Abstract
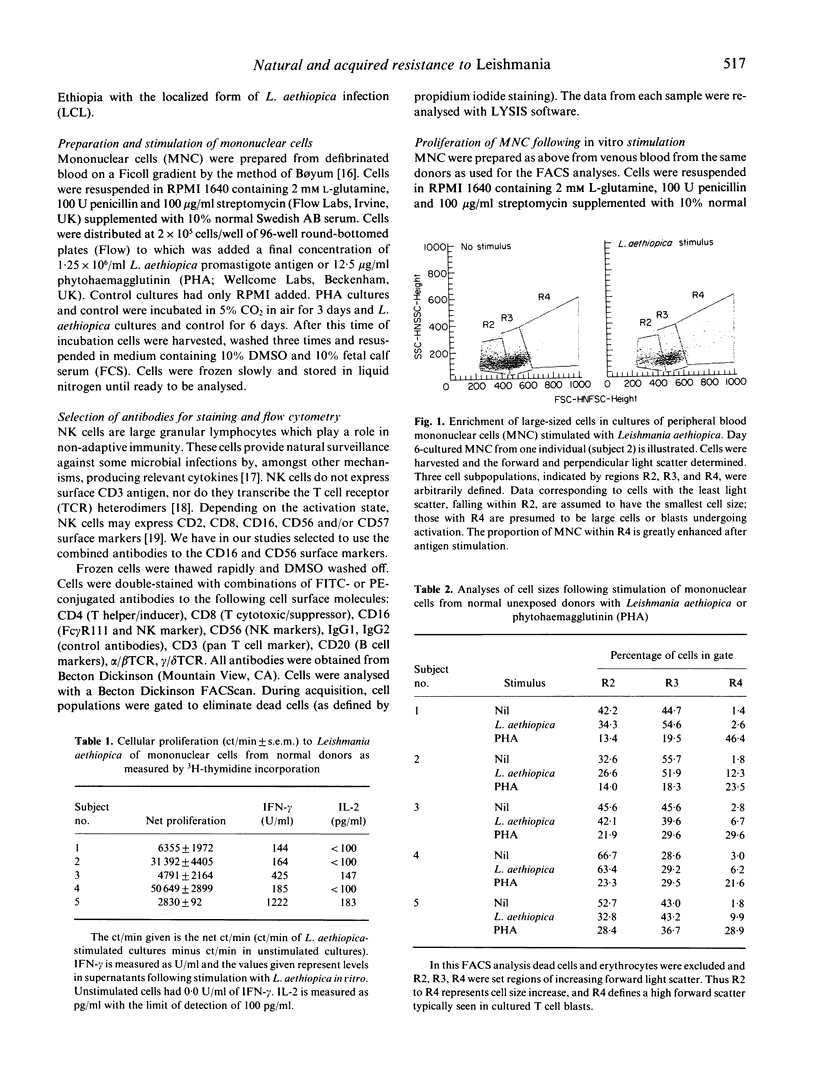
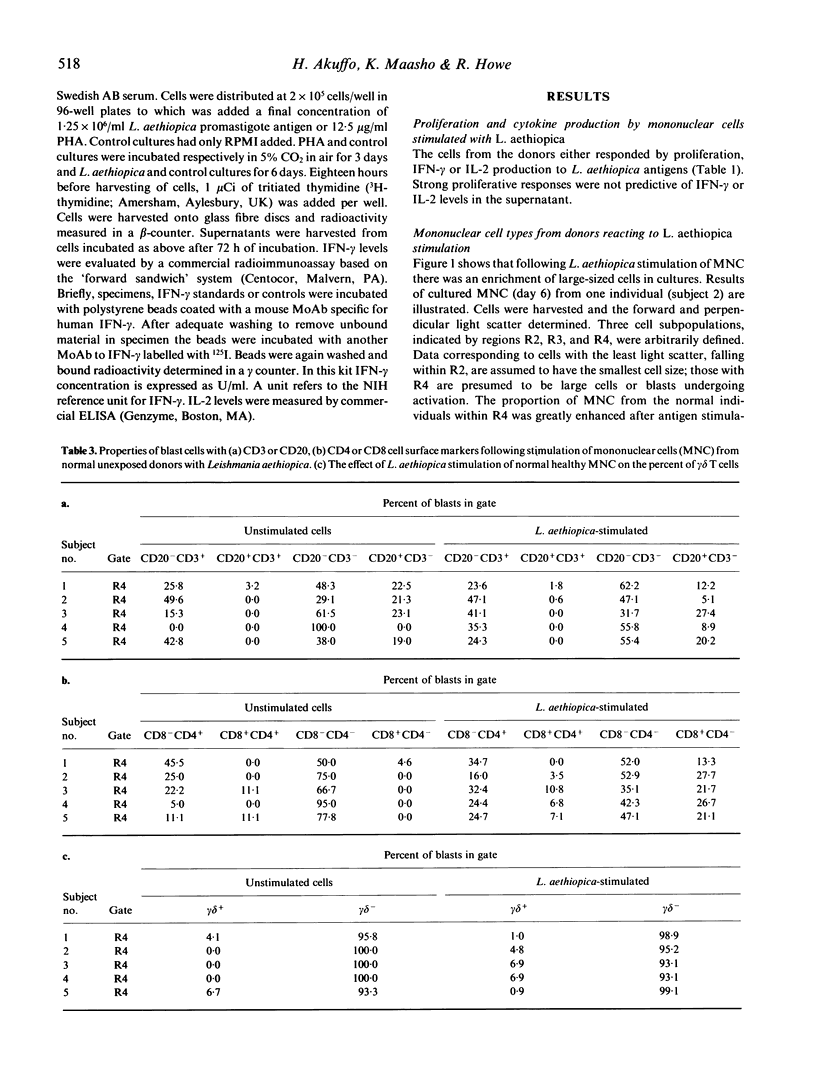
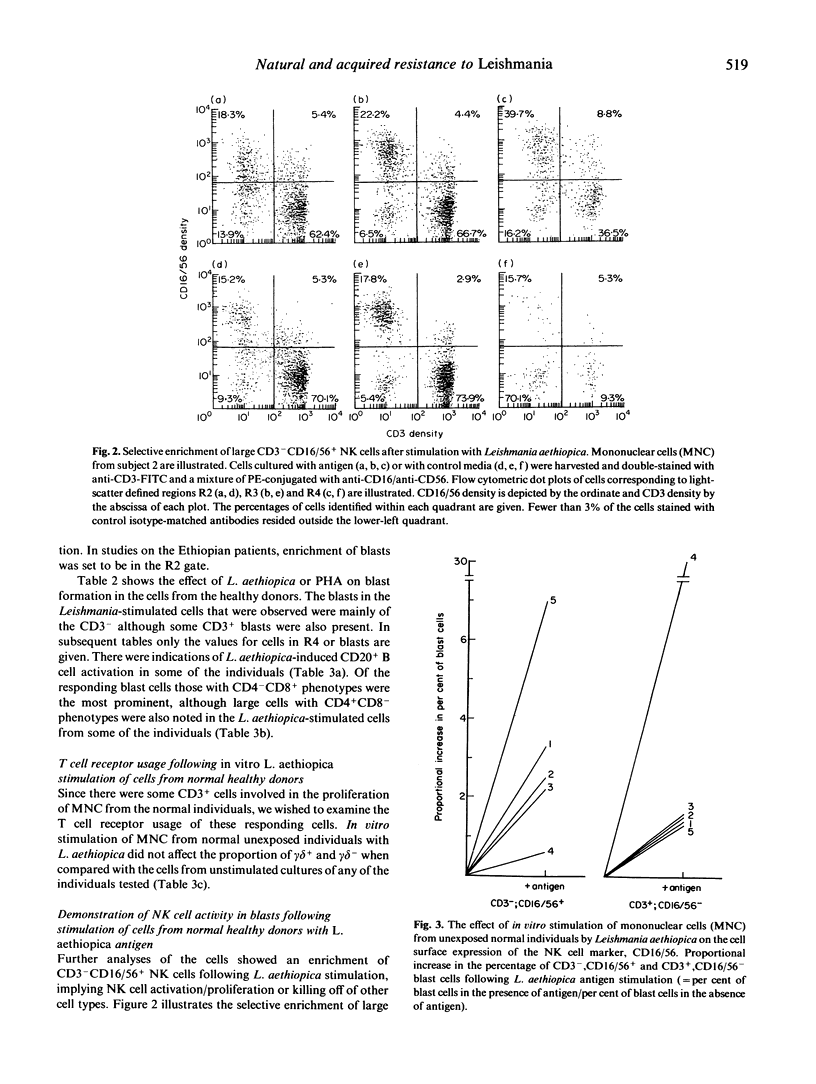
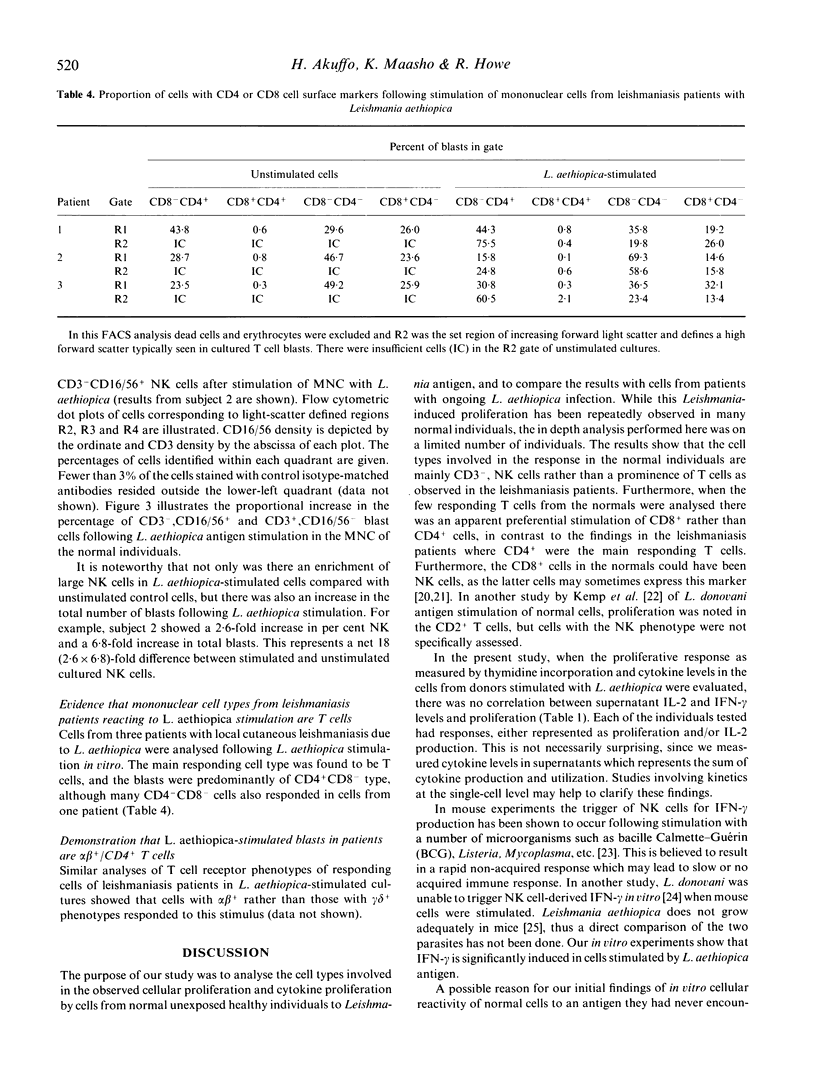
Cells from normal non-Leishmania-exposed individuals could respond in vitro by proliferation and interferon-gamma (IFN-gamma) production to Leishmania aethiopica stimulation. The main cell type that appeared to be activated following such stimulation was CD3-, CD16+/56+, i.e. NK cells. Of the few CD3+ cells responding, an involvement of CD8+ cells was evident in the absence of activation of CD4+ cells in normal individuals, while a different feature was observed when patients' cells were investigated. Cells from patients with L. aethiopica infection did not show this NK response, but rather the CD4+ cells were the prominent responding cells. No evidence of the involvement of superantigens or cells utilizing the gamma delta T cell receptor (gamma delta cells) in the response of unexposed individuals was noted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akuffo H. O., Britton S. F. Contribution of non-Leishmania-specific immunity to resistance to Leishmania infection in humans. Clin Exp Immunol. 1992 Jan;87(1):58–64. doi: 10.1111/j.1365-2249.1992.tb06413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuffo H. O., Fehniger T. E., Britton S. Differential recognition of Leishmania aethiopica antigens by lymphocytes from patients with local and diffuse cutaneous leishmaniasis. Evidence for antigen-induced immune suppression. J Immunol. 1988 Oct 1;141(7):2461–2466. [PubMed] [Google Scholar]
- Akuffo H. O. Non-parasite-specific cytokine responses may influence disease outcome following infection. Immunol Rev. 1992 Jun;127:51–68. doi: 10.1111/j.1600-065x.1992.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Akuffo H. O., Walford C., Nilsen R. The pathogenesis of Leishmania aethiopica infection in BALB/c mice. Scand J Immunol. 1990 Aug;32(2):103–110. doi: 10.1111/j.1365-3083.1990.tb02899.x. [DOI] [PubMed] [Google Scholar]
- Akuffo H., Schurr E., Andersson G., Yamaneberhan T., Britton S. Responsiveness in diffuse versus local cutaneous leishmaniasis is due to parasite differences. Scand J Immunol. 1987 Dec;26(6):717–721. doi: 10.1111/j.1365-3083.1987.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Unanue E. R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991 Dec;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Biassoni R., Ferrini S., Prigione I., Moretta A., Long E. O. CD3-negative lymphokine-activated cytotoxic cells express the CD3 epsilon gene. J Immunol. 1988 Mar 1;140(5):1685–1689. [PubMed] [Google Scholar]
- Hercend T., Reinherz E. L., Meuer S., Schlossman S. F., Ritz J. Phenotypic and functional heterogeneity of human cloned natural killer cell lines. Nature. 1983 Jan 13;301(5896):158–160. doi: 10.1038/301158a0. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Imani F., Soloski M. J. Heat shock proteins can regulate expression of the Tla region-encoded class Ib molecule Qa-1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10475–10479. doi: 10.1073/pnas.88.23.10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Bancroft G. J. Leishmania donovani infection in scid mice: lack of tissue response and in vivo macrophage activation correlates with failure to trigger natural killer cell-derived gamma interferon production in vitro. Infect Immun. 1992 Oct;60(10):4335–4342. doi: 10.1128/iai.60.10.4335-4342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M., Hansen M. B., Theander T. G. Recognition of Leishmania antigens by T lymphocytes from nonexposed individuals. Infect Immun. 1992 Jun;60(6):2246–2251. doi: 10.1128/iai.60.6.2246-2251.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Liew F. Y., Cox F. E. Nonspecific defence mechanism: the role of nitric oxide. Immunol Today. 1991 Mar;12(3):A17–A21. doi: 10.1016/S0167-5699(05)80006-4. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Li Y., Lelchuk R., Chan W. L., Ziltener H. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin (IL)3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989 Jul;19(7):1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- Maasho K., Akuffo H. O. Cells from healthy non-exposed individuals produce cytokines to selected fractions of Leishmania promastigotes. Scand J Immunol Suppl. 1992;11:179–184. doi: 10.1111/j.1365-3083.1992.tb01647.x. [DOI] [PubMed] [Google Scholar]
- Mauël J. Macrophage-parasite interactions in Leishmania infections. J Leukoc Biol. 1990 Feb;47(2):187–193. doi: 10.1002/jlb.47.2.187. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Locksley R. M., Tubbs C., Raff H. V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986 Jan;136(2):655–661. [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Srour E. F., Leemhuis T., Jenski L., Redmond R., Jansen J. Cytolytic activity of human natural killer cell subpopulations isolated by four-color immunofluorescence flow cytometric cell sorting. Cytometry. 1990;11(3):442–446. doi: 10.1002/cyto.990110316. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


