Abstract
The potential of central nervous system (CNS)-derived cells for initiating T cell responses is not known. Using the capacity of unprimed T cells to respond to allogeneic determinants on antigen-presenting cells (APC), we assessed the ability of microglial cells to act as stimulators of primary T cell responses in vitro. For this purpose, microglial cells were activated with lipopolysaccharide (LPS), interferon-gamma (IFN-gamma), or by phagocytosis of progenitor oligodendrocytes and subsequently tested for their ability to induce a proliferative response of naive, resting T cells. Activated microglial cells induced a significant proliferation of virgin, alloreactive CD4+ and CD8+ T lymphocytes, with a more substantial response of highly purified CD4+ than of CD8-expressing T cells. Phagocytosis activation was the most efficient stimulus to induce this APC competence on microglial cells. By contrast, IFN-gamma-pretreated, MHC-expressing astrocytes were unable to induce similar responses of alloreactive CD4+ or CD8+ T cells under the same experimental conditions. Collectively, our data suggest the role of activated microglia as the fully immunocompetent accessory cell population of the CNS.
Full text
PDF

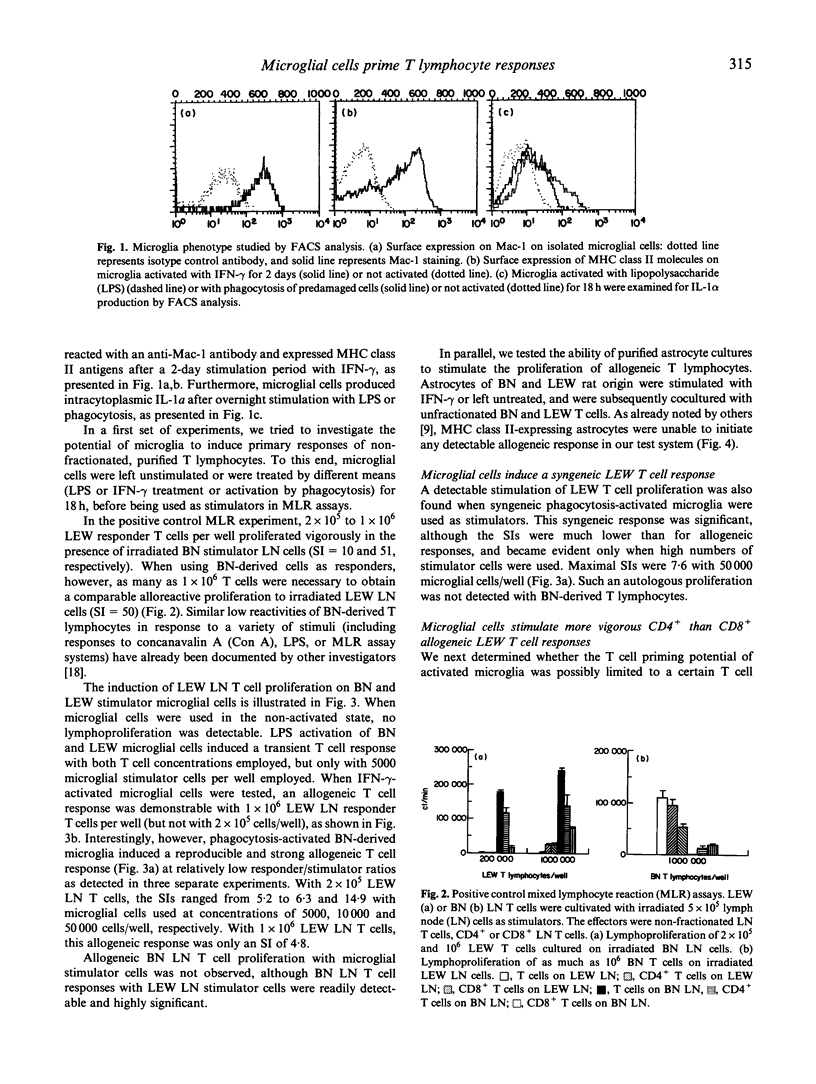
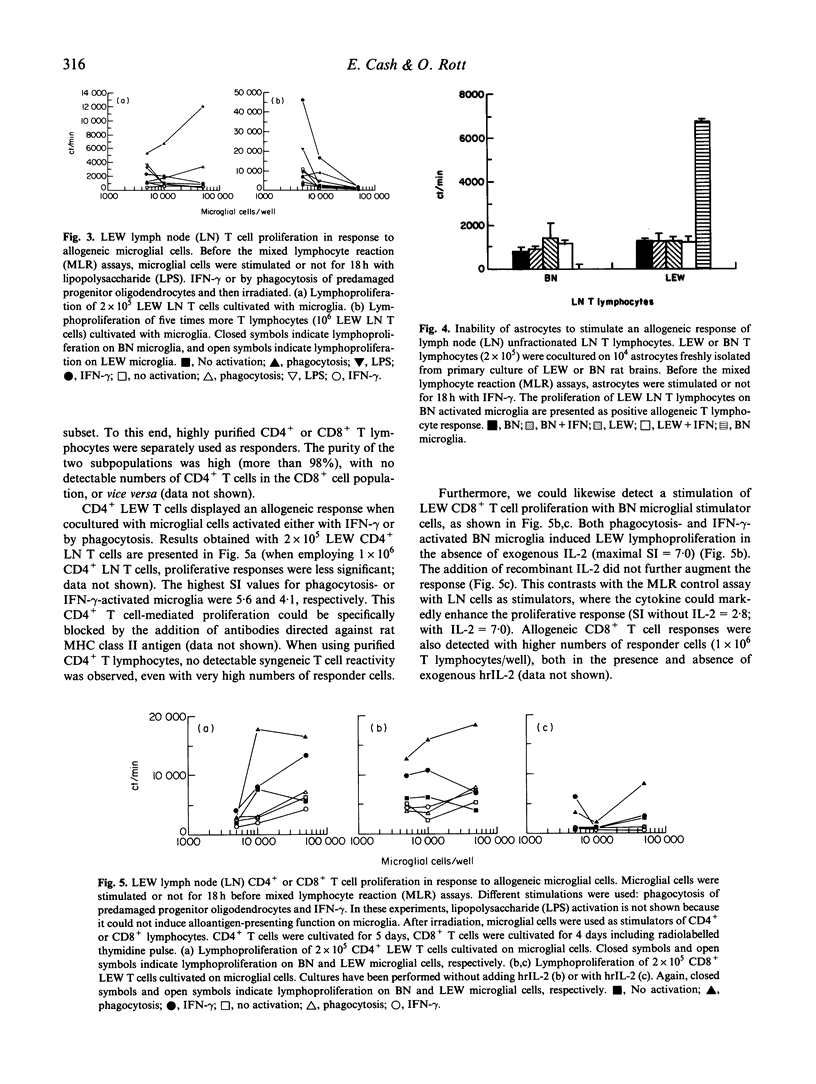


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cash E., Zhang Y., Rott O. Microglia present myelin antigens to T cells after phagocytosis of oligodendrocytes. Cell Immunol. 1993 Mar;147(1):129–138. doi: 10.1006/cimm.1993.1053. [DOI] [PubMed] [Google Scholar]
- Chugani D. C., Kedersha N. L., Rome L. H. Vault immunofluorescence in the brain: new insights regarding the origin of microglia. J Neurosci. 1991 Jan;11(1):256–268. doi: 10.1523/JNEUROSCI.11-01-00256.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craggs R. I., Webster H. D. Ia antigens in the normal rat nervous system and in lesions of experimental allergic encephalomyelitis. Acta Neuropathol. 1985;68(4):263–272. doi: 10.1007/BF00690828. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A., Zhang M., De Vellis J. O2A progenitor cells transplanted into the neonatal rat brain develop into oligodendrocytes but not astrocytes. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):50–54. doi: 10.1073/pnas.90.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18(1):155-71, 132-3. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Benjamin D. S., Burks J., Weiner H. L. Myelin basic protein and proteolipid protein reactivity of brain- and cerebrospinal fluid-derived T cell clones in multiple sclerosis and postinfectious encephalomyelitis. J Immunol. 1987 Jul 1;139(1):68–72. [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hirsch M. R., Wietzerbin J., Pierres M., Goridis C. Expression of Ia antigens by cultured astrocytes treated with gamma-interferon. Neurosci Lett. 1983 Oct 31;41(1-2):199–204. doi: 10.1016/0304-3940(83)90247-1. [DOI] [PubMed] [Google Scholar]
- Hoffman P. M., Dhib-Jalbut S., Mikovits J. A., Robbins D. S., Wolf A. L., Bergey G. K., Lohrey N. C., Weislow O. S., Ruscetti F. W. Human T-cell leukemia virus type I infection of monocytes and microglial cells in primary human cultures. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11784–11788. doi: 10.1073/pnas.89.24.11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Collins M., Vanguri P., Shin M. L. Glutamate differentially inhibits the expression of class II MHC antigens on astrocytes and microglia. J Immunol. 1992 Jun 1;148(11):3391–3397. [PubMed] [Google Scholar]
- Mason D. W., Simmonds S. J. The autonomy of CD8+ T cells in vitro and in vivo. Immunology. 1988 Oct;65(2):249–257. [PMC free article] [PubMed] [Google Scholar]
- McFarland H. F., Dhib-Jalbut S. Multiple sclerosis: possible immunological mechanisms. Clin Immunol Immunopathol. 1989 Jan;50(1 Pt 2):S96–105. doi: 10.1016/0090-1229(89)90116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlay J. P., Puré E., Steinman R. M. Control of the immune response at the level of antigen-presenting cells: a comparison of the function of dendritic cells and B lymphocytes. Adv Immunol. 1989;47:45–116. doi: 10.1016/s0065-2776(08)60662-8. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Kwon E. E., Cho E. S., Sharer L. R. Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann N Y Acad Sci. 1984;436:11–32. doi: 10.1111/j.1749-6632.1984.tb14773.x. [DOI] [PubMed] [Google Scholar]
- Rott O., Tontsch U., Fleischer B. Dissociation of antigen-presenting capacity of astrocytes for peptide-antigens versus superantigens. J Immunol. 1993 Jan 1;150(1):87–95. [PubMed] [Google Scholar]
- Sedgwick J. D., Mössner R., Schwender S., ter Meulen V. Major histocompatibility complex-expressing nonhematopoietic astroglial cells prime only CD8+ T lymphocytes: astroglial cells as perpetuators but not initiators of CD4+ T cell responses in the central nervous system. J Exp Med. 1991 May 1;173(5):1235–1246. doi: 10.1084/jem.173.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Antigen-presenting cells for unprimed T cells. Immunol Today. 1989 Jan;10(1):17–23. doi: 10.1016/0167-5699(89)90060-1. [DOI] [PubMed] [Google Scholar]
- Tontsch U., Rott O. Cortical neurons selectively inhibit MHC class II induction in astrocytes but not in microglial cells. Int Immunol. 1993 Mar;5(3):249–254. doi: 10.1093/intimm/5.3.249. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Weaver C. T., Fuhlbrigge R. C., Kiely J. M., Chaplin D. D. Membrane IL-1: a key protein in antigen presentation. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):489–492. doi: 10.1016/s0769-2625(87)80064-8. [DOI] [PubMed] [Google Scholar]
- Vass K., Lassmann H. Intrathecal application of interferon gamma. Progressive appearance of MHC antigens within the rat nervous system. Am J Pathol. 1990 Oct;137(4):789–800. [PMC free article] [PubMed] [Google Scholar]
- Williams R. M., Moore M. J., Benacerraf B. Genetic control of thymus-derived cell function. IV. Mitogen responsiveness and mixed lymphocyte reactivity of thymus cells and lymph node cells from Lewis and Brown Norway rats. J Immunol. 1973 Nov;111(5):1579–1584. [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]


