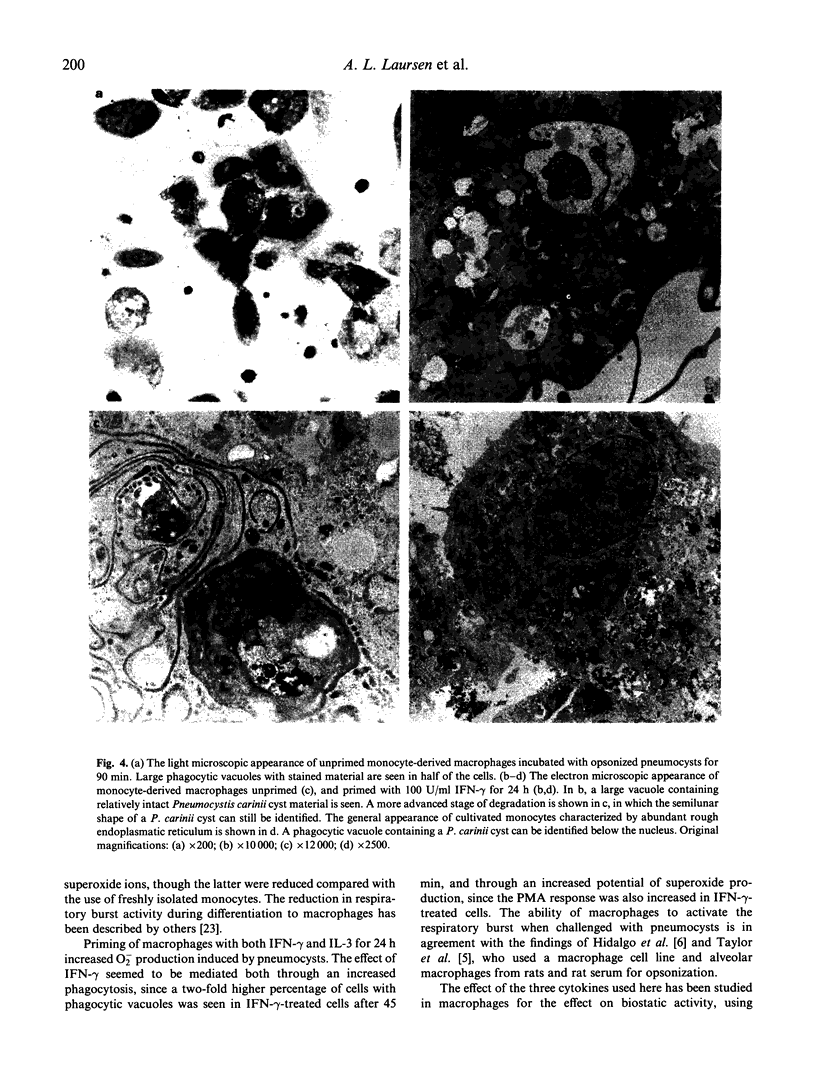


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. M., Liggitt H. D., Brunette E. N., Fuchs H. J., Shellito J. E., Debs R. J. Reduction in intensity of Pneumocystis carinii pneumonia in mice by aerosol administration of gamma interferon. Infect Immun. 1991 Nov;59(11):3859–3862. doi: 10.1128/iai.59.11.3859-3862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J Leukoc Biol. 1990 Jul;48(1):67–73. doi: 10.1002/jlb.48.1.67. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brummer E., Kurita N., Yoshida S., Nishimura K., Miyaji M. Killing of Histoplasma capsulatum by gamma-interferon-activated human monocyte-derived macrophages: evidence for a superoxide anion-dependent mechanism. J Med Microbiol. 1991 Jul;35(1):29–34. doi: 10.1099/00222615-35-1-29. [DOI] [PubMed] [Google Scholar]
- Burke B. A., Good R. A. Pneumocystis carinii infection. Medicine (Baltimore) 1973 Jan;52(1):23–51. doi: 10.1097/00005792-197301000-00002. [DOI] [PubMed] [Google Scholar]
- Capsoni F., Minonzio F., Ongari A. M., Colombo G., Rizzardi G. P., Bonara P., D'Arminio-Monforte A., Zanussi C. Increased expression of IgG Fc receptor type I on neutrophils and monocytes from HIV-infected subjects. Clin Exp Immunol. 1992 Nov;90(2):175–180. doi: 10.1111/j.1365-2249.1992.tb07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen N. O., Larsen C. S., Juhl H., Esmann V. Membrane-associated protein kinase C activity in superoxide-producing human polymorphonuclear leukocytes. J Leukoc Biol. 1988 Jul;44(1):33–40. doi: 10.1002/jlb.44.1.33. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Gregg E. O. Modulation of Mycobacterium avium growth in murine macrophages: reversal of unresponsiveness to interferon-gamma by indomethacin or interleukin-4. J Leukoc Biol. 1991 Jan;49(1):65–72. doi: 10.1002/jlb.49.1.65. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Hausen A., Reibnegger G., Werner E. R., Werner-Felmayer G., Dierich M. P., Wachter H. Interferon-gamma concentrations are increased in sera from individuals infected with human immunodeficiency virus type 1. J Acquir Immune Defic Syndr. 1989;2(2):158–162. [PubMed] [Google Scholar]
- Gigliotti F., Hughes W. T. Passive immunoprophylaxis with specific monoclonal antibody confers partial protection against Pneumocystis carinii pneumonitis in animal models. J Clin Invest. 1988 Jun;81(6):1666–1668. doi: 10.1172/JCI113503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. L., Reed S. G., Sobel J., Arruda S., He S. H., Wick E. A., Grabstein K. H. Interleukin-3 induces antimicrobial activity against Leishmania amazonensis and Trypanosoma cruzi and tumoricidal activity in human peripheral blood-derived macrophages. Infect Immun. 1992 May;60(5):1984–1993. doi: 10.1128/iai.60.5.1984-1993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. D., Jr, Mei B., Cohn Z. A. The separation, long-term cultivation, and maturation of the human monocyte. J Exp Med. 1977 Dec 1;146(6):1613–1626. doi: 10.1084/jem.146.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. A., Halpern J. L., Lundgren B., Swan J. C., Parrillo J. E., Masur H. Monoclonal antibodies to Pneumocystis carinii: identification of specific antigens and characterization of antigenic differences between rat and human isolates. J Infect Dis. 1989 Jan;159(1):60–70. doi: 10.1093/infdis/159.1.60. [DOI] [PubMed] [Google Scholar]
- Laursen A. L., Jensen B. N., Andersen P. L. Local antibodies against Pneumocystis carinii in bronchoalveolar lavage fluid. Eur Respir J. 1994 Apr;7(4):679–685. doi: 10.1183/09031936.94.07040679. [DOI] [PubMed] [Google Scholar]
- Laursen A. L., Obel N., Rungby J., Andersen P. L. Phagocytosis and stimulation of the respiratory burst in neutrophils by Pneumocystis carinii. J Infect Dis. 1993 Dec;168(6):1466–1471. doi: 10.1093/infdis/168.6.1466. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Gootee L. Colony-stimulating factors activate human macrophages to inhibit intracellular growth of Histoplasma capsulatum yeasts. Infect Immun. 1992 Nov;60(11):4593–4597. doi: 10.1128/iai.60.11.4593-4597.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokta M. A., Pollard R. B. Patterns of interferon-gamma production by peripheral blood mononuclear cells from patients with human immunodeficiency virus infection. J Interferon Res. 1990 Apr;10(2):173–181. doi: 10.1089/jir.1990.10.173. [DOI] [PubMed] [Google Scholar]
- Phair J., Muñoz A., Detels R., Kaslow R., Rinaldo C., Saah A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. Multicenter AIDS Cohort Study Group. N Engl J Med. 1990 Jan 18;322(3):161–165. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- Saulsbury F. T., Bernstein M. T., Winkelstein J. A. Pneumocystis carinii pneumonia as the presenting infection in congenital hypogammaglobulinemia. J Pediatr. 1979 Oct;95(4):559–561. doi: 10.1016/s0022-3476(79)80766-0. [DOI] [PubMed] [Google Scholar]
- Smulian A. G., Stringer J. R., Linke M. J., Walzer P. D. Isolation and characterization of a recombinant antigen of Pneumocystis carinii. Infect Immun. 1992 Mar;60(3):907–915. doi: 10.1128/iai.60.3.907-915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szefler S. J., Norton C. E., Ball B., Gross J. M., Aida Y., Pabst M. J. IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol. 1989 Jun 1;142(11):3985–3992. [PubMed] [Google Scholar]
- Taylor M. B., Phillips M., Easmon C. S. Opsonophagocytosis of Pneumocystis carinii. J Med Microbiol. 1992 Apr;36(4):223–228. doi: 10.1099/00222615-36-4-223. [DOI] [PubMed] [Google Scholar]
- Valentin A., Von Gegerfelt A., Matsuda S., Nilsson K., Asjö B. In vitro maturation of mononuclear phagocytes and susceptibility to HIV-1 infection. J Acquir Immune Defic Syndr. 1991;4(8):751–759. [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E., Yoneda K., Stahr B. J. Pneumocystis carinii: new separation method from lung tissue. Exp Parasitol. 1979 Jun;47(3):356–368. doi: 10.1016/0014-4894(79)90088-2. [DOI] [PubMed] [Google Scholar]
- Wehle K., Schirmer M., Dünnebacke-Hinz J., Küpper T., Pfitzer P. Quantitative differences in phagocytosis and degradation of Pneumocystis carinii by alveolar macrophages in AIDS and non-HIV patients in vivo. Cytopathology. 1993;4(4):231–236. doi: 10.1111/j.1365-2303.1993.tb00093.x. [DOI] [PubMed] [Google Scholar]



